Abstract
Hybrid Peripheral Nerve Sheath Tumors (HPNSTs) are rare benign neoplasms that exhibit a combination of histological features from multiple types of benign peripheral nerve sheath tumors, including schwannomas, neurofibromas, and perineuriomas. These tumors present a diagnostic challenge due to their morphological and histological variability. In this article, we aim to summarize the key morphological, histological, and molecular characteristics of HPNSTs, providing insights into their diagnostic approaches. We review the different hybrid subtypes, including schwannoma–perineurioma, schwannoma–neurofibroma, and perineurioma–neurofibroma, emphasizing their clinical features, genetic associations, and the role of surgical excision as the gold-standard treatment.
1. Introduction
Hybrid Peripheral Nerve Sheath Tumors (HPNSTs) are benign neoplasms that exhibit histological features of more than one type of peripheral nerve sheath tumor. The three types of peripheral nerve sheath tumors are schwannoma, neurofibroma, and perineurioma. Although recognized for some time, these tumors were only officially included in the World Health Organization (WHO) Classification of Tumors of Soft Tissue and Bone separately for the first time in the 4th edition and continued in the 5th edition of the WHO classification of CNS tumors, as well as soft tissue and bone tumors [1,2,3]. HPNSTs have been reported across all age groups, with a higher prevalence in young adults and no significant gender predilection. They exhibit a wide anatomical distribution, predominantly affecting somatic soft tissues, although cases involving bone have also been documented. A recent report by Chow et al. described an HPNST located in the femur [4]. The most common sites include the fingers (digits), especially in schwannoma/perineurioma hybrids, where they typically present as painless subcutaneous or dermal masses. Macroscopically, these tumors appear as well-circumscribed, firm, nodular lesions with a grayish cut surface, ranging from 1 to 8 cm in size [5]. In Table 1, all the main clinical and histological characteristics of the three types of PNSTs (schwannoma, neurofibroma, perineurioma) have been summarized.

Table 1.
Classic and distinguishing features of each of the 3 main types of PNSTs.
Histologically, HPNSTs retain the morphologic and immunohistochemical features of their individual components, typically displaying a dual composition. Hybrid schwannoma/perineurioma tumors resemble perineuriomas in their lamellar, storiform, or whorled architecture, but their cellular morphology is predominantly schwannoma-like, with spindle-shaped cells, wavy nuclei, pale eosinophilic cytoplasm, and indistinct borders. Hybrid schwannoma/neurofibroma tumors, on the other hand, contain schwannoma-like Antoni A areas with nuclear palisading and Verocay bodies, alongside neurofibroma-like regions composed of fibroblasts, elongated wavy nuclei, and a collagenous or mucin-rich myxoid matrix arranged in an architecture that may be plexiform, albeit with a lack of consensus on diagnostic criteria [6]. The rare hybrid neurofibroma/perineurioma tumors present areas of perineuriomatous differentiation adjacent to plexiform neurofibroma-like regions [7]. Immunohistochemical (IHC) analysis confirmed these dual components: schwannoma/perineurioma hybrids were positive for S100 and SOX10 in schwannomatous regions, while perineuriomatous areas expressed EMA, Claudin-1, and GLUT-1. Schwannoma/neurofibroma hybrids exhibit strong S100 and SOX10 positivity in schwannomatous areas, whereas the neurofibroma component expresses S100, SOX10, EMA, and GLUT-1. In neurofibroma/perineurioma hybrids, the neurofibroma component follows the above-mentioned immunoprofile, while perineuriomatous components lack S100 expression [8]. HPNSTs are frequently associated with tumor syndromes, particularly NF2-related schwannomatosis, other schwannomatoses, and less frequently neurofibromatosis type 1 (NF1). More than 70% of patients with schwannomatoses develop single or multiple hybrid neurofibroma/schwannoma tumors, while over 25% of patients with NF2-related schwannomatosis exhibit at least one hybrid neurofibroma/schwannoma tumor. Hybrid neurofibroma/perineurioma tumors occur almost exclusively in NF1 [8]. Given their association with NF1, these tumors may undergo malignant transformation into malignant peripheral nerve sheath tumors (MPNSTs), although the precise recurrence and malignancy rates remain unclear. While rare, a few case reports describe local recurrence, and at least two cases of malignant transformation have been documented [9]. By analyzing the specific characteristics of this large group of tumors, this review aims to highlight the main diagnostic, prognostic, and therapeutic aspects, in order to improve the overall knowledge of and approach to these relatively uncommon entities.
