Current Trends and Impact of Liver Biopsy on Survival in Hepatocellular Carcinoma: A Korean Multicenter Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Group Classification
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Changes in Biopsy Rates over Time
3.3. First-Line Treatment Choices for HCC Based on Biopsy Status
3.4. Comparison of OS and TFS Based on Biopsy Status
3.5. Risk Factors for OS and TFS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AASLD | American Association for the Study of Liver Diseases |
| BCLC | Barcelona Clinic Liver Cancer |
| CI | Confidence Interval |
| CT | Computed Tomography |
| HCC | Hepatocellular Carcinoma |
| HR | Hazard Ratio |
| iCCA | Intrahepatic Cholangiocarcinoma |
| IQR | Interquartile Range |
| KPLCR | Korean Primary Liver Cancer Registry |
| LT | Liver Transplantation |
| MRI | Magnetic Resonance Imaging |
| mUICC | Modified Union for International Cancer Control |
| OS | Overall Survival |
| PT-INR | Prothrombin Time-International Normalized Ratio |
| SPSS | Statistical Package for the Social Sciences |
| TACE | Trans-Arterial Chemoembolization |
| TFS | Transplant-Free Survival |
| mUICC | Modified Union for International Cancer Control |
References
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [PubMed]
- Toh, M.R.; Wong, E.Y.T.; Wong, S.H.; Ng, A.W.T.; Loo, L.H.; Chow, P.K.; Ngeow, J. Global Epidemiology and Genetics of Hepatocellular Carcinoma. Gastroenterology 2023, 164, 766–782. [Google Scholar] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar]
- Suddle, A.; Reeves, H.; Hubner, R.; Marshall, A.; Rowe, I.; Tiniakos, D.; Hubscher, S.; Callaway, M.; Sharma, D.; See, T.C.; et al. British Society of Gastroenterology guidelines for the management of hepatocellular carcinoma in adults. Gut 2024, 73, 1235–1268. [Google Scholar] [PubMed]
- Rho, Y.S.; Pagano, I.; Wong, L.L.; Kwee, S.A.; Acoba, J.D. Factors and Survival Implications associated with biopsy of hepatocellular carcinoma. HPB 2021, 23, 1054–1060. [Google Scholar]
- Jain, D. Tissue diagnosis of hepatocellular carcinoma. J. Clin. Exp. Hepatol. 2014, 4, S67–S73. [Google Scholar]
- Childs, A.; Zakeri, N.; Ma, Y.T.; O’Rourke, J.; Ross, P.; Hashem, E.; Hubner, R.A.; Hockenhull, K.; Iwuji, C.; Khan, S.; et al. Biopsy for advanced hepatocellular carcinoma: Results of a multicentre UK audit. Br. J. Cancer 2021, 125, 1350–1355. [Google Scholar]
