1. Introduction
Positron emission tomography (PET) radiopharmaceuticals targeting prostate specific membrane antigen (PSMA) receptors on tumor cells can visualize smaller lesions than conventional imaging methods (such as magnetic resonance imaging [MRI] or computed tomography [CT]) [
1,
2,
3], helping to facilitate prostate cancer diagnosis [
4] and early detection of recurrent disease [
5].
PET-positive results can be confidently verified with histopathology standard of truth (SoT) measures [
6,
7,
8]. Verifying PET-positive findings from an investigational agent without histopathology data can be challenging, however, obtaining tissue samples may not always be possible in patients with recurrent disease where performing multiple biopsies could be impractical or unsafe [
7,
9]. In such patients, follow-up with conventional imaging is often used as an alternative SoT despite its potential for lower accuracy than the investigational agent [
7,
10]. Standardizing image interpretations within and, where possible, between clinical trials is important because the SoT methodology used to verify PET findings can substantially impact endpoint measures [
11].
The diagnostic PSMA-targeting PET radiopharmaceutical
18F-flotufolastat (
18F-rhPSMA-7.3) is currently approved for clinical use in the USA in patients with recurrent prostate cancer, following findings from the phase 3 SPOTLIGHT study (NCT04186845) [
12]. SPOTLIGHT took place during the COVID-19 pandemic, which widely restricted patient visits to clinical practice [
13,
14,
15]. One quarter of patients in the SPOTLIGHT efficacy analysis population (EAP) had no histopathology or post PET confirmatory imaging data for verification of PET-positive results, leaving investigators with only baseline/historic conventional imaging for SoT assessments [
7]. Considering the recognized challenges associated with verifying PET-positive lesions of investigational PSMA-targeting radiopharmaceuticals, we conducted an analysis of data from a subset of patients in the SPOTLIGHT study to examine the potential impact of SoT methodology on efficacy endpoints traditionally used in clinical trials of diagnostic radiopharmaceuticals.
2. Materials and Methods
The methods of SPOTLIGHT have been published previously [
7]. Briefly, SPOTLIGHT enrolled adult men with elevated prostate-specific antigen (PSA) levels (≥0.2 ng/mL) and suspected recurrent prostate cancer, who underwent PET 50–70 min after intravenous administration of 296 MBq
18F-flotufolastat.
For SoT assessment, the protocol required histopathology ≤60 days post-PET or confirmatory conventional imaging (MRI, CT, 18F-fluciclovine-PET, or 18F-sodium fluoride-PET per site standard of care) ≤90 days post-PET. Three blinded readers evaluated PET scans, and majority-read values represented agreement between ≥2 readers. SoT was established by histopathology when biopsy data were available; alternatively, a separate Truth Panel established SoT following review of conventional imaging data.
Our analysis included all patients from the SPOTLIGHT EAP who underwent 18F-flotufolastat imaging and had data for SoT determination, and with baseline PSA ≤ 5 ng/mL. Efficacy endpoint data were stratified by the SoT methodology used to verify PET-positive lesions.
Key endpoints traditionally used in diagnostic clinical trials were evaluated: patient-level verified detection rate (VDR) and patient-level positive predictive value (PPV); and region-level PPV in the prostate/prostate bed, pelvic lymph nodes, and other extrapelvic sites (lymph nodes, bones, and soft tissue/parenchyma).
Differences between SoT groups for each endpoint were compared using a chi-square test. p-values < 0.0167 were considered statistically significant; the threshold for statistical significance was adjusted from <0.05 to account equally for each of 3 pairwise comparisons (histopathology vs. baseline/historic imaging; histopathology vs. post-PET confirmatory imaging; post-PET confirmatory imaging vs. baseline/historic imaging) per endpoint.