2. Materials and Methods
2.1. Search Strategy
A literature review was conducted using PubMed/MEDLINE and Scopus to identify all English-language published cases of Hybrid Nerve Sheath Tumors in both adult and pediatric patients from 2014 to the present. The research utilized the following Medical Subject Headings (MeSH) terms: Hybrid Peripheral Nerve Sheath Tumor, Benign Neurofibroma/Schwannoma, Schwannoma/Perineurioma, and Neurofibroma/Perineurioma. Additionally, we manually reviewed the reference lists of relevant studies to identify articles that may have been missed during the electronic search.
2.2. Inclusion and Exclusion Criteria
The timeframe for the selected studies ranged from January 2014 to December 2024. Articles not addressing HPNSTs or those discussing them from a perspective other than their clinical, morphological, immunohistochemical, and genetic features were excluded.
Both case series (studies reporting at least two cases) and single case reports on this topic were included in the review, while comments, perspectives, guidelines, editorials, systematic reviews and/or meta-analyses, and manuscripts in languages other than English were excluded.
Papers available only as abstracts or those with text appearing too brief or non-informative were not included in the present review.
The clinical parameters analyzed included sex, age (median and range), anatomical location, median follow-up, HPNST type, genetic associations, tumor syndromes, histological and imaging features, local recurrence, and treatment (Table 2).

Table 2.
Cases of HPNSTs reported in the literature to date.
3. Results
To the best of our knowledge, about 112 cases of HPNSTs have been published to date. The analysis across 112 documented cases revealed a nearly equal distribution between genders, with 43% of patients being female (n = 48) and 37% male (n = 43). In 21 cases, the patients’ gender was not specified. The average age varied, but most cases fell within the 24–50 age range, with extremes spanning from 2 to 85 years old. Tumors were most commonly found in the limbs, accounting for 42% (47/112 cases), followed by spinal and peripheral nerves (22%; 25/112 cases), other soft tissue sites (retroperitoneum, vestibule, thigh, etc.) for 6% (8/112 cases), head and neck region (9%; 10/112 cases), and the gastrointestinal tract (3%; 4/112 cases). In the remaining 18 cases, the anatomical location of the lesion was not available (Figure 1).
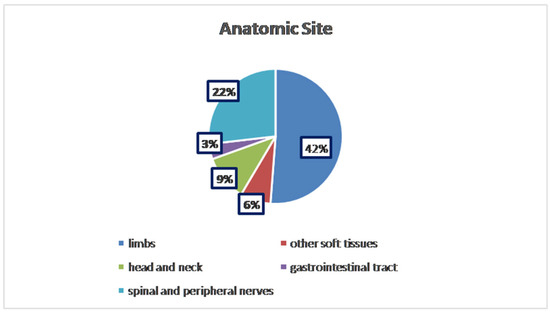
Figure 1.
Distribution of anatomical sites.
From a histopathological perspective, the majority of tumors were classified as schwannoma–perineurioma hybrids, making up approximately three-quarters of the reported cases (75.89%; 85/112 cases). Other hybrid variants, including neurofibroma–schwannoma and perineurioma–neurofibroma, were less frequent (19.64%, 22/112 cases; 2.68%, 3/112 cases, respectively). To the best of our knowledge, only two reported cases described a hybrid tumor composed of three distinct components: schwannoma, neurofibroma, and perineurioma (1.79%; 2/112) [16] (Figure 2).
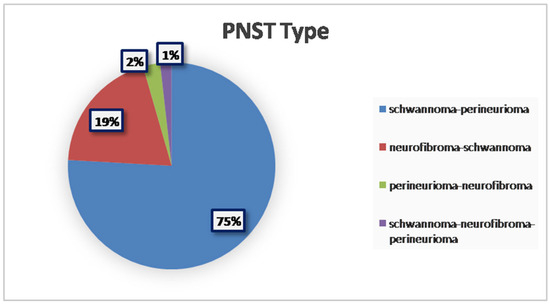
Figure 2.
Prevalence of different PNST types.
Regardless of histological subtype or anatomical location, all cases were treated surgically, reinforcing the fact that surgical excision with free margins remains the gold-standard treatment. The prognosis was highly favorable, with only one reported case of local recurrence, due to the presence of infiltrative margins and incomplete initial excision.