- Di Tommaso, L.; Spadaccini, M.; Donadon, M.; Personeni, N.; Elamin, A.; Aghemo, A.; Lleo, A. Role of liver biopsy in hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 6041–6052. [Google Scholar]
- EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [CrossRef]
- Roberts, L.R.; Sirlin, C.B.; Zaiem, F.; Almasri, J.; Prokop, L.J.; Heimbach, J.K.; Murad, M.H.; Mohammed, K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology 2018, 67, 401–421. [Google Scholar]
- Kim, H.; Park, Y.N. Role of biopsy sampling for diagnosis of early and progressed hepatocellular carcinoma. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 813–829. [Google Scholar] [PubMed]
- Beaufrère, A.; Calderaro, J.; Paradis, V. Combined hepatocellular-cholangiocarcinoma: An update. J. Hepatol. 2021, 74, 1212–1224. [Google Scholar]
- Chang, Y.; Kim, J.I.; Lee, B.; Kim, S.G.; Jung, M.J.; Kim, Y.S.; Jeong, S.W.; Jang, J.Y.; Yoo, J.J. Clinical application of ultrasonography-guided percutaneous liver biopsy and its safety over 18 years. Clin. Mol. Hepatol. 2020, 26, 318–327. [Google Scholar] [PubMed]
- Wang, Y.; Deng, B. Hepatocellular carcinoma: Molecular mechanism, targeted therapy, and biomarkers. Cancer Metastasis Rev. 2023, 42, 629–652. [Google Scholar]
- Sim, H.W.; Knox, J. Hepatocellular carcinoma in the era of immunotherapy. Curr. Probl. Cancer 2018, 42, 40–48. [Google Scholar]
- Lehrich, B.M.; Zhang, J.; Monga, S.P.; Dhanasekaran, R. Battle of the biopsies: Role of tissue and liquid biopsy in hepatocellular carcinoma. J. Hepatol. 2024, 80, 515–530. [Google Scholar]
- Kysela, P.; Kala, Z.; Zatloukal, M.; Raudenská, M.; Brančíková, D. Hepatocellular carcinoma—Prognostic criteria of individualized treatment. Klin. Onkol. 2022, 35, 100–113. [Google Scholar]
- Kim, B.H.; Yun, E.H.; Lee, J.H.; Hong, G.; Park, J.Y.; Shim, J.H.; Kim, E.; Kong, H.J.; Jung, K.W.; Lim, Y.S. Advancing Korean nationwide registry for hepatocellular carcinoma: A systematic sampling approach utilizing the Korea Central Cancer Registry database. J. Liver Cancer 2024, 24, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, J.Y.; Lee, S.K. Heavy smoking increases early mortality risk in patients with hepatocellular carcinoma after curative treatment. J. Liver Cancer 2024, 24, 253–262. [Google Scholar]