3. Results
3.1. Baseline Characteristics
Of 420 patients enrolled in SPOTLIGHT, 391 underwent
18F-flotufolastat-PET, 389 had an evaluable scan, and 366 with data for SoT assessment were included in the EAP. Of patients in the SPOTLIGHT EAP, 297 had baseline PSA ≤ 5 ng/mL (median baseline PSA = 0.8 ng/mL [range = 0.0–5.0]) and were included in this study: 56% (
n = 166) had post-PET confirmatory imaging, 26% (
n = 78) had baseline/historic conventional imaging only, and 18% (
n = 53) had histopathological confirmation of ≥1 PET-positive lesion. Patient baseline characteristics are presented in
Table 1.
3.2. Impact of SoT Methodology on Efficacy Endpoints
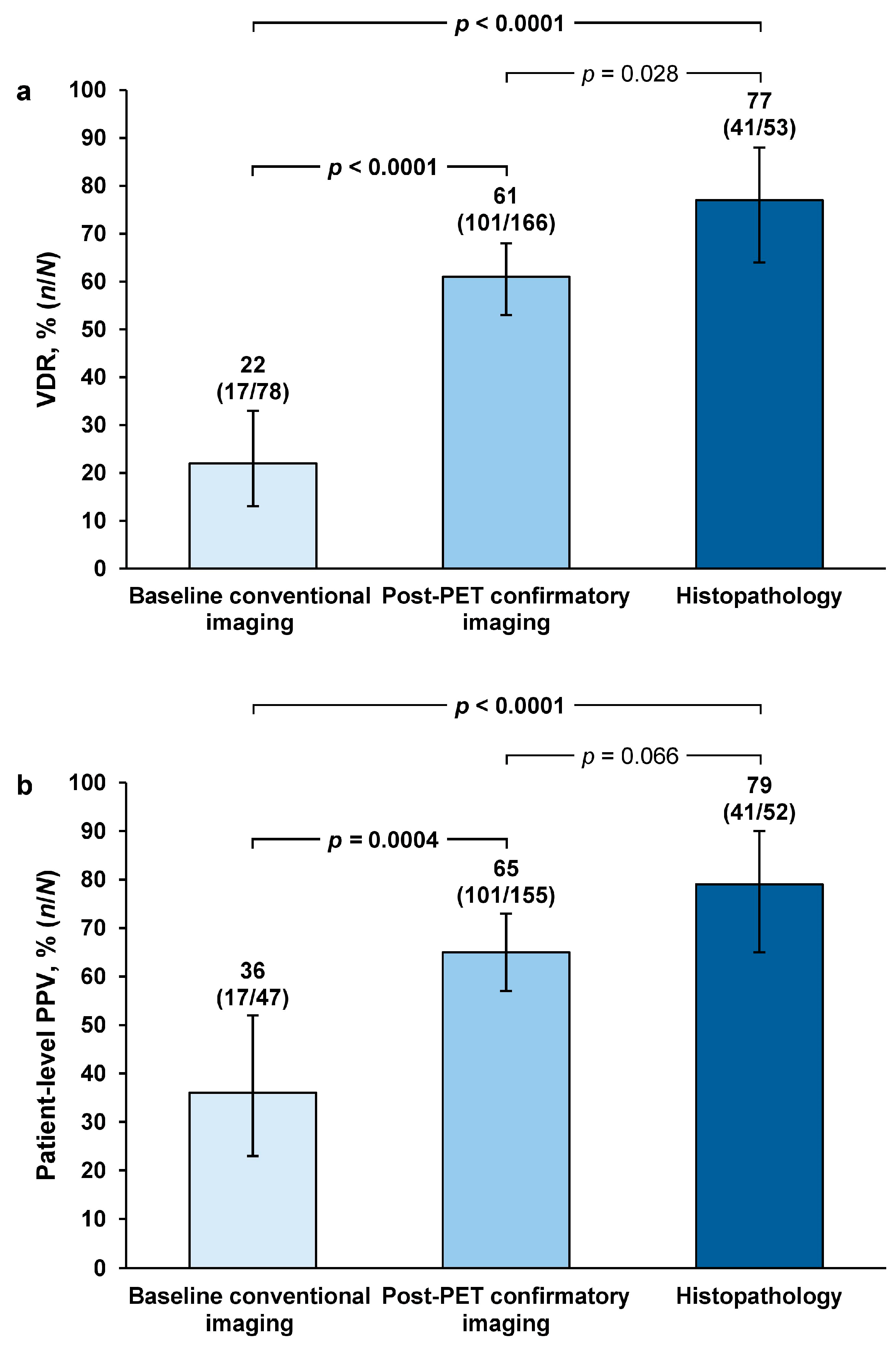
The highest majority-read values were obtained with histopathology across all evaluated endpoints.