Genetic analysis was performed on 15% of the 112 cases. For a majority of these, no identifiable recognized genetic condition or related constitutional gene variant was found; however, as well as for the NF1 gene in neurofibromatosis, there are now known to be several genes in which mutations can underlie NF2 or schwannomatosis, including SMARCB1, LZTR1, or NF2 genes [27], and not all of the cases which had genetic analysis may have been tested for all relevant genes. However, a few instances exhibited somatic variants in genes such as BRAF, TERT, and NF2. Imaging findings varied based on tumor location, with MRI typically revealing well-circumscribed, lobulated masses. In cases affecting bone, osteolytic lesions were often present, while tumors in the gastrointestinal tract sometimes led to obstructive complications. In five cases, the patients were affected by neurofibromatosis type 1, and in fourteen cases, by schwannomatosis.
Furthermore, pertinent data were extracted and arranged in a narrative manner.
4. Discussion
4.1. Schwannoma–Perineurioma
The schwannoma–perineurioma hybrid is the most reported subtype and typically occurs sporadically. Macroscopically, these tumors are well circumscribed but unencapsulated and are composed of spindle cells with wavy, tapering nuclei, eosinophilic cytoplasm, and indistinct cell borders. Histologically, they display a perineurioma-like pattern, with storiform, lamellar, or whorled growth, while still maintaining a schwannoma-like cytomorphology (Figure 3). Degenerative changes similar to those seen in ancient schwannomas, such as myxoid degeneration and cystic alterations, may also be present. Immunohistochemically, these tumors exhibit S100 and SOX10 immunoreactivity in schwannomatous areas, while perineuriomatous components express EMA, Claudin-1, and GLUT-1 (Figure 4). Unlike schwannoma–neurofibroma hybrids, these tumors are usually sporadic and not strongly associated with genetic syndromes. They are considered benign with an extremely low recurrence risk, although rare cases of low-grade malignant potential have been reported in the literature [5]. It has also been reported that the majority of hybrid schwannoma–perineuriomas harbor VGLL3 rearrangements [28,29].
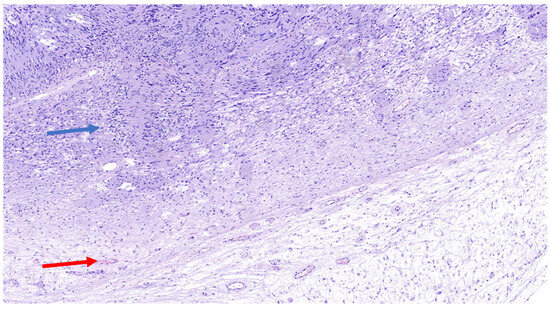
Figure 3.
H&E (100× magnification) of a peripheral nerve sheath tumor with features of schwannoma and perineurioma. The schwannomatous component: areas of spindle-shaped cells arranged in bundles, with Verocay bodies (blue arrow), moderate cellularity. The perineurial component: uniform spindle cells arranged in a storiform pattern within a denser collagenous matrix (red arrow).
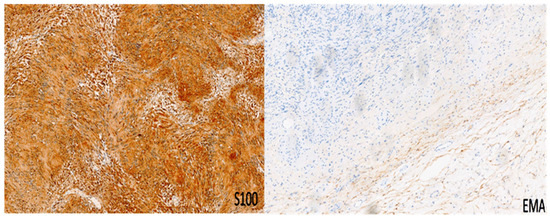
Figure 4.
Immunohistochemistry for S100 and EMA in a peripheral nerve sheath tumor with features of schwannoma and perineurioma. S100 shows immunoreactivity in schwannomatous areas, while perineuriomatous components express EMA. Immunoperoxidase; original magnifications: 100×.
4.2. Schwannoma–Neurofibroma
The schwannoma–neurofibroma hybrid is one of the more commonly described HPNST variants and is frequently associated with NF1- and NF2-related schwannomatosis, as well as other forms of schwannomatosis. Although accurate diagnostic criteria have not yet been established [6], these tumors exhibit distinct cellular components: Schwann-cell nodules exhibiting Antoni A areas with nuclear palisading and Verocay bodies, and strong immunoreactivity for SOX10 and S100, while intermixed neurofibroma-like regions consist of elongated, wavy nuclei, fibroblasts, and a collagen-rich myxoid matrix, often organized in a plexiform arrangement (Figure 5), with scattered S100/SOX10 staining, and entrapped NFP-positive axons. As mentioned above, immunohistochemically, schwannomatous areas are strongly positive for S100 and SOX10, whereas the neurofibroma component expresses S100, SOX10, CD34, EMA, and GLUT-1 (Figure 6). These tumors are usually slow-growing and asymptomatic, and they have a low recurrence rate following surgical excision. However, when associated with NF1, there is a potential risk of malignant transformation into MPNSTs [7].