- Yoon, J.S.; Lee, H.A.; Kim, H.Y.; Sinn, D.H.; Lee, D.H.; Hong, S.K.; Cho, J.Y.; Choi, J.; Chang, Y.; Kong, H.J.; et al. Hepatocellular Carcinoma in Korea: An Analysis of the 2015 Korean Nationwide Cancer Registry. J. Liver Cancer 2021, 21, 58–68. [Google Scholar]
- Dahdaleh, F.S.; Naffouje, S.A.; Sherman, S.K.; Kamarajah, S.K.; Salti, G.I. Tissue Diagnosis Is Associated With Worse Survival in Hepatocellular Carcinoma: A National Cancer Database Analysis. Am. Surg. 2022, 88, 1234–1243. [Google Scholar] [PubMed]
- Russo, F.P.; Imondi, A.; Lynch, E.N.; Farinati, F. When and how should we perform a biopsy for HCC in patients with liver cirrhosis in 2018? A review. Dig. Liver Dis. 2018, 50, 640–646. [Google Scholar] [PubMed]
- Chan, L.L.; Chan, S.L. The evolving role of lenvatinib at the new era of first-line hepatocellular carcinoma treatment. Clin. Mol. Hepatol. 2023, 29, 909–923. [Google Scholar] [PubMed]
- Galle, P.R.; Dufour, J.F.; Peck-Radosavljevic, M.; Trojan, J.; Vogel, A. Systemic therapy of advanced hepatocellular carcinoma. Future Oncol. 2021, 17, 1237–1251. [Google Scholar]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar]
- Wang, P.; Meng, Z.Q.; Chen, Z.; Lin, J.H.; Ping, B.; Wang, L.F.; Wang, B.H.; Liu, L.M. Diagnostic value and complications of fine needle aspiration for primary liver cancer and its influence on the treatment outcome-a study based on 3011 patients in China. Eur. J. Surg. Oncol. 2008, 34, 541–546. [Google Scholar]


| Variable | All (n = 18,304) | Pre-Treatment Biopsy (−) of Liver Cancer (n = 16,528) | Pre-Treatment Biopsy (+) of Liver Cancer (n = 1776) | p |
|---|---|---|---|---|
| Age (years) | 61.36 ± 11.61 | 61.28 ± 11.56 | 62.14 ± 12.12 | 0.004 |
| Male | 14,497 (79.20%) | 13,042 (78.91%) | 1455 (81.96%) | 0.003 |
| Height (cm) | 164.47 ± 8.36 | 164.48 ± 8.38 | 164.39 ± 8.19 | 0.680 |
| Weight (kg) | 64.97 ± 11.25 | 65.10 ± 11.25 | 63.84 ± 11.19 | <0.001 |
| Laboratory findings | ||||
| Platelet (109/L) | 162.28 ± 90.47 | 159.04 ± 89.33 | 192.11 ± 95.35 | <0.001 |
| AST (IU/L) | 79.16 ± 122.42 | 74.94 ± 89.37 | 76.36 ± 4.35 | 0.877 |
| ALT (IU/L) | 56.13 ± 109.27 | 55.79 ± 96.01 | 59.27 ± 192.54 | 0.207 |
