Lactate Levels in a Replanted Limb as an Early Biomarker for Assessing Post-Surgical Evolution: A Case Report
Abstract
1. Introduction
2. Case Description
2.1. Signs, Symptoms, and Management
2.2. Biochemical Monitoring
2.3. Statistical Analysis
2.4. Biochemical Parameter Results
2.4.1. Biochemical Evaluation
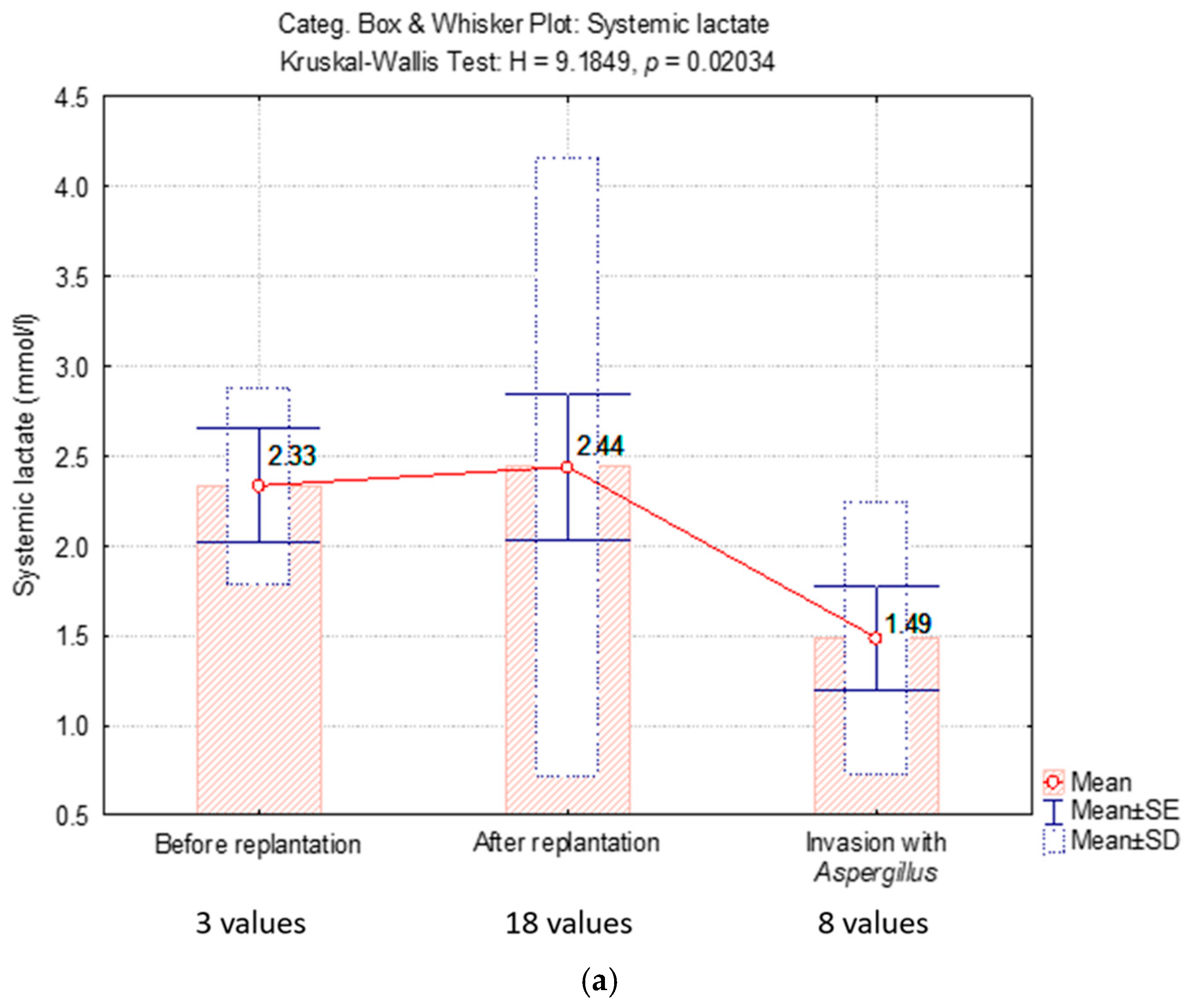
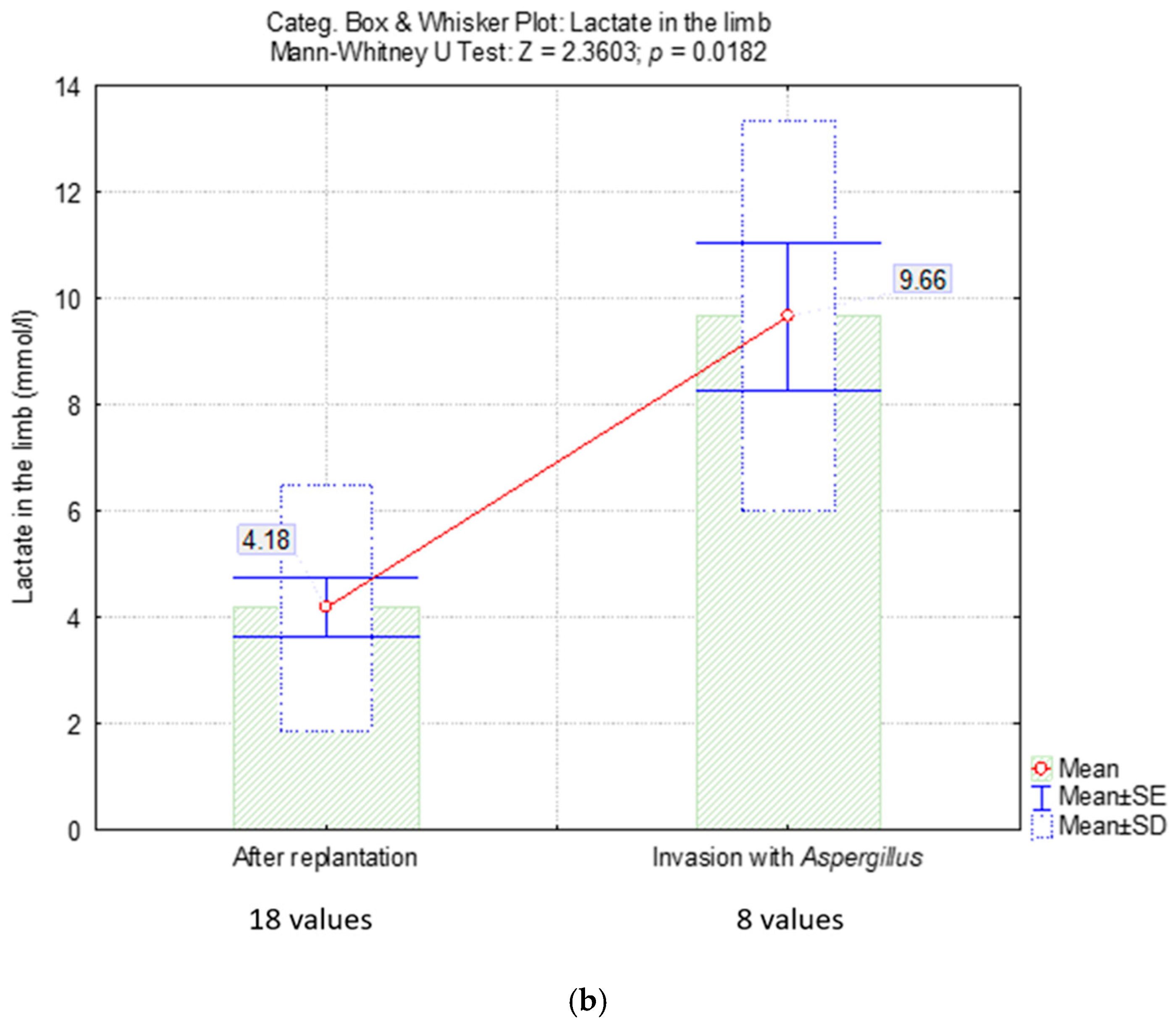
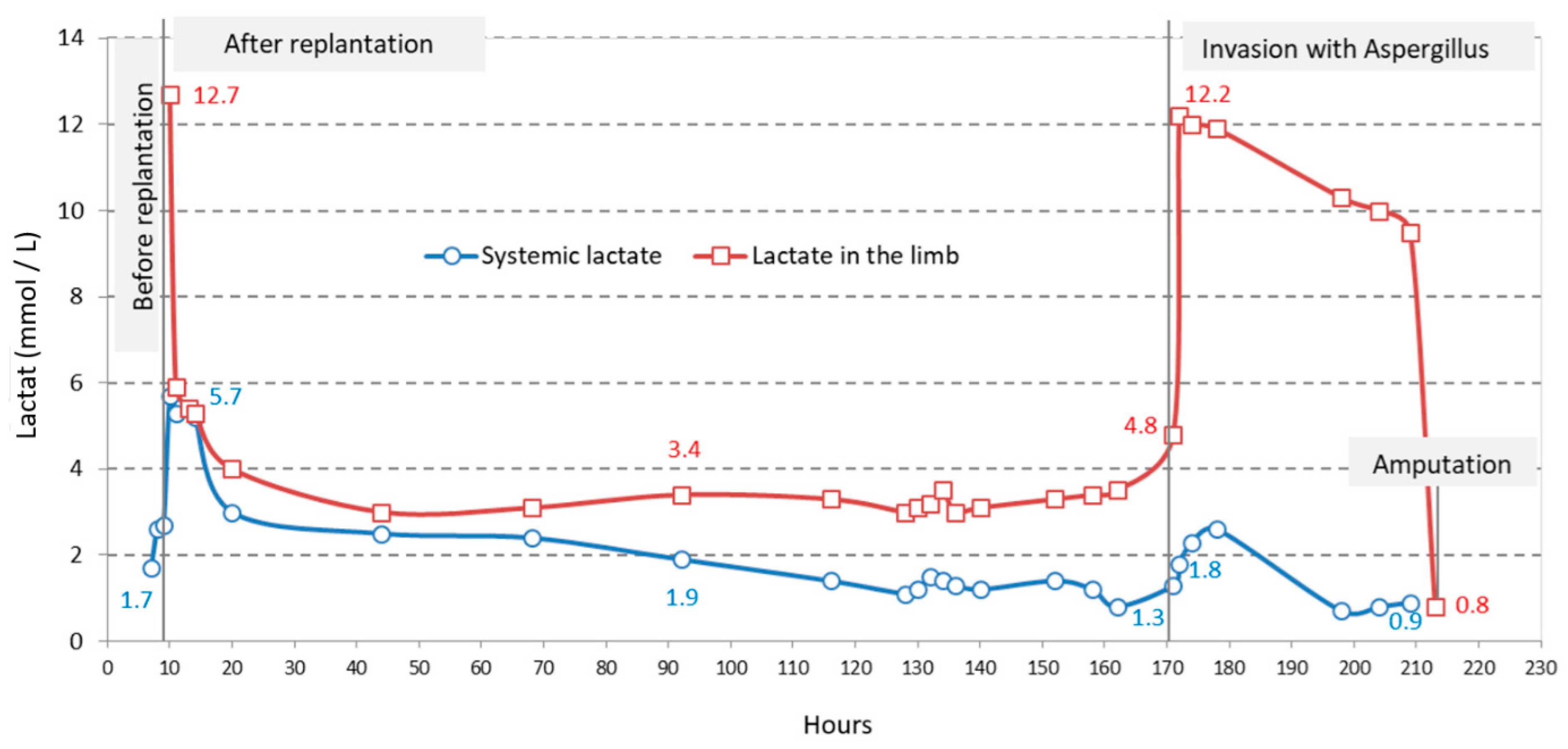
2.4.2. Lactate Level
2.4.3. Partial Pressure of Oxygen (PaO2)
2.4.4. Venous Oxygen Saturation (SvO2)
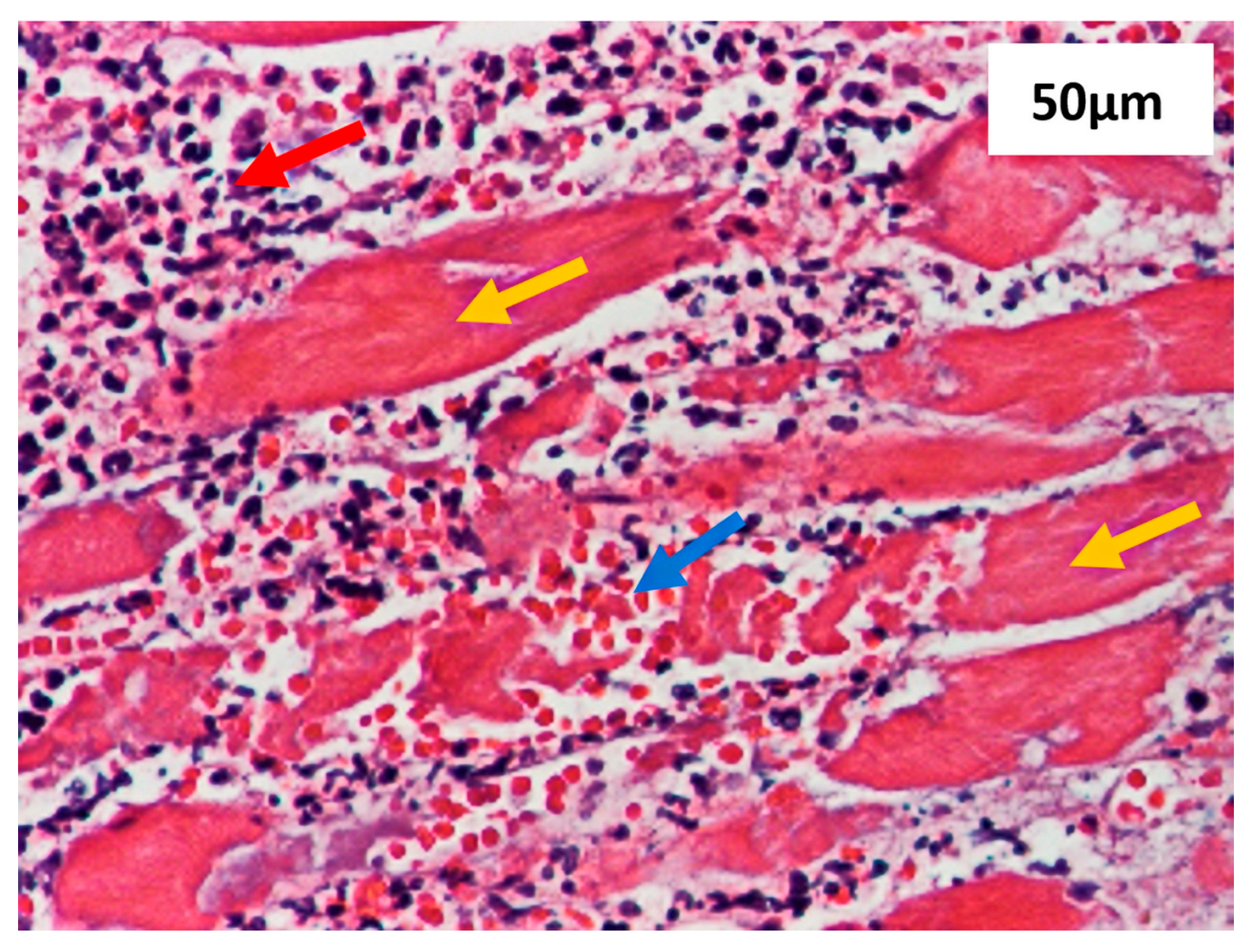
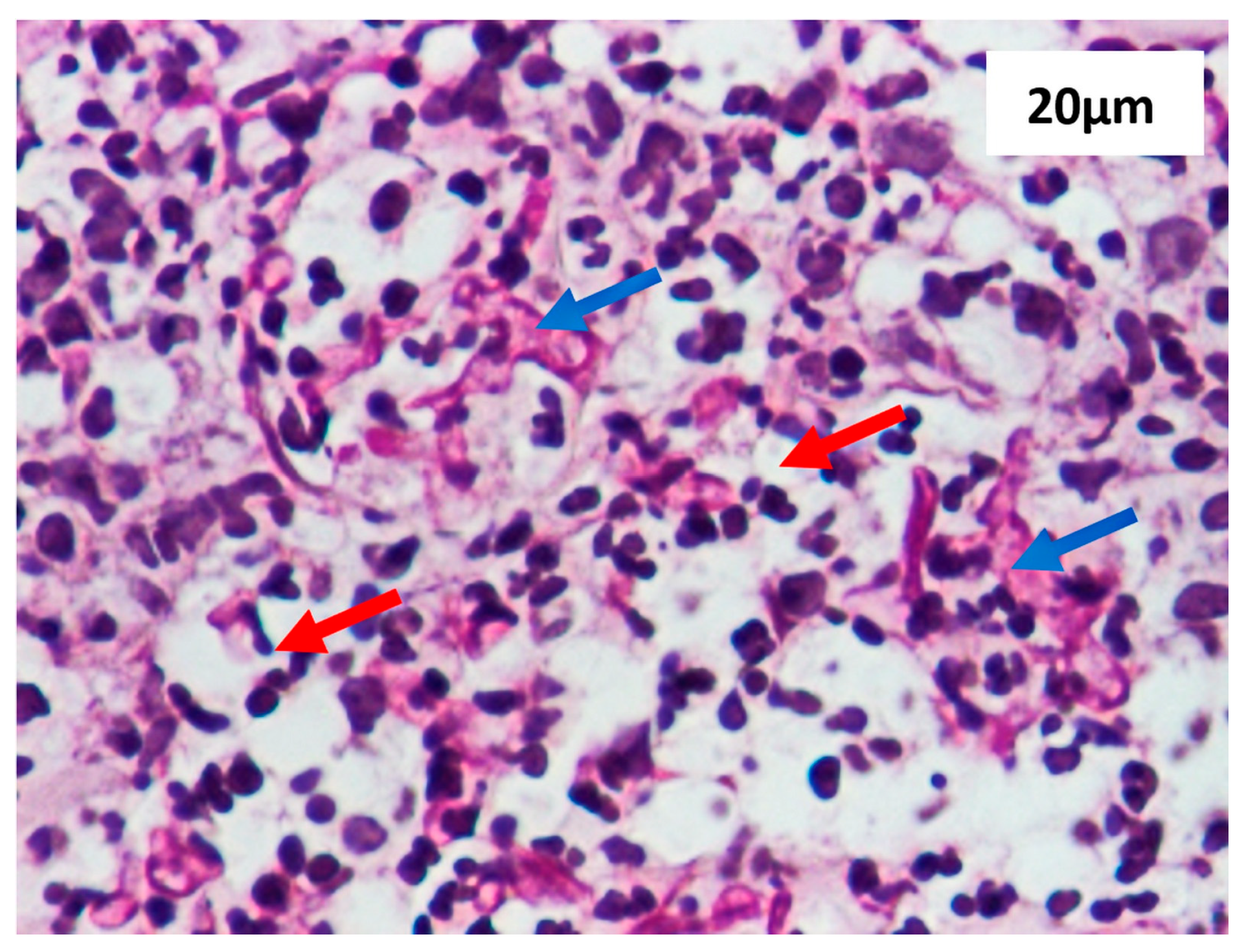
2.4.5. Methods Section Describing the Histological Analysis
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Black, C.K.; Ormiston, L.D.; Fan, K.L.; Kotha, V.S.; Attinger, C.; Evans, K.K. Amputations versus Salvage: Reconciling the Differences. J. Reconstr. Microsurg. 2021, 37, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Barla, M.; Gavanier, B.; Mangin, M.; Parot, J.; Bauer, C.; Mainard, D. Is amputation a viable treatment option in lower extremity trauma? Orthop. Traumatol. Surg. Res. 2017, 103, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Glass, G.E.; Ahmadi, H.; Mackey, S.; Simmons, J.; Hettiaratchy, S.; Pearse, M.; Nanchahal, J. Delayed amputation following trauma increases residual lower limb infection. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 531–537. [Google Scholar] [CrossRef]
- Yeh, H.K.; Fang, F.; Lin, Y.T.; Lin, C.H.; Lin, C.H.; Hsu, C.C. The effect of systemic injury score on the decision making of mangled lower extremities. Injury 2016, 47, 2127–2130. [Google Scholar] [CrossRef]
- Gattinoni, L.; Vasques, F.; Camporota, L.; Meessen, J.; Romitti, F.; Pasticci, I.; Duscio, E.; Vassalli, F.; Forni, L.G.; Payen, D.; et al. Understanding Lactatemia in Human Sepsis. Potential Impact for Early Management. Am. J. Respir. Crit. Care Med. 2019, 200, 582–589. [Google Scholar] [CrossRef]