Patient-level VDR was 77% (
n = 41/53; 95% confidence interval [CI] = 64–88) among the 53 patients with histopathology data for SoT. This was 3.6-fold higher than the VDR estimate using baseline/historic conventional imaging as SoT. Using post-PET confirmatory imaging as SoT gave a 2.8-fold higher VDR estimate than baseline/historic conventional imaging. VDR estimates with histopathology and post-PET confirmatory imaging were significantly greater than VDR estimates with baseline/historic imaging (both
p < 0.0001). Histopathology SoT gave a 1.3-fold higher VDR estimate than post-PET confirmatory imaging, but this difference was not of statistical significance (
p = 0.028) (
Figure 1a).
Patient-level PPV estimates with histopathology and post-PET confirmatory imaging were significantly greater than PPV estimates with baseline/historic conventional imaging: PPV was 2.2-fold higher with histopathology (
p < 0.0001) and 1.8-fold higher when using post-PET confirmatory imaging (
p = 0.0004) compared with baseline/historic conventional imaging. Histopathology SoT gave a 1.2-fold higher VDR estimate than post-PET confirmatory imaging, but this difference was not of statistical significance (
p = 0.066) (
Figure 1b).
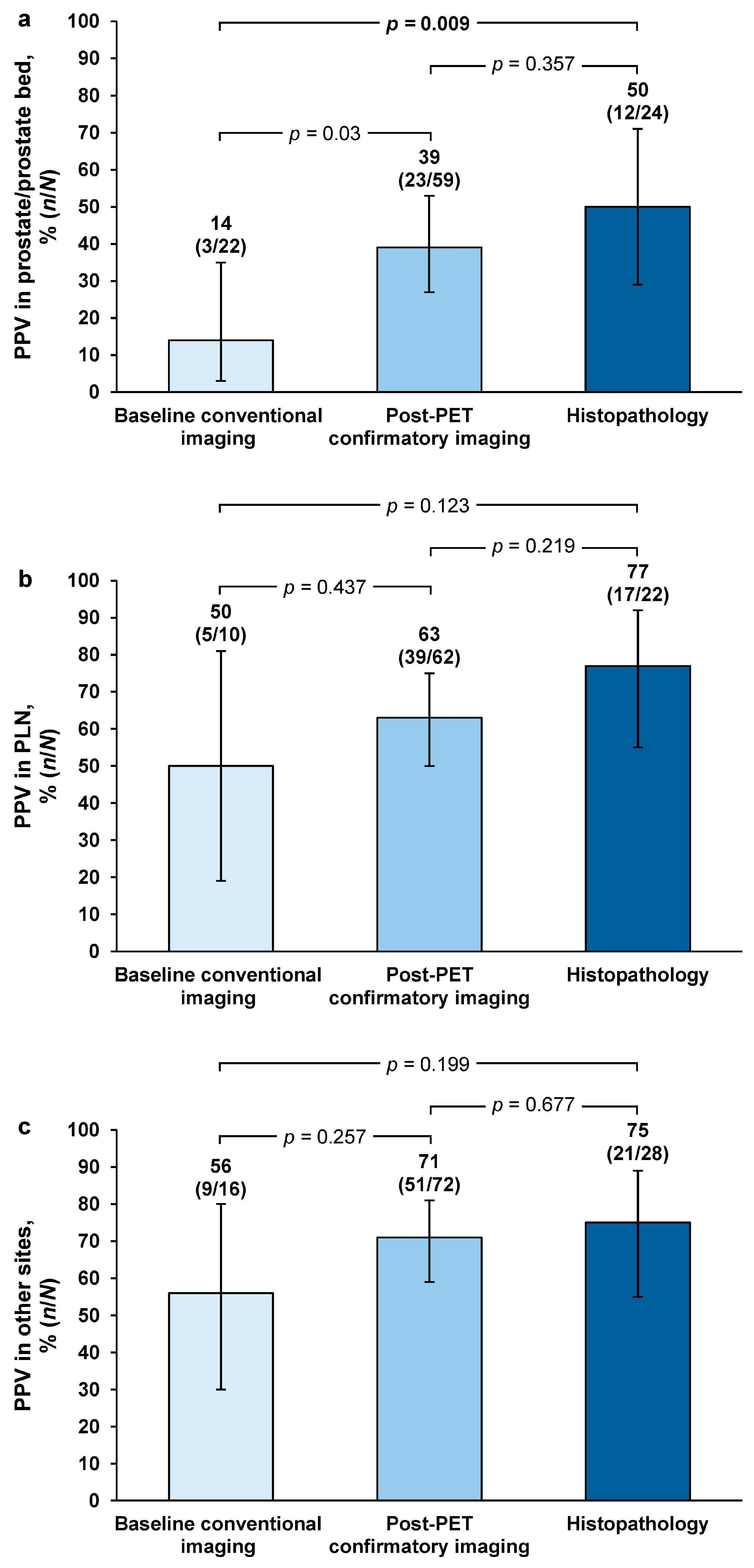
For region-level PPV, majority-read values were highest with histopathology in the prostate/prostate bed (50%;
n = 12/24 [95% CI = 29–71]) and pelvic lymph nodes (77%;
n = 17/22 [95% CI = 55–92), and at other sites (75%;
n = 21/28 [95% CI = 55–89]) (
Figure 2).