| Total bilirubin (mg/dL) | 1.66 ± 3.05 | 1.68 ± 3.07 | 1.45 ± 2.83 | 0.003 |
| Creatinine (mg/dL) | 0.98 ± 0.78 | 0.99 ± 0.80 | 0.95 ± 0.55 | 0.066 |
| Albumin (mg/dL) | 3.74 ± 0.67 | 3.74 ± 0.68 | 3.81 ± 0.60 | <0.001 |
| PT-INR | 1.16 ± 0.60 | 1.17 ± 0.23 | 1.11 ± 0.16 | <0.001 |
| mUICC stage | ||||
| Stage I | 2787 (15.34%) | 2642 (16.10%) | 145 (8.25%) | <0.001 |
| Stage II | 6787 (37.37%) | 6198 (37.78%) | 589 (33.50%) | <0.001 |
| Stage III | 4626 (25.47%) | 4204 (25.62%) | 422 (24.00%) | <0.001 |
| Stage IV-A | 2142 (11.79%) | 1884 (11.48%) | 258 (14.68%) | <0.001 |
| Stage IV-B | 1822 (10.03%) | 1478 (9.01%) | 344 (19.57%) | <0.001 |
| BCLC stage | ||||
| Stage 0 | 1400 (9.07%) | 1310 (9.45%) | 90 (8.25%) | <0.001 |
| Stage A | 3928 (25.46%) | 3669 (26.47%) | 259 (16.49%) | <0.001 |
| Stage B | 2882 (18.68%) | 4204 (18.47%) | 322 (20.50%) | <0.001 |
| Stage C | 6036 (39.12%) | 1884 (37.48%) | 841 (53.53%) | <0.001 |
| Stage D | 1184 (7.67%) | 1478 (8.12%) | 59 (3.76%) | <0.001 |
| Group | Total (n = 18,304) | Pre-Treatment Biopsy (−) of Liver Cancer (n = 16,528) | Pre-Treatment Biopsy (+) of Liver Cancer (n = 1776) | p |
|---|---|---|---|---|
| Total | 53.47 ± 0.45 | 54.58 ± 0.48 | 43.13 ± 1.29 | <0.001 |
| mUICC stage | ||||
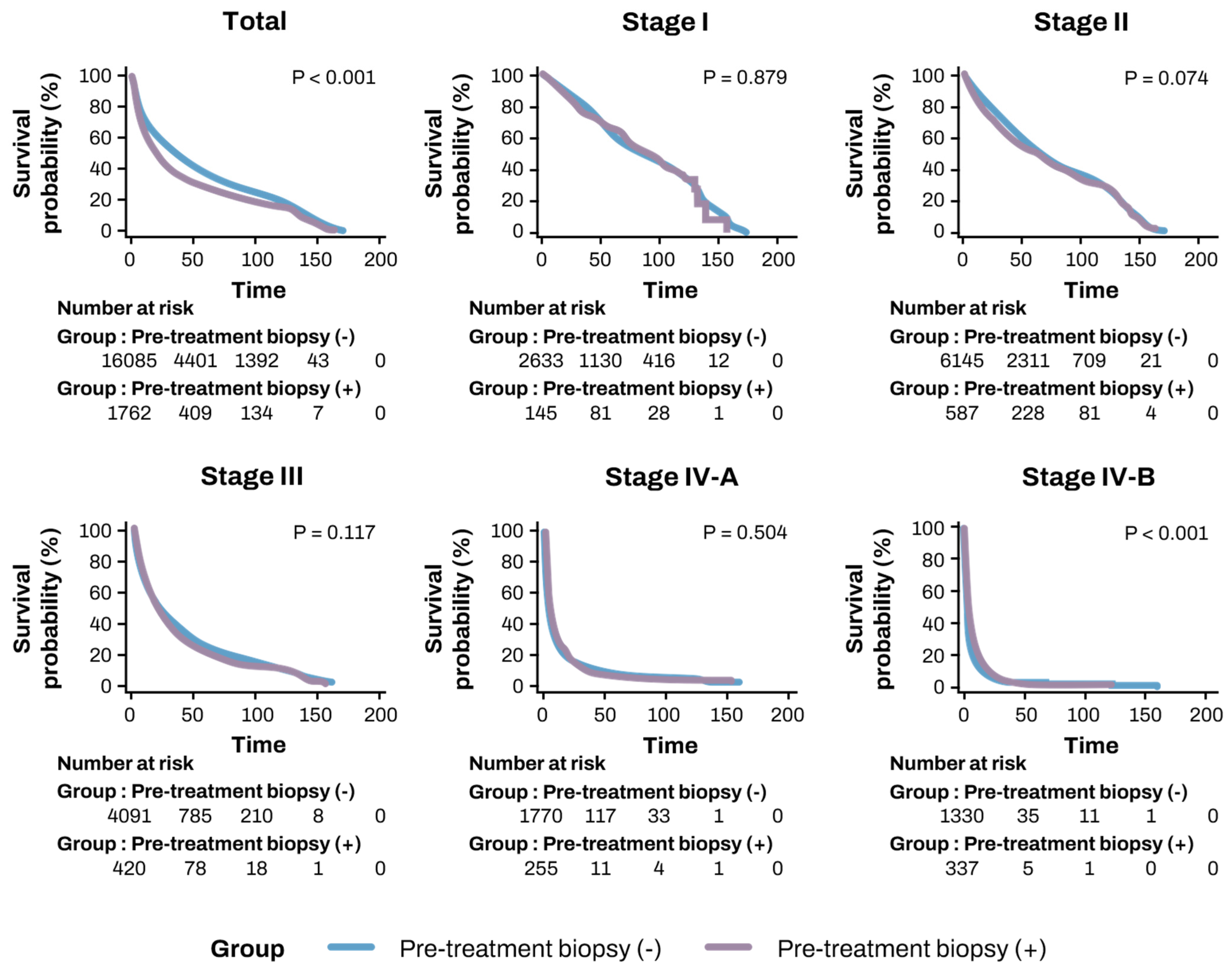
| Stage I (n = 2787) | 88.49 ± 1.35 | 88.66 ± 1.40 | 86.40 ± 4.80 | 0.879 |
| Stage II (n = 6787) | 73.92 ± 0.77 | 74.22 ± 0.81 | 70.24 ± 2.52 | 0.074 |
| Stage III (n = 4626) | 39.26 ± 0.71 | 39.60 ± 0.75 | 35.93 ± 2.20 | 0.117 |
| Stage IV-A (n = 2142) | 15.12 ± 0.68 | 15.11 ± 0.72 | 14.63 ± 1.84 | 0.504 |
| Stage IV-B (n = 1822) | 8.42 ± 0.53 | 8.21 ± 0.60 | 9.08 ± 0.84 | <0.001 |
| BCLC stage | ||||
| Stage 0 (n = 1400) | 100.29 ± 1.82 | 101.02 ± 1.91 | 91.67 ± 6.08 | 0.247 |
| Stage A (n = 3928) | 84.67 ± 1.03 | 84.25 ± 1.08 | 88.47 ± 3.65 | 0.233 |
| Stage B (n = 2882) | 47.36 ± 0.97 | 48.38 ± 1.04 | 38.30 ± 2.35 | 0.001 |
| Stage C (n = 6036) | 28.04 ± 0.58 | 28.68 ± 0.63 | 23.75 ± 1.37 | 0.004 |
| Stage D (n = 1184) | 14.13 ± 0.49 | 14.20 ± 1.06 | 10.70 ± 2.21 | 0.300 |
| 1st line treatment | ||||
| Resection (n = 3726) | 98.98 ± 1.17 | 99.11 ± 1.23 | 95.78 ± 3.85 | 0.383 |
| Liver transplantation (n = 182) | 107.33 ± 5.18 | 107.27 ± 5.33 | 87.375 ± 20.01 | 0.801 |
| Radiofrequency ablation (n = 1963) | 86.06 ± 1.41 | 86.64 ± 1.54 | 80.87 ± 3.83 | 0.112 |