- Kimmoun, A.; Novy, E.; Auchet, T.; Ducrocq, N.; Levy, B. Hemodynamic consequences of severe lactic acidosis in shock states: From bench to bedside. Crit. Care 2015, 19, 175. [Google Scholar] [CrossRef]
- Alivanoglou, A.; Marvaki, C.; Toylia, G.; Vasilopoulos, G. The prognostic value of lactate acid in sepsis. J. Health Res. J. 2018, 4, 119–138. [Google Scholar] [CrossRef]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Levy, B.; Gibot, S.; Franck, P.; Cravoisy, A.; Bollaert, P.E. Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: A prospective study. Lancet 2005, 365, 871–875, Erratum in: Lancet 2005, 366, 122. [Google Scholar] [CrossRef]
- Suetrong, B.; Walley, K.R. Lactic Acidosis in Sepsis: It’s Not All Anaerobic: Implications for Diagnosis and Management. Chest 2016, 149, 252–261. [Google Scholar] [CrossRef]
- Srikanth, R. Free Tissue Transfer in Pediatric Lower Limb Trauma. Indian J. Plast. Surg. 2019, 52, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Jabir, S.; Sheikh, F.; Fitzgerald O’Connor, E.; Griffiths, M.; Niranjan, N. A systematic review of the applications of free tissue transfer for paediatric lower limb salvage following trauma. J. Plast. Surg. Hand Surg. 2015, 49, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Kundal, V.K.; Debnath, P.R.; Sen, A. Epidemiology of pediatric trauma and its pattern in urban India: A tertiary care hospital-based experience. J. Indian Assoc. Pediatr. Surg. 2017, 22, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, A.K.; Agarwal, H.; Priyadarshini, P.; Kumar, A.; Kumar, S.; Gupta, A.; Mishra, B.; Aggarwal, R.; Soni, K.D.; Mathur, P.; et al. Primary vs delayed primary closure in patients undergoing lower limb amputation following trauma: A randomised control study. Int. Wound J. 2020, 17, 419–428. [Google Scholar] [CrossRef]