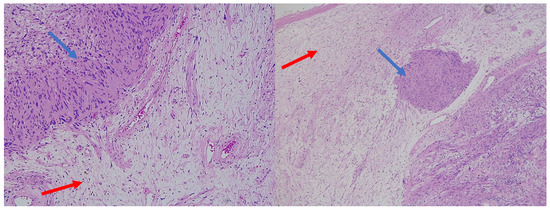
Figure 5.
H&E (100× magnification) of a peripheral nerve sheath tumor with features of schwannoma and neurofibroma. The schwannomatous component: elongated cellular bundles, Verocay bodies (blue arrow), and an Antoni A/B pattern. The neurofibromatous component: heterogeneous cellular population, scattered spindle cells within a more myxoid matrix (red arrow).
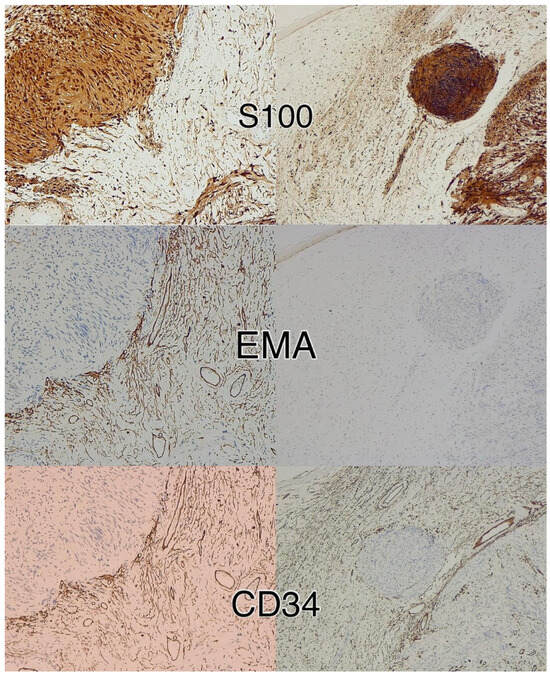
Figure 6.
Immunohistochemistry for S100, CD34, and EMA in a peripheral nerve sheath tumor with features of schwannoma and neurofibroma. Schwannomatous areas are strongly positive for S100, whereas the neurofibroma component expresses S100, CD34, and EMA. Immunoperoxidase; original magnifications 100×.
4.3. Perineurioma–Neurofibroma
The perineurioma–neurofibroma hybrid is the rarest subtype of HPNSTs and is almost always associated with NF1. These tumors contain both perineuriomatous and neurofibromatous regions, with the latter frequently exhibiting a plexiform architecture. The perineurioma component consists of spindle cells with elongated nuclei, arranged in thin fascicles or whorled patterns, whereas the neurofibroma component comprises fibroblasts, Schwann cells, and an extracellular matrix rich in mucin and collagen fibers. Immunohistochemically, the neurofibroma component is positive for S100, SOX10, EMA, Claudin-1, and GLUT-1, while the perineurioma component does not express S100. This subtype has been reported less frequently than other hybrid PNSTs, and its clinical behavior is not well characterized. However, given its strong association with NF1, there is a potential risk of malignant transformation, similar to other NF1-associated tumors [7]. Due to their rarity, their classification is still to be defined, and it is not clear whether they represent a “true” distinct tumor category or simply neurofibromas rich in perineurial cells.
