Abstract
Background/Objectives: The aim of the study was to evaluate interobserver variability in the determination of the primary tumor for radiotherapy treatment planning in esophageal squamous cell carcinoma (ESCC). Methods: Sixteen patients with locally advanced ESCC were included in the analysis. In all patients positron emission tomography with computed tomography (PETC/CT) and magnetic resonance (MR) scans for radiotherapy planning were performed. Five experienced radiation oncologists delineated the primary tumor based on CT alone, MR alone, PET/CT, CT with fused MR and PET/CT with fused MR. Mean tumor volumes were calculated for each patient and imaging modality. The generalized conformity index (CIgen) was calculated to assess agreement in tumor determination. Results: The mean tumor volumes and CIgen for CT alone, MR alone, PET/CT, CT with fused MR and PET/CT with fused MR were 33.1 cm3, 30.2 cm3, 38.1 cm3, 31.9 cm3, 36.2 cm3 and 0.59, 0.64, 0.66, 0.63, 0.71, respectively. CIgen was significantly higher using PET/CT with fused MR compared to CT (p < 0.001) and PET/CT (p = 0.002) and using PET/CT compared to CT (alone) (p = 0.003). Conclusions: Our study showed higher agreement in primary tumor determination in ESCC using PET/CT compared to CT alone. Higher agreement was also found using PET/CT with fused MR compared to CT alone and PET/CT.
1. Introduction
Imaging is essential for the diagnosis, staging, treatment planning, and post-treatment follow-up of esophageal squamous cell carcinoma (ESCC). Endoscopic ultrasound (EUS) is generally effective in evaluation of the clinical T stage of ESCC, though caution is needed when differentiating between Tis, T1a, T1b, and in T4 stages [1]. Computed tomography (CT) is commonly utilized for staging of ESCC; however, its accuracy in determining T stage remains limited [2].
Positron emission tomography (PET) combined with computed tomography (CT) is now established as a crucial diagnostic tool for the initial staging of ESCC. This is due to its high accuracy in detecting metastases, including pathological lymph nodes, with a sensitivity of 66% and a specificity of 96% [3]. The tracer most commonly used in ESCC diagnostics is 18-F-fluorodeoxyglucose (FDG), a glucose analog that accumulates in tissues with increased metabolic activity [4,5]. Another important application of FDG-PET/CT in the diagnosis of ESCC is the determination of the extent of the primary tumor. Studies have shown that there is a strong correlation between the tumor length measured on preoperative FDG-PET/CT scans and the actual dimensions in histopathological samples obtained after surgery [6,7]. However, several studies investigating interobserver variability in determining the primary tumor in radiotherapy have shown contradictory results. While some studies suggest potential benefits, others have shown no significant impact of FDG-PET/CT on improving interobserver agreement [8,9].
Magnetic resonance (MR) imaging is promising in the diagnostics of ESCC as it offers better resolution and soft tissue contrast compared to CT imaging. However, MR imaging of areas such as the upper abdomen or mediastinum is challenging due to motion artefacts caused by organ movement and the central location of these regions. In recent years, advances in technology have significantly minimized these imaging artefacts and improved overall image quality [10,11,12]. Hou et al. compared tumor lengths of ESCC determined by various imaging modalities, including CT, T2-weighted MR (T2-MR), and diffusion-weighted imaging (DWI), with pathological lesion length. Their results showed that DWI had a stronger correlation with pathological lesion length than CT or T2 MRI, making it the most accurate imaging method among the methods studied [13]. Moreover, Vollenbrock et al. concluded that agreement in determination of the primary tumor with MR is comparable to that with FDG-PET/CT [14].
Radiotherapy is a cornerstone in the preoperative and definitive treatment of non-metastatic esophageal cancer [15,16,17,18]. However, a recently published landmark study has shown that perioperative chemotherapy improves the survival of patients with resectable esophageal adenocarcinoma (EAC) compared to preoperative chemoradiotherapy [19]. Consequently, radiotherapy remains crucial for the treatment of ESCC only. Accuracy is fundamental in radiotherapy as it may impact local control and the incidence of toxic adverse events [20,21]. Uncertainties in delineation of the target and consequent deviations from the treatment protocol may lead to impaired local control [22]. The aim of our study was to analyze the role of PET/CT and MR on the determination of the primary tumor in radiotherapy for ESCC.