- Lumley, E.S.; Ramsay, G.; Wohlgemut, H.S.; Jansen, J.O. Complex lower limb trauma and reconstructive surgery: Understanding health service resource use. Eur. J. Plast. Surg. 2021, 44, 679–686. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D.; CARE Group. The CARE guidelines: Consensus-based clinical case reporting guideline development. Headache 2013, 53, 1541–1547. [Google Scholar] [CrossRef]
- Luo, X.; Yilihamu, Y.; Liu, A.; Huang, Y.; Ou, C.; Zou, Y.; Zhang, X. Replantation and Lengthening of a Lower Leg in a 7-Year-Old Child: A Case Report. J. Foot Ankle Surg. 2019, 58, 1273–1275. [Google Scholar] [CrossRef]
- Li, X.L.; Wang, W.; Liu, F.; Hu, W.; Liang, D.S. Successful Lower Limb Replantation of Knee-Level Amputation in a Child: A Case Report. J. Foot Ankle Surg. 2020, 59, 427–430. [Google Scholar] [CrossRef]
- Karanas, Y.L.; Nigriny, J.; Chang, J. The timing of microsurgical reconstruction in lower extremity trauma. Microsurgery 2008, 28, 632–634. [Google Scholar] [CrossRef]
- Momeni, A.; Lanni, M.; Levin, L.S.; Kovach, S.J. Microsurgical reconstruction of traumatic lower extremity defects in the pediatric population. Plast. Reconstr. Surg. 2017, 139, 998–1004. [Google Scholar] [CrossRef]
- Piccolo, C.L.; Galluzzo, M.; Trinci, M.; Ianniello, S.; Tonerini, M.; Brunese, L.; Miele, V. Lower Limbs Trauma in Pediatrics. Semin. Musculoskelet. Radiol. 2017, 21, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Wagels, M.; Rowe, D.; Senewiratne, S.; Theile, D.R. History of lower limb reconstruction after trauma. ANZ J. Surg. 2013, 83, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Zosimas, D.; Abukar, A.; Srilekha, A. A rare case of peripheral vascular graft infection by Aspergillus fumigatus and review of the literature. Ann. R. Coll. Surg. Engl. 2017, 99, e34–e35. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 7th ed.; WH Freeman: New York, NY, USA, 2017; pp. 658–659. [Google Scholar]