Majority-read PPV for histopathology versus baseline/historic conventional imaging was 3.7-fold higher in the prostate/prostate bed, which was of statistical significance (
p = 0.009). Majority-read PPV for histopathology versus baseline/historic imaging was 1.5-fold higher in the pelvic lymph nodes (
p = 0.123) and 1.3-fold higher at other sites (
p = 0.199). For post-PET confirmatory imaging versus baseline/historic imaging, majority-read PPV was 2.9-fold higher in the prostate/prostate bed (
p = 0.03) and 1.3-fold higher in the pelvic lymph nodes (
p = 0.437) and other regions (
p = 0.257). For histopathology versus post-PET confirmatory imaging, majority-read values were 1.3-fold higher in the prostate/prostate bed (
p = 0.357), 1.2-fold higher in the pelvic lymph nodes (
p = 0.219), and 1.1-fold higher in other regions (
p = 0.677) (
Figure 2).
4. Discussion
SoT using histopathology consistently achieved the highest majority-read values for all evaluated endpoints. Post PET confirmatory imaging also provided increased majority-read estimates for all endpoints compared with baseline/historic conventional imaging. The SPOTLIGHT study noted that verification of PET-positive scans without histopathology could increase the rate of “false false positives”, and furthermore showed an increase in VDR and patient-level PPV in the per-protocol population, which excluded patients without histopathology or post-PET confirmatory imaging [
7]. This highlighted the unsuitability of using only single-timepoint imaging for robust verification of PSMA-avid lesions, as also demonstrated by the results of our additional analysis.