| Trans-arterial chemoembolization (n = 7424) | 48.92 ± 0.59 | 50.06 ± 0.62 | 36.11 ± 1.72 | <0.001 |
| Systemic chemotherapy (n = 1146) | 12.66 ± 0.76 | 12.49 ± 0.87 | 13.08 ± 1.52 | 0.423 |
| Radiotherapy (n = 283) | 22.71 ± 2.57 | 24.80 ± 2.97 | 10.37 ± 1.45 | 0.035 |
| Best supportive care (n = 3580) | 14.23 ± 0.52 | 14.23 ± 0.54 | 14.03 ± 1.50 | 0.112 |
| Years of diagnosis | ||||
| 2008–2014 (n = 10,733) | 55.10 ± 0.56 | 55.72 ± 0.59 | 49.43 ± 1.71 | 0.001 |
| 2015–2018 (n = 7571) | 34.06 ± 0.35 | 35.13 ± 0.37 | 23.89 ± 0.96 | <0.001 |
| Group | Total (n = 18,304) | Pre-Treatment Biopsy (−) of Liver Cancer (n = 16,528) | Pre-Treatment Biopsy (+) of Liver Cancer (n = 1776) | p |
|---|---|---|---|---|
| Total | 51.59 ± 0.44 | 52.57 ± 0.47 | 42.45 ± 1.28 | <0.001 |
| mUICC stage | ||||
| Stage I (n = 2787) | 85.66 ± 1.34 | 85.81 ± 1.40 | 83.9 ± 4.81 | 0.902 |
| Stage II (n = 6787) | 71.55 ± 0.76 | 71.62 ± 0.80 | 70.24 ± 2.52 | 0.486 |
| Stage III (n = 4626) | 37.53 ± 0.69 | 37.82 ± 0.73 | 34.77 ± 2.16 | 0.202 |
| Stage IV-A (n = 2142) | 14.77 ± 0.66 | 14.89 ± 0.72 | 13.32 ± 1.62 | 0.733 |
| Stage IV-B (n = 1822) | 8.41 ± 0.53 | 8.20 ± 0.60 | 9.20 ± 0.84 | <0.001 |
| BCLC stage | ||||
| Stage 0 (n = 1400) | 98.88 ± 1.82 | 99.68 ± 1.91 | 89.27 ± 6.09 | 0.171 |
| Stage A (n = 3928) | 82.33 ± 1.03 | 81.79 ± 1.08 | 87.69 ± 3.67 | 0.105 |
| Stage B (n = 2882) | 45.88 ± 0.97 | 46.78 ± 1.02 | 37.75 ± 2.33 | 0.003 |
| Stage C (n = 6036) | 27.11 ± 0.58 | 27.71 ± 0.62 | 23.11 ± 1.34 | 0.008 |
| Stage D (n = 1184) | 10.19 ± 0.49 | 10.14 ± 0.80 | 10.74 ± 2.21 | 0.045 |
| 1st line treatment | ||||
| Resection (n = 3726) | 98.49 ± 1.17 | 98.59 ± 1.23 | 95.79 ± 3.85 | 0.470 |
| Radiofrequency ablation (n = 1963) | 84.89 ± 1.40 | 85.37 ± 1.53 | 80.44 ± 3.82 | 0.168 |
| Trans-arterial chemoembolization (n = 7424) | 47.51 ± 0.58 | 48.59 ± 1.04 | 36.00 ± 1.72 | <0.001 |
| Systemic chemotherapy (n = 1146) | 11.68 ± 0.68 | 15.15 ± 2.50 | 14.19 ± 1.50 | 0.911 |
| Radiotherapy (n = 283) | 21.56 ± 2.47 | 23.42 ± 1.29 | 10.37 ± 1.46 | 0.051 |
| Best supportive care (n = 3580) | 14.23 ± 0.52 | 14.23 ± 0.55 | 14.18 ± 1.51 | 0.112 |
| Years of diagnosis | ||||
| 2008–2014 (n = 10,733) | 53.26 ± 0.55 | 53.78 ± 0.58 | 48.50 ± 1.69 | 0.015 |