- Haller, H.L.; Sander, F.; Popp, D.; Rapp, M.; Hartmann, B.; Demircan, M.; Nischwitz, S.P.; Kamolz, L.P. Oxygen, pH, Lactate, and Metabolism—How Old Knowledge and New Insights Might Be Combined for New Wound Treatment. Medicina 2021, 57, 1190. [Google Scholar] [CrossRef]
- Mutschler, M.; Nienaber, U.; Brockamp, T.; Wafaisade, A.; Fabian, T.; Paffrath, T.; Paffrath, T.; Bouillon, B.; Maegele, M.; Trauma Register DGU. Renaissance of base deficit for the initial assessment of trauma patients: A base deficit-based classification for hypovolemic shock developed on data from 16,305 patients derived from the TraumaRegister DGU®. Crit. Care 2013, 17, R42. [Google Scholar] [CrossRef]
- Fine-Goulden, M.R.; Durward, A. How to use lactate. Arch. Dis. Child. Educ. Pract. 2014, 99, 17–22. [Google Scholar] [CrossRef]
- Guyther, J.; Cantwell, L. Big Tests in Little People. Emerg. Med. Clin. N. Am. 2021, 39, 467–478. [Google Scholar] [CrossRef]
- Lawton, L.; Crouch, R.; Voegeli, D. Is lactate an effective clinical marker of outcome for children with major trauma? —A literature review. Int. Emerg. Nurs. 2016, 28, 39–45. [Google Scholar] [CrossRef][Green Version]
- Weber, B.; Lackner, I.; Braun, C.K.; Kalbitz, M.; Huber-Lang, M.; Pressmar, J. Laboratory Markers in the Management of Pediatric Polytrauma: Current Role and Areas of Future Research. Front. Pediatr. 2021, 9, 622753. [Google Scholar] [CrossRef]
- Pattharanitima, P.; Thongprayoon, C.; Petnak, T.; Srivali, N.; Gembillo, G.; Kaewput, W.; Chesdachai, S.; Vallabhajosyula, S.; O’Corragain, O.A.; Mao, M.A.; et al. Machine Learning Consensus Clustering Approach for Patients with Lactic Acidosis in Intensive Care Units. J. Pers. Med. 2021, 11, 1132. [Google Scholar] [CrossRef]