While histopathology is the ideal SoT for prostate cancer diagnosis, challenges can arise where tissue biopsies are considered unfeasible or unsafe in patients with recurrent disease. Patients may not consent to undergo one or multiple tissue biopsies if they consider the procedure as uncomfortable or stressful [
16], and undergoing multiple biopsies increases the risk of complications and infections [
9]. Additionally, some PSMA-avid lesions may be too small to yield sufficient tissue samples for analysis. The nature of recurrent disease could have influenced the low proportion of patients with histopathology SoT data in SPOTLIGHT and, subsequently, in our analysis of SPOTLIGHT data (18%). It is also important to consider that patient visits to clinical practice at the time of the SPOTLIGHT study were limited by restrictions imposed during the COVID-19 pandemic [
17,
18]. Approximately 25% of patients in our analysis of SPOTLIGHT data did not have post-PET confirmatory imaging or histopathological follow-up, which was a likely consequence of these restrictions in patient visits. As our data show, reliance on only baseline or historic conventional imaging to verify imaging findings results in significantly lower endpoint estimates which ultimately would have impacted the overall study results. This is a clear limitation of SPOTLIGHT [
7], given that such restrictions on patient visits to clinical practice did not affect earlier registration trials of PSMA-targeting radiopharmaceuticals [
6,
8].
Although post-PET confirmatory imaging could be considered a suitable alternative to histopathology when verifying an investigational PSMA-PET radiopharmaceutical, conventional imaging methodologies are notably less sensitive than PSMA-PET [
1,
2,
3], and this was further supported by our data showing lower majority-read values for patient- and region-level endpoints with post-PET confirmatory imaging than with histopathology SoT. It should also be noted that use of
18F-fluciclovine-PET, which is more sensitive than CT in prostate cancer [
19,
20] and which was the most sensitive conventional imaging methodology available during SPOTLIGHT, was limited per US Food and Drug Administration request, and only 17% of lesions in SPOTLIGHT were verified with
18F-fluciclovine [
7] (by contrast, 54% of patients in the
18F-DCFPyL registration trial had post-PET confirmatory imaging with
18F-fluciclovine [
8]). Such a limitation on SPOTLIGHT SoT methodology, combined with varying rates of histopathology between SPOTLIGHT and other registration trials of PSMA-targeting radiopharmaceuticals [
6,
8], may have impacted performance estimates in the trials.
In our analysis of SPOTLIGHT data, statistical analysis of region-level data was limited by small sample sizes. Furthermore, slight differences in patient characteristics between groups were noted, including median baseline PSA values; although these differences between groups were minimal, any potential impact of these on the endpoint estimates would need to be determined by future prospective studies.
5. Conclusions
Our analysis of SPOTLIGHT data demonstrates the significant impact that SoT methods used to verify positive scan findings can have on clinical trial endpoints. These data highlight a limitation of evaluating endpoints with different SoT methodologies in diagnostic clinical trials; standardizing SoT measures in clinical trials could help improve the robustness of findings. Histopathology was the optimal SoT methodology that achieved significantly higher majority-read patient-level endpoint estimates than imaging SoT.
Author Contributions
B.H.L. contributed to data acquisition, A.C. planned and supervised analysis of the data, and P.D. helped with study design and was the medical monitor for the study sponsor. All authors have read and agreed to the published version of the manuscript.
Funding
SPOTLIGHT was funded by Blue Earth Diagnostics Ltd., Oxford, UK. No funding was provided for this observational study.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Formal consent was not required for this observational study.
Data Availability Statement
The original contributions presented in this study are included within the article itself. Further inquiries can be directed to the corresponding author.
Acknowledgments
Medical writing support was provided by Joanna Wilson, Blue Earth Diagnostics Ltd., Oxford, UK.
Conflicts of Interest
Benjamin H. Lowentritt acted as a consultant/advisor for AstraZeneca, Blue Earth Diagnostics Ltd., Merck, Pfizer, and Sumitomo Pharma, and has served as a consultant/advisor for Myovant. Albert Chau acted as a consultant for Blue Earth Diagnostics Ltd. Phillip Davis is employed by Blue Earth Diagnostics Inc. Blue Earth Diagnostics Ltd. provided funding for SPOTLIGHT, no funding was provided for this observational study.