2. Materials and Methods
The study received approval from the National Medical Ethics Committee (Approval No. 0120-620/2019/3) on 21 January 2020, and was conducted in compliance with the Declaration of Helsinki. It was also registered in the ClinicalTrials.gov database under the identifier NCT05611658. Written informed consent was obtained from all participants prior to their inclusion in the study.
2.1. Patients
Previously, we had conducted a prospective analysis of 23 patients with carcinoma of the esophagus (adenocarcinoma and squamous cell carcinoma) [23]. For this analysis, we included data from patients with ESCC only. Patients had to fulfill the following inclusion criteria: locally advanced ESCC, planned preoperative or definitive chemoradiotherapy and no contraindications for MR imaging.
2.2. PET/CT
In all patients, an FDG-PET/CT scan was performed in the treatment position for radiotherapy using a Siemens Biograph™ mCT 40 PET-CT simulator (Siemens Healthineers, Erlangen, Germany) according to a standard preparation protocol. The administered activity of 18F-FDG was 3.7 MBq/kg via intravenous injection. Approximately 60 min later, a CT scan was performed with the following parameters: 120 kV, 200 mAs, a rotation time of 1 s, a pitch of 0.8 and a slice thickness of 3 mm. Before the CT scan, an iodine-based intravenous contrast agent was administered. Subsequently, a PET scan was performed in 3-dimensional mode with a duration of 2 min per bed position.
2.3. MR
MR was performed with a 1.5T Optima™ MR450w GE MR simulator (General Electric, Chicago, IL, USA). Patients were scanned in the treatment position before the start of radiotherapy, without the use of an intravenous contrast agent. T2 images in the transverse plane with a slice thickness of 3 mm and DWI were obtained for each patient.
2.4. Tumor Determination
Five radiation oncologists, each with more than five years of experience in the treatment of esophageal carcinoma, determined the gross tumor volume (GTV) using the Eclipse™ planning system (Palo Alto, CA, USA). To ensure a uniform approach, an initial meeting was held to familiarize the observers with MR images of esophageal carcinoma. Under the guidance of an experienced radiologist, they practiced the delineation of GTV on MR images on two pilot cases. The observers were given relevant information about the location and characteristics of the tumor. The GTV was contoured separately based on several imaging modalities: CT, PET/CT, MR, CT fused with MR, and PET/CT fused with MR. All study images were anonymized to maintain objectivity. GTV was defined as the tumor visible on imaging that encompassed the entire circumference of the esophagus without including regional pathological lymph nodes. To reduce the likelihood of recall bias, contouring was performed with different imaging modalities at least two weeks apart.
During contouring on PET/CT images, observers initially delineated the GTV on CT images and then corrected the volume as needed using PET images. The GTVPET was defined as 20% of the maximum standardized uptake value (SUV). The PET and CT images were fused, and the visible tumor was contoured as GTV, with adjustments made as needed to include GTVPET (Figure 1).
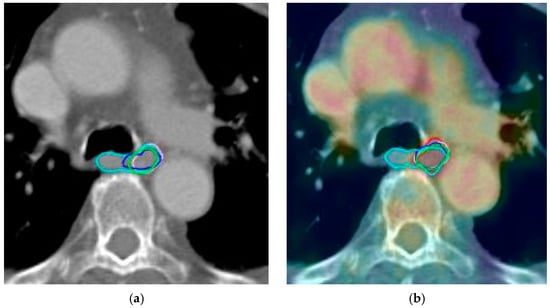
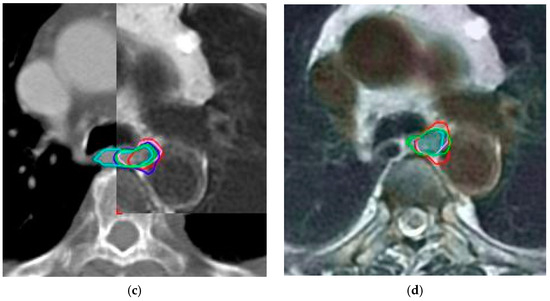
Figure 1.
Green, cyan, red, purple, blue and magenta lines represent delineations of the tumor by each observer in case 9 based on: (a) computed tomography alone; (b) positron emission tomography with computed tomography; (c) computed tomography with fused magnetic resonance; (d) positron emission tomography with computed tomography and magnetic resonance.