- Cobianchi, L.; Peloso, A.; Filisetti, C.; Mojoli, F.; Sciutti, F. Serum lactate level as a useful predictor of clinical outcome after surgery: An unfulfilled potential? J. Thorac. Dis. 2016, 8, E295–E297. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Bai, K.; Liu, C.J. The impact of admission serum lactate on children with moderate to severe traumatic brain injury. PLoS ONE 2019, 14, e0222591. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, E.; Seghers, L.; Hoek, R.A.S.; van den Akker, J.P.C.; Bode, L.G.M.; Rijnders, B.J. A. Aspergillus in Critically Ill COVID-19 Patients: A Scoping Review. J. Clin. Med. 2021, 10, 2469. [Google Scholar] [CrossRef]
- Lieberman, M.; Peet, A. Mark’ Basic Medical Biochemistry: A Clinical Approach, 5th ed.; Wolters Kluwer: Philadelphia, PA, USA; Alphen aan den Rijn, The Netherlands, 2018; pp. 320–324. [Google Scholar]
- Odom, S.R.; Howell, M.D.; Silva, G.S.; Nielsen, V.M.; Gupta, A.; Shapiro, N.I.; Talmor, D. Lactate clearance as a predictor of mortality in trauma patients. J. Trauma Acute Care Surg. 2013, 74, 999–1004. [Google Scholar] [CrossRef]
- Kushima, H.; Tsunoda, T.; Matsumoto, T.; Kinoshita, Y.; Izumikawa, K.; Shirasawa, S.; Fujita, M.; Ishii, H. Effects of Aspergillus fumigatus Conidia on Apoptosis and Proliferation in an In Vitro Model of the Lung Microenvironment. Microorganisms 2021, 9, 1435. [Google Scholar] [CrossRef] [PubMed]
- Elbaiah, A.H.; Taha, M.; Saker, A.; Aziz, S.; Shahate, A. Patterns of extremities trauma in children and their management in emergency department in Suez Canal University hospital Ismailia Egypt. Int. Surg. J. 2016, 3, 887–892. [Google Scholar] [CrossRef][Green Version]
- Mocanu, M.; Vâță, D.; Alexa, A.I.; Trandafir, L.; Patrașcu, A.I.; Hâncu, M.F.; Gheucă-Solovăstru, L. Atopic Dermatitis-Beyond the Skin. Diagnostics 2021, 11, 1553. [Google Scholar] [CrossRef]
- Wezensky, S.J.; Cramer, R.A., Jr. Implications of hypoxic microenvironments during invasive aspergillosis. Med. Mycol. 2011, 49, S120–S124. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.; Namvar, S.; Gago, S.; Labram, B.; Bowyer, P.; Richardson, M.D.; Herrick, S.E. Differential Proinflammatory Responses to Aspergillus fumigatus by Airway Epithelial Cells In Vitro Are Protease Dependent. J. Fungi 2021, 7, 468. [Google Scholar] [CrossRef]