| 2015–2018 (n = 7571) | 33.10 ± 0.35 | 34.08 ± 0.37 | 23.77 ± 0.96 | <0.001 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Biopsy | ||||
| Pre-treatment biopsy (−) | 1 (ref) | 1 (ref) | ||
| Pre-treatment biopsy (+) | 1.287 (1.217–1.361) | <0.001 | 1.021 (0.961–1.085) | 0.502 |
| Age | 1.017 (1.016–1.019) | <0.001 | 1.030 (1.013–1.011) | <0.001 |
| Sex | ||||
| Female | 1 (ref) | 1 (ref) | ||
| Male | 1.160 (1.109–1.212) | <0.001 | 1.091 (1.040–1.145) | <0.001 |
| Etiology | ||||
| HBV | 1 (ref) | 1 (ref) | ||
| HCV | 1.263 (1.193–1.338) | <0.001 | 1.072 (1.003–1.146) | 0.040 |
| Non-B, Non-C | 1.334 (1.281–1.389) | <0.001 | 1.050 (1.003–1.098) | 0.036 |
| mUICC stage | ||||
| Stage I | 1 (ref) | 1 (ref) | ||
| Stage II | 1.401 (1.313–1.495) | <0.001 | 1.413 (1.316–1.517) | <0.001 |
| Stage III | 3.131 (2.933–3.342) | <0.001 | 2.407 (2.238–2.589) | <0.001 |
| StageIV-A | 7.442 (6.921–8.002) | <0.001 | 4.519 (4.162–4.907) | <0.001 |
| StageIV-B | 11.423 (10.589–12.322) | <0.001 | 5.970 (5.471–6.514) | <0.001 |
| ChildPugh class | ||||
| Class A | 1 (ref) | 1 (ref) | ||
| Class B | 2.763 (2.652–2.879) | <0.001 | 1.820 (1.741–1.902) | <0.001 |
| Class C | 4.650 (4.318–5.007) | <0.001 | 3.021 (2.786–3.276) | <0.001 |
| 1st line Treatment | ||||
| Best supportive care | 1 (ref) | 1 (ref) | ||
| Resection | 0.097 (0.091−0.104) | <0.001 | 0.194 (0.180–0.209) | <0.001 |
| Liver transplantation | 0.094 (0.072–0.122) | <0.001 | 0.114 (0.087–0.150) | <0.001 |
| Radiofrequency ablation | 0.132 (0.123–0.143) | <0.001 | 0.284 (0.261–0.310) | <0.001 |
| Trans-arterial chemoembolization | 0.305 (0.292–0.319) | <0.001 | 0.444 (0.422–0.467) | <0.001 |
| Systemic chemotherapy | 1.028 (0.960–1.101) | 0.429 | 0.823 (0.763–0.887) | <0.001 |
| Radiotherapy | 0.702 (0.617–0.800) | <0.001 | 0.739 (0.644–0.846) | <0.001 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Biopsy | ||||
| Pre-treatment biopsy (−) | 1 (ref) | 1 (ref) | ||
| Pre-treatment biopsy (+) | 1.247 (1.180–1.319) | <0.001 | 1.013 (0.954–1.076) | 0.674 |
| Age | 1.015 (1.013–1.016) | <0.001 | 1.012 (1.010–1.013) | <0.001 |
| Sex | ||||
| Female | 1 (ref) | 1 (ref) | ||
| Male | 1.075 (1.052–1.099) | <0.001 | 1.081 (1.030–1.133) | <0.001 |
| Etiology | ||||
| HBV | 1 (ref) | 1 (ref) | ||
| HCV | 1.228 (1.160–1.301) | <0.001 | 1.061 (0.997–1.129) | 0.060 |