2.5. Data Analysis
GTV volumes were measured for each observer, each patient and all imaging modalities. Average volumes were then calculated for each patient and imaging modality. The generalized conformity index (CIgen) was calculated to assess agreement in tumor determination. This index is independent of the number of volumes analyzed. A CIgen of 1 means perfect agreement between the observers, while CIgen = 0 means that there is no overlap between the delineations [24].
The CIgen was determined for each patient and then averaged across all patients for each imaging modality.
For the original prospective trial, we determined the sample size based on data from existing studies. We calculated that approximately 21 patients would be required, with a significance level of 0.05 and a statistical power of 0.8 [9,14,25]. For the purpose of this analysis, we included only 16 patients with ESCC form the original group. The Wilcoxon signed rank test was used to analyze statistically significant differences. For statistical analysis, we used Statistical Package for the Social Sciences, version 29.0 (SPSS Inc., Chicago, IL, USA).
3. Results
A total of 16 patients diagnosed with ESCC and treated at our institute between April 2020 and May 2021 were included in this additional analysis. Thirteen were men and three were women; the average age was sixty-one years. The characteristics of the patients are presented in Table 1.

Table 1.
Characteristics of the patients with squamous cell esophageal cancer included in the study.
Tumor delineation using PET/CT imaging resulted in significantly larger mean volumes (38.10 cm3) compared to CT (33.06 cm3, p = 0.004). Delineations based on MR were significantly smaller compared to CT (p = 0.007). Volumes based on PET/CT with MR were significantly larger than on CT alone (p = 0.044) (Table 2).

Table 2.
Volume of tumor per imaging modality.
CIgen was significantly higher using PET/CT MR compared to CT (p < 0.001) and PET/CT (p = 0.002). Statistically significant higher agreement was seen for PET/CT compared to planning CT alone (p = 0.003). No statistically significant difference was seen between CT alone and MR (p = 0.796) or CT fused with MR (p = 0.49) (Table 3).

Table 3.
Generalized conformity index per imaging modality.
4. Discussion
Our analysis showed that PET/CT significantly improves interobserver agreement in determining the primary tumor of ESCC compared to CT, and the agreement is even higher when MR is added. These results are in contrast to our previous analysis, which also included patients with EAC. The highest interobserver agreement was observed for PET/CT fused with MR, whereas CT alone showed the lowest agreement. However, only the difference in CIgen between MR alone and CT alone was marginally significant (p = 0.048) [23]. Interestingly, studies examining interobserver agreement in assessing the primary tumor, mainly in EAC, also found no significant differences between PET/CT, MRI, and CT. Schreurs et al. analyzed interobserver variation of target volumes between PET/CT and CT in majority of EAC and found no significant difference [26]. Nowee et al. also found no difference in the delineation of GTV between PET/CT and CT in six patients with esophageal cancer, three of whom had EAC and three of whom had ESCC [9]. Another study analyzing the contouring of GTV on MR and PET/CT images in six patients with esophageal cancer (three EAC, three ESCC) showed no significant differences [14]. On the other hand, Toya et al. analyzed 10 patients with only ESCC in their study. They compared the delineation of GTV using PET/CT and CT and found higher agreement with PET/CT (p = 0.005) [8]. We can assume that ESCCs are more avid tumors for FDG than EACs, so tumor assessment on PET/CT is more accurate. In addition, the majority of EACs are tumors of the distal part of the esophagus, which is more prone to movement, and the borders of the tumor are more difficult to assess even with various imaging techniques. Interestingly, MR improves the agreement of tumor assessment in ESCC even more when added to PET/CT. We can explain this by the fact that PET/CT improves the assessment of the location of the tumor, while MR improves the accuracy of tumor margin determination due to its superior soft tissue contrast.
To our knowledge, our study was the first to compare all three imaging modalities to determine interobserver variability. The fusion of all three modalities showed the highest agreement. This could be due to the fact that the observers obtained the most information about the characteristics and extent of the tumor from all three imaging modalities together and therefore determined the tumor with greater agreement. In contrast, in two other similar studies, only one imaging modality was compared with the other, without fusion, and consequently no improvement in agreement was observed [9,14].
Our study has some limitations. Firstly, we did not compare the extent of primary tumors on different imaging techniques with histopathological specimens, making it impossible to determine which imaging method was closest to the “ground truth”. Interestingly, there was only one study that examined primary tumors and regional lymph nodes with PET/MR, PET/CT, MR, and contrast-enhanced CT (CECT), with postoperative pathology serving as the reference standard for assessing diagnostic accuracy. PET/MRI was superior to PET/CT, MRI and CECT [27].