- Wasyluk, W.; Zwolak, A. Metabolic Alterations in Sepsis. J. Clin. Med. 2021, 10, 2412. [Google Scholar] [CrossRef]
- Duceac, L.D.; Tarca, E.; Ciuhodaru, M.I.; Tantu, M.M.; Goroftei, R.E.B.; Banu, E.A.; Damir, D.; Glod, M.; Luca, A.C. Study on the Mechanism of Antibiotic Resistance. Rev. Chim. 2019, 70, 199–201. [Google Scholar] [CrossRef]
- Aguado, J.M.; Valle, R.; Arjona, R.; Ferreres, J.C.; Gutierrez, J.A. Aortic bypass graft infection due to Aspergillus: Report of a case and review. Clin. Infect. Dis. 1992, 14, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, D.; Bashir, M.; Gailey, M.P. Ascending aortic graft thrombosis and diffuse embolization from early endoluminal Aspergillus infection. Ann. Thorac. Surg. 2012, 94, 1337–1339. [Google Scholar] [CrossRef]
- Paris, S.; Boisvieux-Ulrich, E.; Crestani, B.; Houcine, O.; Taramelli, D.; Lombardi, L.; Latgé, J.P. Internalization of Aspergillus fumigatus conidia by epithelial and endothelial cells. Infect. Immun. 1997, 65, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.; Loeffler, J.; Ebel, F. Aspergillus fumigatus: Contours of an opportunistic human pathogen. Cell. Microbiol. 2010, 12, 1535–1543. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Gresnigt, M.S.; Romani, L.; Netea, M.G.; Latgé, J.P. Aspergillus fumigatus morphology and dynamic host interactions. Nat. Rev. Microbiol. 2017, 15, 661–674. [Google Scholar] [CrossRef]
- Ruhnke, M.; Behre, G.; Buchheidt, D.; Christopeit, M.; Hamprecht, A.; Heinz, W.; Heussel, C.P.; Horger, M.; Kurzai, O.; Karthaus, M.; et al. Diagnosis of invasive fungal diseases in haematology and oncology: 2018 update of the recommendations of the infectious diseases working party of the German society for hematology and medical oncology (AGIHO). Mycoses 2018, 61, 796–813. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Wang, T.; Yin, C.; Sun, Y.; Xu, D.; Wang, Z.; Luan, L.; Hou, J.; Li, S. Aspergillus spondylitis: Case series and literature review. BMC Musculoskelet. Disord. 2020, 21, 572. [Google Scholar] [CrossRef]
- Binder, U.; Lass-Flörl, C. New insights into invasive aspergillosis—From the pathogen to the disease. Curr. Pharm. Des. 2013, 19, 3679–3688. [Google Scholar] [CrossRef]
- Duceac, L.D.; Marcu, C.; Ichim, D.L.; Ciomaga, I.M.; Tarca, E.; Iordache, A.C.; Ciuhodaru, M.I.; Florescu, L.; Tutunaru, D.; Luca, A.C.; et al. Antibiotic Molecules Involved in Increasing Microbial Resistance. Rev. Chim. 2019, 70, 2622–2626. [Google Scholar] [CrossRef]
- Collazos, J.; Mayo, J.; Martínez, E.; Ibarra, S. Prosthetic vascular graft infection due to Aspergillus species: Case report and literature review. Eur. J. Clin. Microbiol. Infect. Dis. 2001, 20, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Belu, A.; Trandafir, L.M.; Țarcă, E.; Cojocaru, E.; Frăsinariu, O.; Stârcea, M.; Moscalu, M.; Tiutiuca, R.C.; Luca, A.C.; Galaction, A. Variations in Biochemical Values under Stress in Children with SARS-CoV-2 Infection. Diagnostics 2022, 12, 1213. [Google Scholar] [CrossRef] [PubMed]
| Before Replantation (7–10 h After the Accident) | After Replantation (10–171 h After the Accident) | Massive Invasion with Aspergillus spp. (171–213 h After the Accident) | |
|---|---|---|---|
| Monitoring parameters | |||
| PaO2 (mmHg) | |||
| mean ± SD | 51.7 ± 2.5 | 42.6 ± 5.4 | 46.3 ± 12.6 |
| median (Q1; Q3) | 52 (49; 54) | 42.5 (39; 45) | 49.5 (45.5; 53.5) |
| Glycemia (g/dL) | |||
| mean ± SD | 169.7 ± 57.4 | 107.4 ± 33.5 | 81.1 ± 28.4 |
| median (Q1; Q3) | 177 (109; 223) | 100 (95; 122) | 90.5 (77.5; 98) |
| Hct (%) | |||
| mean ± SD | 23 ± 5 | 26.4 ± 5.8 | 29.1 ± 4.9 |