5. Conclusions
HPNSTs are a rare and complex group of neoplasms that primarily affect young to middle-aged adults and have no significant gender predisposition. They most commonly occur in the limbs and soft tissues and are predominantly composed of schwannoma–perineurioma hybrids. Surgical excision remains the gold standard for treatment, with a favorable prognosis and an extremely low recurrence rate. Despite the lack of consistent genetic associations, some cases exhibit somatic mutations in genes such as BRAF, TERT, and NF2, suggesting potential molecular underpinnings of these tumors. The association with genetic syndromes, particularly NF1, warrants careful surveillance, as there is a possibility of malignant transformation, although this outcome remains unusual. Our findings align with those of Hornick et al. [13], who reported a predominance of schwannoma–perineurioma hybrids and an extremely low rate of local recurrence (probably due to infiltrative margins on histology and a lack of complete surgical resection). It must be mentioned that rare cases of benign cutaneous plexiform hybrid tumors of perineurioma and cellular neurothekeoma (BCPHTPCNs) have been recently described [30,31,32]; these lesions tend to arise as solitary papules in the perioral area [16,28,29]. BCPHTPCNs exhibit combined morphologic features of both perineurioma and cellular neurothekeoma, frequently arranged in a plexiform pattern [30,31,32]. Neurothekeomas are rare, benign soft tissue tumors that typically appear on the head and neck. The histological patterns of neurothekeomas can vary, including myxoid, cellular, or mixed types, with the variation primarily dependent on the amount of myxoid matrix present [33]. However, as neurothekeomas are not properly considered peripheral nerve sheath tumors, we excluded this hybrid form from the present review.
Finally, further research into the molecular characteristics (e.g., NF2, BRAF, and TERT mutations) of these tumors may offer greater insight into their pathogenesis and potential therapeutic targets. Overall, the current data support a highly favorable outcome for most patients, particularly when treated surgically, although long-term monitoring is essential for those with genetic predispositions.
Author Contributions
Conceptualization, S.S. and G.B.; methodology, R.C.; software, S.S.; validation, G.M., R.C., V.B. and G.B.; formal analysis, M.Z. (Magda Zanelli); investigation, A.P.; resources, M.Z. (Maurizio Zizzo); data curation, N.K.; writing—original draft preparation, S.S.; writing—review and editing, G.B.; visualization, I.B.; supervision, G.B.; project administration, M.Z. (Magda Zanelli); funding acquisition, M.Z. (Magda Zanelli). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
This study was partially supported by the Italian Ministry of Health—Ricerca Corrente Annual Program 2025. We wish to thank Sabrina Francesca Vinci, Giovanni Mattia and Virginia Dolcini from Struttura Semplice Grant Office & Research Administration S.C. Infrastruttura Ricerca-Statistica, Direzione Scientifica Azienda USL-IRCCS Reggio Emilia.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HPNSTs | Hybrid Peripheral Nerve Sheath Tumors |
| WHO | World Health Organization |
| PNSTs | Peripheral nerve sheath tumors |
| MPNSTs | Malignant peripheral nerve sheath tumors |
| MeSH | Medical Subject Headings |
| IHC | Immunohistochemical |
| NF1 | Neurofibromatosis type 1 |
| BCPHTPCN | Benign cutaneous plexiform hybrid tumor of perineurioma and cellular neurothekeoma |
References
- Central Nervous System Tumors, 5th ed.; WHO Press: Geneva, Switzerland, 2021.
- Soft Tissue and Bone Tumors, 5th ed.; WHO Press: Geneva, Switzerland, 2020.