Secondly, our study involved fewer observers compared to two similar studies. The study by Nowee et al. involved 20 physicians from 14 different centers, whereas the study by Vollenbrock et al. involved 10 physicians from two centers [9,14]. As a result, our group was less heterogeneous and there was a possibility that some observers learned from others. This may limit the validity of our results and may not fully reflect the potential for adoption in routine clinical practice.
Thirdly, we analyzed data from only 16 patients. In the original prospective study, the sample was calculated for the analysis of patients with EAC and ESCC. In this “post hoc” analysis, we included data from patients with ESCC only, so the results may not be robust enough. Therefore, to confirm our results, a study with larger number of patients is warranted. However, two studies that used similar methods and imaging modalities both analyzed data from only six patients [9,14]. Neither study found a statistically significant difference in interobserver variability, which could be due to the fact that not enough real-world clinical scenarios were included.
Fourthly, the delineation of primary tumors began with CT alone, followed by MR alone, PET/CT, CT fused with MR, and finally PET/CT fused with MR. The poorest agreement was observed with CT, while the highest agreement was seen with PET/CT fused with MR. This could be partly due to the fact that the observers were able to memorize the anatomical details of the cases during successive contouring sessions, despite a minimum interval of 14 days—often longer—between sessions. Notably, the entire contouring process was completed within a period of almost two years.
Despite these limitations, we can conclude that we found significantly higher agreement in the determination of the primary tumor with PET/CT compared to CT alone, and that the agreement was even higher when we added MR to PET/CT. Interobserver variability could have an important clinical impact as it may influence the occurrence of toxic side effects and local control. Studies investigating the impact of contouring uncertainties on radiation dose distribution help us to identify which differences might be clinically relevant. Several studies on different tumors have shown that interobserver variability has a significant impact on dose coverage to the target or organs at risk [20]. For example, in the treatment of cervical cancer, the effect of interobserver variability on the dose distribution of MR-guided brachytherapy was studied. They found that uncertainty in delineation affected the dose distribution to the target by ±5 Gy and to the organs at risk by ±2–3 Gy [28]. In breast cancer, interobserver variability had no effect on target coverage, while the percentage of lung volume irradiated with 20 Gy (V20) varied between 5% and 25%, heart V20 varied between 0% and 7% and heart V10 varied between 2% and 20% [29]. We found no studies in the literature examining the effects of contouring uncertainties on the dosimetric parameters of radiation treatment plans for esophageal cancer. This could be a starting point for further research, as late toxic effects after radiation treatment for esophageal cancer may lead to poorer survival. The incidence of grade 3 or higher cardiovascular complications following radiotherapy for esophageal cancer is estimated to be between 10.8% and 16.3% and may be related to the dose received by cardiac structures, particularly the coronary arteries [30,31,32]. Pulmonary complications are also frequently observed after radiotherapy of thoracic tumors. In patients treated for esophageal cancer with neoadjuvant chemoradiation therapy and surgery, the mean lung dose was most strongly associated with postoperative pulmonary complications, which occurred in 22.1% of cases [33]. Both cardiovascular and pulmonary complications can have a significant impact on patient survival and quality of life.
Esophageal cancer is not a very common tumor, but it has high mortality and morbidity [34]. Appropriate treatment, including radiotherapy, is important to achieve local control of the disease, which may also have an impact on overall survival. Since higher interobserver agreement could potentially improve local control, it seems reasonable to additionally use MR and PET/CT in routine practice to facilitate the determination of the primary tumor for radiotherapy treatment planning. As esophageal cancer is not a common cancer, this would not present a major financial burden in most centers. However, it should be emphasized that optimization of imaging protocols and further research are needed.
5. Conclusions
In conclusion, our study demonstrated that PET/CT fused with MR achieved the highest agreement in identifying the primary tumor in ESCC. Considering the fact that ESCC is a relatively rare cancer, adopting PET/CT and MR for radiotherapy treatment planning and primary tumor determination seems feasible.