| median (Q1; Q3) | 23 (18; 28) | 26 (24; 31) | 31 (26.5; 32.5) |
| SvO2 (%) | |||
| mean ± SD | 79.3 ± 2.1 | 71.4 ± 11.5 | 74.4 ± 6.7 |
| median (Q1; Q3) | 80 (77; 81) | 75 (62; 78) | 74 (69.5; 79) |
| Monitoring parameters—potential prognostic factors for the reimplanted limb | |||
| Systemic lactate (mmol/L) | |||
| mean ± SD | 2.3 ± 0.5 | 2.4 ± 1.72 | 1.4 ± 0.7 |
| median (Q1; Q3) | 2.6 (1.7; 2.7) | 1.4 (1.2; 3) | 1.3 (0.8; 2.3) |
| Lactate in the limb (mmol/L) | - | ||
| mean ± SD | 4.1 ± 2.3 | 9.6 ± 3.7 | |
| median (Q1; Q3) | 3.4 (3.1; 4) | 10.3 (9.5; 12) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belu, A.; Țarcă, V.; Filip, N.; Țarcă, E.; Trandafir, L.M.; Heredea, R.E.; Chifan, S.; Parteni, D.E.; Bernic, J.; Cojocaru, E. Lactate Levels in a Replanted Limb as an Early Biomarker for Assessing Post-Surgical Evolution: A Case Report. Diagnostics 2025, 15, 688. https://doi.org/10.3390/diagnostics15060688
Belu A, Țarcă V, Filip N, Țarcă E, Trandafir LM, Heredea RE, Chifan S, Parteni DE, Bernic J, Cojocaru E. Lactate Levels in a Replanted Limb as an Early Biomarker for Assessing Post-Surgical Evolution: A Case Report. Diagnostics. 2025; 15(6):688. https://doi.org/10.3390/diagnostics15060688
Chicago/Turabian StyleBelu, Alina, Viorel Țarcă, Nina Filip, Elena Țarcă, Laura Mihaela Trandafir, Rodica Elena Heredea, Silviana Chifan, Diana Elena Parteni, Jana Bernic, and Elena Cojocaru. 2025. "Lactate Levels in a Replanted Limb as an Early Biomarker for Assessing Post-Surgical Evolution: A Case Report" Diagnostics 15, no. 6: 688. https://doi.org/10.3390/diagnostics15060688
APA StyleBelu, A., Țarcă, V., Filip, N., Țarcă, E., Trandafir, L. M., Heredea, R. E., Chifan, S., Parteni, D. E., Bernic, J., & Cojocaru, E. (2025). Lactate Levels in a Replanted Limb as an Early Biomarker for Assessing Post-Surgical Evolution: A Case Report. Diagnostics, 15(6), 688. https://doi.org/10.3390/diagnostics15060688