- Michal, M.; Kazakov, D.V.; Belousova, I.; Bisceglia, M.; Zamecnik, M.; Mukensnabl, P. A benign neoplasm with histopathological features of both schwannoma and retiform perineurioma (benign schwannoma-perineurioma): A report of six cases of a distinctive soft tissue tumor with a predilection for the fingers. Virchows Arch. 2004, 445, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.T.C. Primary intraosseous hybrid nerve sheath tumor of femur: A hitherto undescribed occurrence in bone with secondary aneurysmal bone cyst formation resulting in pathological fracture. Pathol. Res. Pract. 2015, 211, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Magro, G.; Broggi, G.; Angelico, G.; Puzzo, L.; Vecchio, G.M.; Virzì, V.; Salvatorelli, L.; Ruggieri, M. Practical Approach to Histological Diagnosis of Peripheral Nerve Sheath Tumors: An Update. Diagnostics 2022, 12, 1463. [Google Scholar] [CrossRef]
- Katsumi, T.; Hayashi, R.; Takei, S.; Ansai, O.; Takatsuka, S.; Takenouchi, T.; Saito, K.; Suda, K.; Yoshihara, K.; Nagai, T.; et al. Reevaluating hybrid neurofibroma/schwannoma: Predominance of schwannoma features despite CD34 positivity and initial neurofibroma diagnosis. J. Dermatol. 2024, 51, 1461–1469. [Google Scholar] [CrossRef]
- Feany, M.B.; Anthony, D.C.; Fletcher, C.D. Nerve sheath tumours with hybrid features of neurofibroma and schwannoma: A conceptual challenge. Histopathology 1998, 32, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Kacerovska, D.; Michal, M.; Kuroda, N.; Tanaka, A.; Sima, R.; Denisjuk, N.; Kreuzberg, B.; Ricarova, R.; Kazakov, D.V. Hybrid peripheral nerve sheath tumors, including a malignant variant in type 1 neurofibromatosis. Am. J. Dermatopathol. 2013, 35, 641–649. [Google Scholar] [CrossRef]
- Evans, D.G.R.; Baser, M.E.; McGaughran, J.; Sharif, S.; Howard, E.; Moran, A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J. Med. Genet. 2002, 39, 311–314. [Google Scholar] [CrossRef]
- Agaimy, A.; Michal, M. Hybrid schwannoma-perineurioma of the gastrointestinal tract: A clinicopathologic study of 2 cases and reappraisal of perineurial cells in gastrointestinal schwannomas. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 454–459. [Google Scholar] [CrossRef]
- Bergamini, M.; Costa, A.; Noberto, L.; Torres, G.; Soares, H.; Martins, F.; de Souza, S.; Braz-Silva, P. Primary intra-osseous Hybrid Schwannoma-Perineurioma in the mandible. J. Clin. Exp. Dent. 2020, 12, e888–e891. [Google Scholar] [CrossRef]
- Lang, Y.; Liu, D.; Xiang, P.; Wang, J.; Li, Y. Primary intraosseous hybrid epithelioid schwannoma/perineurioma in the proximal tibia: A case report of benign hybrid neoplasm with local hypercellularity. Diagn. Pathol. 2019, 14, 51. [Google Scholar] [CrossRef]
- Hornick, J.L.; Bundock, E.A.; Fletcher, C.D.M. Hybrid schwannoma/perineurioma: Clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am. J. Surg. Pathol. 2009, 33, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Choong, P.; Ali, M.; Lindsay, D.; Saifuddin, A. Hybrid peripheral nerve sheath tumours: MRI features with pathological correlation in 24 cases. Br. J. Radiol. 2023, 97, 126–134. [Google Scholar] [CrossRef]
- Din, N.U.; Ahmad, Z.; Abdul-Ghafar, J.; Ahmed, R. Hybrid peripheral nerve sheath tumors: Report of five cases and detailed review of literature. BMC Cancer 2017, 17, 349. [Google Scholar] [CrossRef]
- Kuroda, N.; Kazakov, D.V.; Hes, O.; Michal, M.; Goda, M.; Miyazaki, K.; Hayashi, Y.; Okamoto, S.; Lee, G.-H. Hybrid peripheral nerve sheath tumor of the nasal cavity showing schwannomatous, neurofibromatous, and perineuriomatous areas. Med. Mol. Morphol. 2010, 43, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Chijiiwa, Y.; Sano, J.; Okamura, K.; Nishio, J. Intramuscular Hybrid Nerve Sheath Tumor of the Thigh: Case Report and Literature Review. In Vivo 2024, 38, 971–974. [Google Scholar] [CrossRef]