Author Contributions
Conceptualization, A.S.-E. and B.S.; methodology, A.S.-E. and B.S.; formal analysis, A.S.-E. and P.P.; data curation, A.S.-E., V.V., A.J.-P., J.B.-H. and F.A.; writing—original draft preparation, A.S.-E.; writing—review and editing, A.S.-E. and B.S.; visualization, A.S.-E.; supervision, B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the National Medical Ethics Committee of the Republic of Slovenia of on 21 January 2020 (Approval No. 0120-620/2019/3).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ESCC | Esophageal squamous cell carcinoma |
| EUS | Endoscopic ultrasound |
| CT | Computed tomography |
| PET | Positron emission tomography |
| FDG | 18-F-fluorodeoxyglucose |
| EAC | Esophageal adenocarcinoma |
| MR | Magnetic resonance |
| T2 | T2-weighted |
| DWI | Diffusion-weighted imaging |
| GTV | Gross tumor volume |
| SUV | Standardized uptake value |
| CIgen | Generalized conformity index |
| V20 | Percentage of target volume irradiated with 20 Gy |
References
- Yang, J.; Luo, G.Y.; Liang, R.B.; Zeng, T.S.; Long, H.; Fu, J.H.; Xu, G.L.; Yang, M.Z.; Li, S.; Zhang, L.J.; et al. Efficacy of Endoscopic Ultrasonography for Determining Clinical T Category for Esophageal Squamous Cell Carcinoma: Data From 1434 Surgical Cases. Ann. Surg. Oncol. 2018, 25, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.; Zhao, Q.Y.; Li, X.Q.; Wang, L.; Wang, N.N.; Wang, J.Z.; Wang, Q. Esophageal wall thickness on CT scans: Can it predict the T stage of primary thoracic esophageal squamous cell carcinoma? Esophagus 2022, 19, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Chen, Y.; Zhu, Y.; Xu, Y. Systematic review and meta-analysis of the accuracy of 18F-FDG PET/CT for detection of regional lymph node metastasis in esophageal squamous cell carcinoma. J. Thorac. Dis. 2018, 10, 6066–6076. [Google Scholar] [CrossRef]
- Lu, J.; Sun, X.-D.; Yang, X.; Tang, X.-Y.; Qin, Q.; Zhu, H.-C.; Cheng, H.-Y.; Sun, X.-C. Impact of PET/CT on radiation treatment in patients with esophageal cancer: A systematic review. Crit. Rev. Oncol./Hematol. 2016, 107, 128–137. [Google Scholar] [CrossRef]
- Gauthé, M.; Richard-Molard, M.; Cacheux, W.; Michel, P.; Jouve, J.L.; Mitry, E.; Alberini, J.L.; Lièvre, A.; Fédération Francophone de Cancérologie Digestive (FFCD). Role of fluorine 18 fluorodeoxyglucose positron emission tomography/computed tomography in gastrointestinal cancers. Dig. Liver Dis. 2015, 47, 443–454. [Google Scholar] [CrossRef]
- Han, D.; Yu, J.; Yu, Y.; Zhang, G.; Zhong, X.; Lu, J.; Yin, Y.; Fu, Z.; Mu, D.; Zhang, B.; et al. Comparison of 18F-Fluorothymidine and 18F-Fluorodeoxyglucose PET/CT in Delineating Gross Tumor Volume by Optimal Threshold in Patients with Squamous Cell Carcinoma of Thoracic Esophagus. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1235–1241. [Google Scholar] [CrossRef]
- Zhong, X.; Yu, J.; Zhang, B.; Mu, D.; Zhang, W.; Li, D.; Han, A.; Song, P.; Li, H.; Yang, G.; et al. Using 18F-Fluorodeoxyglucose Positron Emission Tomography to Estimate the Length of Gross Tumor in Patients with Squamous Cell Carcinoma of the Esophagus. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 136–141. [Google Scholar] [CrossRef]
- Toya, R.; Matsuyama, T.; Saito, T.; Imuta, M.; Shiraishi, S.; Fukugawa, Y.; Iyama, A.; Watakabe, T.; Sakamoto, F.; Tsuda, N.; et al. Impact of hybrid FDG-PET/CT on gross tumor volume definition of cervical esophageal cancer: Reducing interobserver variation. J. Radiat. Res. 2019, 60, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Nowee, M.E.; Voncken, F.E.M.; Kotte, A.N.T.J.; Goense, L.; van Rossum, P.S.N.; van Lier, A.L.H.M.W.; Heijmink, S.W.; Aleman, B.M.P.; Nijkamp, J.; Meijer, G.J.; et al. Gross tumour delineation on computed tomography and positron emission tomography-computed tomography in oesophageal cancer: A nationwide study. Clin. Transl. Radiat. Oncol. 2018, 14, 33–39. [Google Scholar] [CrossRef]