| Non-B, Non-C | 1.293 (1.243–1.346) | <0.001 | 1.036 (0.991–1.084) | 0.122 |
| mUICC stage | ||||
| Stage I | 1 (ref) | 1 (ref) | ||
| Stage II | 1.376 (1.291–1.467) | <0.001 | 1.415 (1.320–1.517) | <0.001 |
| Stage III | 3.031 (2.843–3.232) | <0.001 | 2.352 (2.190–2.525) | <0.001 |
| StageIV-A | 6.891 (6.415–7.402) | <0.001 | 4.289 (3.955–4.651) | <0.001 |
| StageIV-B | 10.317 (9.574–11.117) | <0.001 | 5.660 (5.193–6.169) | <0.001 |
| ChildPugh class | ||||
| Class A | 1 (ref) | 1 (ref) | ||
| Class B | 2.898 (2.783–3.019) | <0.001 | 1.855 (1.775–1.938) | <0.001 |
| Class C | 5.696 (5.294–6.129) | <0.001 | 2.994 (2.763–3.244) | <0.001 |
| 1st-line Treatment | ||||
| Best supportive care | 1 (ref) | 1 (ref) | ||
| Resection | 0.099 (0.093–0.106) | <0.001 | 0.193 (0.179–0.208) | <0.001 |
| Liver transplantation | 4.308 (3.701–5.014) | <0.001 | 7.213 (6.133–8.412) | <0.001 |
| Radiofrequency ablation | 0.137 (0.127–0.147) | <0.001 | 0.287 (0.263–0.312) | <0.001 |
| Trans-arterial chemoembolization | 0.316 (0.302–0.330) | <0.001 | 0.454 (0.432–0.478) | <0.001 |
| Systemic chemotherapy | 1.064 (0.993–1.139) | 0.077 | 0.849 (0.787–0.915) | <0.001 |
| Radiotherapy | 0.724 (0.636–0.824) | <0.001 | 0.757 (0.661–0.868) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, S.J.; Yoo, J.-J.; Kim, S.G.; Kim, Y.-S. Current Trends and Impact of Liver Biopsy on Survival in Hepatocellular Carcinoma: A Korean Multicenter Analysis. Diagnostics 2025, 15, 818. https://doi.org/10.3390/diagnostics15070818
Chun SJ, Yoo J-J, Kim SG, Kim Y-S. Current Trends and Impact of Liver Biopsy on Survival in Hepatocellular Carcinoma: A Korean Multicenter Analysis. Diagnostics. 2025; 15(7):818. https://doi.org/10.3390/diagnostics15070818
Chicago/Turabian StyleChun, Seong Joon, Jeong-Ju Yoo, Sang Gyune Kim, and Young-Seok Kim. 2025. "Current Trends and Impact of Liver Biopsy on Survival in Hepatocellular Carcinoma: A Korean Multicenter Analysis" Diagnostics 15, no. 7: 818. https://doi.org/10.3390/diagnostics15070818
APA StyleChun, S. J., Yoo, J.-J., Kim, S. G., & Kim, Y.-S. (2025). Current Trends and Impact of Liver Biopsy on Survival in Hepatocellular Carcinoma: A Korean Multicenter Analysis. Diagnostics, 15(7), 818. https://doi.org/10.3390/diagnostics15070818