- Leite, A.A.; Mariz, B.A.L.A.; Oliveira, L.A.; Júnior, J.N.R.A.; de Almeida, O.P.; Vargas, P.A. Hybrid Neurofibroma/Schwannoma of the Oral Cavity: A Rare Case Report and Literature Review. Int. J. Surg. Pathol. 2023, 31, 695–701. [Google Scholar] [CrossRef]
- Emanuel, P.; Pertsemlidis, D.S.; Gordon, R.; Xu, R. Benign hybrid perineurioma-schwannoma in the colon. A case report. Ann. Diagn. Pathol. 2006, 10, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Colazo, J.M.; Perez, A.N.; Judice, A.D.; Quirion, J.; Prieto-Granada, C.N.; Holt, G.E. Benign Neurofibroma/Schwannoma Hybrid Peripheral Nerve Sheath Tumor of the Ulnar Nerve Harboring a Metastatic Papillary Thyroid Carcinoma Deposit: A Case Report of Tumor-to-Tumor Metastasis. Case Rep. Pathol. 2022, 2022, 9038222. [Google Scholar] [CrossRef]
- Goyal-Honavar, A.; Gupta, A.; Chacko, G.; Chacko, A.G. Trigeminal hybrid nerve sheath tumor—A case report and literature review. Br. J. Neurosurg. 2023, 37, 1326–1329. [Google Scholar] [CrossRef]
- Hong, S.; Hara, T. Hybrid nerve sheath tumor in the orbit: A case report and review of literature. Surg. Neurol. Int. 2019, 10, 250. [Google Scholar] [CrossRef]
- Mitsui, Y.; Ishida, E.; Ogawa, K.; Fukumoto, T.; Asada, H. Hybrid Epithelioid Schwannoma/Neurofibroma: A Report of 3 Cases. Am. J. Dermatopathol. 2025, 47, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Inatomi, Y.; Ito, T.; Nagae, K.; Yamada, Y.; Kiyomatsu, M.; Nakano-Nakamura, M.; Uchi, H.; Oda, Y.; Furue, M. Hybrid perineurioma-neurofibroma in a patient with neurofibromatosis type 1, clinically mimicking malignant peripheral nerve sheath tumor. Eur. J. Dermatol. 2014, 24, 412–413. [Google Scholar] [CrossRef] [PubMed]
- Harder, A.; Wesemann, M.; Hagel, C.; Schittenhelm, J.; Fischer, S.; Tatagiba, M.; Nagel, C.; Jeibmann, A.; Bohring, A.; Mautner, V.-F.; et al. Hybrid neurofibroma/schwannoma is overrepresented among schwannomatosis and neurofibromatosis patients. Am. J. Surg. Pathol. 2012, 36, 702–709. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, C.T.; Kaffenberger, B.H.; Gru, A.A. A hybrid tumor with schwannoma-perineurioma-neurofibroma morphology. J. Cutan. Pathol. 2015, 42, 911–913. [Google Scholar] [CrossRef]
- Evans, D.G.; Bowers, N.L.; Tobi, S.; Hartley, C.; Wallace, A.J.; King, A.T.; Lloyd, S.K.W.; Rutherford, S.A.; Hammerbeck-Ward, C.; Pathmanaban, O.N.; et al. Schwannomatosis: A genetic and epidemiological study. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Dickson, B.C.; Antonescu, C.R.; Demicco, E.G.; Leong, I.; Anderson, N.D.; Swanson, D.; Zhang, L.; Fletcher, C.D.M.; Hornick, J.L. Hybrid schwannoma–perineurioma frequently harbors VGLL3 rearrangement. Mod. Pathol. 2021, 34, 1116–1124. [Google Scholar] [CrossRef]
- Nihous, H.; Baud, J.; Azmani, R.M.; Michot, A.; Perret, R.; Mayeur, L.B.; de Pinieux, G.; Milin, S.; Angot, E.; Duquenne, S.; et al. Clinicopathologic and Molecular Study of Hybrid Nerve Sheath Tumors Reveals Their Common Association With Fusions Involving VGLL3. Am. J. Surg. Pathol. 2022, 46, 591–602. [Google Scholar] [CrossRef]
- Linos, K.; Stuart, L.; Goncharuk, V.; Edgar, M. Benign cutaneous biphasic hybrid tumor of perineurioma and cellular neurothekeoma: A case report expanding the clinical and histopathologic features of a recently described entity. Am. J. Dermatopathol. 2015, 37, 319–322. [Google Scholar] [CrossRef]
- Requena, L.; Sitthinamsuwan, P.; Fried, I.; Kaddu, S.; Schirren, C.G.; Schärer, L.; Hantschke, M.; Cerroni, L.; McCalmont, T.H.; Kutzner, H. A benign cutaneous plexiform hybrid tumor of perineurioma and cellular neurothekeoma. Am. J. Surg. Pathol. 2013, 37, 845–852. [Google Scholar] [CrossRef]
- Yamada, S.; Kitada, S.; Nabeshima, A.; Noguchi, H.; Sasaguri, Y.; Hisaoka, M. Benign cutaneous plexiform hybrid tumor of perineurioma and cellular neurothekeoma arising from the nose. Diagn. Pathol. 2013, 8, 165. [Google Scholar] [CrossRef]
- Kao, E.Y.; Kernig, M.L. Neurothekeoma. In StatPearls [Internet]. (2022); StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).