- Pellat, A.; Dohan, A.; Soyer, P.; Veziant, J.; Coriat, R.; Barret, M. The role of magnetic resonance imaging in the management of esophageal cancer. Cancers 2022, 14, 1141. [Google Scholar] [CrossRef]
- Van Rossum, P.S.N.; van Lier, A.L.H.M.W.; Lips, I.M.; Meijer, G.J.; Reerink, O.; van Vulpen, M.; Lam, M.G.E.H.; van Hillegersberg, R.; Ruurda, J.P. Imaging of oesophageal cancer with FDG-PET/CT and MRI. Clin. Radiol. 2015, 70, 81–95. [Google Scholar] [CrossRef]
- Metcalfe, P.; Liney, G.P.; Holloway, L.; Walker, A.; Barton, M.; Delaney, G.P.; Vinod, S.; Tomé, W. The potential for an enhanced role for MRI in radiation-therapy treatment planning. Technol. Cancer Res. Treat. 2013, 12, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.-L.; Shi, G.-F.; Gao, X.-S.; Asaumi, J.; Li, X.-Y.; Liu, H.; Yao, C.; Chang, J.Y. Improved longitudinal length accuracy of gross tumor volume delineation with diffusion weighted magnetic resonance imaging for esophageal squamous cell carcinoma. Radiat. Oncol. 2013, 8, 169. [Google Scholar] [CrossRef]
- Vollenbrock, S.E.; Nowee, M.E.; Voncken, F.E.M.; Kotte, A.N.T.J.; Goense, L.; van Rossum, P.S.N.; van Lier, A.L.H.M.W.; Heijmink, S.W.; Bartels-Rutten, A.; Wessels, F.J.; et al. Gross Tumor Delineation in Esophageal Cancer on MRI Compared With 18F-FDG-PET/CT. Adv. Radiat. Oncol. 2019, 4, 596–604. [Google Scholar] [CrossRef] [PubMed]
- van Hagen, P.; Hulshof, M.C.; van Lanschot, J.J.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Eyck, B.M.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van der Wilk, B.J.; Shapiro, J.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: The randomized controlled CROSS trial. J. Clin. Oncol. 2021, 39, 1995–2004. [Google Scholar] [CrossRef]
- Hulshof, M.C.C.M.; Geijsen, E.D.; Rozema, T.; Oppedijk, V.; Buijsen, J.; Neelis, K.J.; Nuyttens, J.J.M.E.; van der Sangen, M.J.C.; Jeene, P.M.; Reinders, J.G.; et al. Randomized study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer (ARTDECO Study). J. Clin. Oncol. 2021, 39, 2816–2824. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, B.; Zhu, W.; Li, J.; Huang, R.; Sun, Z.; Yang, X.; Liu, L.; He, H.; Liao, Z.; et al. A Phase III multicenter randomized clinical trial of 60 Gy versus 50 Gy radiation dose in concurrent chemoradiotherapy for inoperable esophageal squamous cell carcinoma. Clin. Cancer Res. 2022, 28, 1792–1799. [Google Scholar] [CrossRef]
- Hoeppner, J.; Brunner, T.; Schmoor, C.; Bronsert, P.; Kulemann, B.; Claus, R.; Utzolino, S.; Izbicki, J.R.; Gockel, I.; Gerdes, B.; et al. Perioperative chemotherapy or preoperative chemoradiotherapy in esophageal Cancer. N. Engl. J. Med. 2025, 392, 323–335. [Google Scholar] [CrossRef]
- Vinod, S.K.; Jameson, M.G.; Min, M.; Holloway, L.C. Uncertainties in volume delineation in radiation oncology: A systematic review and recommendations for future studies. Radiother. Oncol. 2016, 121, 169–179. [Google Scholar] [CrossRef]
- Segedin, B.; Petric, P. Uncertainties in target volume delineation in radiotherapy—Are they relevant and what can we do about them? Radiol. Oncol. 2016, 50, 254–262. [Google Scholar] [CrossRef]
- Weber, D.C.; Tomsej, M.; Melidis, C.; Hurkmans, C.W. QA makes a clinical trial stronger: Evidence-based medicine in radiation therapy. Radiother. Oncol. 2012, 105, 4–8. [Google Scholar] [CrossRef]
- Secerov-Ermenc, A.; Peterlin, P.; Anderluh, F.; But-Hadzic, J.; Jeromen-Peressutti, A.; Velenik, V.; Segedin, B. Inter-observer variation in gross tumour volume delineation of oesophageal cancer on MR, CT and PET/CT. Radiol. Oncol. 2024, 58, 580–587. [Google Scholar] [CrossRef]
- Kouwenhoven, E.; Giezen, M.; Struikmans, H. Measuring the similarity of target volume delineations independent of the number of observers. Phys. Med. Biol. 2009, 54, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Machiels, M.; Jin, P.; van Hooft, J.E.; Gurney-Champion, O.J.; Jelvehgaran, P.; Geijsen, E.D.; Jeene, P.M.; Willemijn Kolff, M.; Oppedijk, V.; Rasch, C.R.N. Reduced inter-observer and intra-observer delineation variation in esophageal cancer radiotherapy by use of fiducial markers. Acta Oncol. 2019, 58, 943–950. [Google Scholar] [CrossRef]
- Schreurs, L.M.A.; Busz, D.M.; Paardekooper, G.M.R.M.; Beukema, J.C.; Jager, P.L.; Van der Jagt, E.J.; Van Dam, G.M.; Groen, H.; Plukker, J.T.M.; Langendijk, J.A. Impact of 18-fluorodeoxyglucose positron emission tomography on computed tomography defined target volumes in radiation treatment planning of esophageal cancer: Reduction in geographic misses with equal inter-observer variability. Dis. Esophagus 2010, 23, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guo, R.; Zhang, Y.; Yu, B.; Meng, X.; Kong, H.; Yang, Y.; Yang, Z.; Li, N. Value of 18F-FDG PET/MRI in the Preoperative Assessment of Resectable Esophageal Squamous Cell Carcinoma: A Comparison With 18F-FDG PET/CT, MRI, and Contrast-Enhanced CT. Front. Oncol. 2022, 12, 844702. [Google Scholar] [CrossRef] [PubMed]
- Hellebust, T.P.; Tanderup, K.; Lervåg, C.; Fidarova, E.; Berger, D.; Malinen, E.; Pötter, R.; Petrič, P. Dosimetric impact of interobserver variability in MRI-based delineation for cervical cancer brachytherapy. Radiother. Oncol. 2013, 107, 13–19. [Google Scholar] [CrossRef]
- Li, X.A.; Tai, A.; Arthur, D.W.; Buchholz, T.A.; Macdonald, S.; Marks, L.B.; Moran, J.M.; Pierce, L.J.; Rabinovitch, R.; Taghian, A.; et al. Radiation therapy oncology group multi-institutional and multiobserver study. Variability of target and normal structure delineation for breast cancer radiotherapy: An RTOG multi-institutional and multiobserver Study. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 944–951. [Google Scholar] [CrossRef]
- Cai, G.; Li, C.; Li, J.; Yang, J.; Li, C.; Sun, L.; Li, J.; Yu, J.; Meng, X. Cardiac substructures dosimetric predictors for cardiac toxicity after definitive radiotherapy in esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 366–381. [Google Scholar] [CrossRef]
- Beukema, J.C.; van Luijk, P.; Widder, J.; Langendijk, J.A.; Muijs, C.T. Is cardiac toxicity a relevant issue in the radiation treatment of esophageal cancer? Radiother. Oncol. 2015, 114, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Iijima, H.; Isohashi, F.; Tsujii, Y.; Fujinaga, T.; Nagai, K.; Yoshii, S.; Sakatani, A.; Hiyama, S.; Shinzaki, S.; et al. The heart’s exposure to radiation increases the risk of cardiac toxicity after chemoradiotherapy for superficial esophageal cancer: A retrospective cohort study. BMC Cancer 2019, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.K.; Oh, D.; Kim, H.K.; Ahn, Y.C.; Noh, J.M.; Shim, Y.M.; Zo, J.I.; Choi, Y.S.; Sun, J.M.; Lee, S.H.; et al. Dosimetric predictors for postoperative pulmonary complications in esophageal cancer following neoadjuvant chemoradiotherapy and surgery. Radiother. Oncol. 2019, 133, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Uhlenhopp, D.J.; Then, E.O.; Sunkara, T.; Gaduputi, V. Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 2020, 13, 1010–1021. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).