Abbreviations
The following abbreviations are used in this manuscript:
| PET | Positron emission tomography |
| PSMA | Prostate-specific membrane antigen |
| MRI | Magnetic resonance imaging |
| CT | Computed tomography |
| SoT | Standard-of-truth |
| 18F | Fluorine-18 |
| EAP | Efficacy analysis population |
| PSA | Prostate-specific antigen |
| VDR | Verified detection rate |
| PPV | Positive predictive value |
| CI | Confidence interval |
| ISUP | International Society of Urological Pathology |
References
- Chausse, G.; Ben-Ezra, N.; Stoopler, M.; Levett, J.Y.; Niazi, T.; Anidjar, M.; Abikhzer, G.; Probst, S. Diagnostic performance of 18F-DCFPyL positron emission tomography/computed tomography for biochemically recurrent prostate cancer and change-of-management analysis. Can. Urol. Assoc. J. 2021, 15, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Meijer, D.; Jansen, B.H.E.; Wondergem, M.; Bodar, Y.J.L.; Srbljin, S.; Vellekoop, A.E.; Keizer, B.; van der Zant, F.M.; Hoekstra, O.S.; Nieuwenhuijzen, J.A.; et al. Clinical verification of 18F-DCFPyL PET-detected lesions in patients with biochemically recurrent prostate cancer. PLoS ONE 2020, 15, e0239414. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Kwon, D.H.; Velazquez, A.I.; de Kouchkovsky, I. PSMA PET Scan. JAMA Oncol. Patient Page 2022, 8, 1860. [Google Scholar] [CrossRef] [PubMed]
- Wondergem, M.; Jansen, B.H.E.; van der Zant, F.M.; van der Sluis, T.M.; Knol, R.J.J.; van Kalmthout, L.W.M.; Hoekstra, O.S.; van Moorselaar, R.J.A.; Oprea-Lager, D.E.; Vis, A.N. Early lesion detection with 18F-DCFPyL PET/CT in 248 patients with biochemically recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: A prospective single-arm clinical trial. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Jani, A.B.; Ravizzini, G.; Gartrell, B.A.; Siegel, B.A.; Twardowski, P.; Saltzstein, D.; Fleming, M.T.; Chau, A.; Davis, P.; Chapin, B.F.; et al. Diagnostic performance and safety of 18F-rhPSMA-7.3 PET in men with suspected prostate cancer recurrence: Results from a phase 3, prospective, multicenter study (SPOTLIGHT). J. Urol. 2023, 210, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Rowe, S.P.; Gorin, M.A.; Saperstein, L.; Pouliot, F.; Josephson, D.; Wong, J.Y.C.; Pantel, A.R.; Cho, S.Y.; Gage, K.L.; et al. Diagnostic performance of 18F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: Results from the CONDOR phase III, multicenter study. Clin. Cancer Res. 2021, 27, 3674–3682. [Google Scholar] [CrossRef] [PubMed]
- Kopp, R.P.; Stroup, S.P.; Schroeck, F.R.; Freedland, S.J.; Millard, F.; Terris, M.K.; Aronson, W.J.; Presti, J.C., Jr.; Amling, C.L.; Kane, C.J. Are repeat prostate biopsies safe? A cohort analysis from the SEARCH database. J. Urol. 2012, 187, 2056–2060. [Google Scholar] [CrossRef][Green Version]
- Calais, J.; Ceci, F.; Eiber, M.; Hope, T.A.; Hofman, M.S.; Rischpler, C.; Bach-Gansmo, T.; Nanni, C.; Savir-Baruch, B.; Elashoff, D.; et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: A prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019, 20, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- U.S Department of Health and Human Services Food and Drug Administration: Center for Drug Evaluation and Research Center for Biologics Evaluation and Research. Clinical Trial Imaging Endpoint Process Standards guidance for Industry; CDER: Silver Spring, MD, USA; CBER: Silver Spring, MD, USA, 2018. Available online: https://www.fda.gov/media/81172/download (accessed on 6 January 2025).
- U.S Department of Health and Human Services Food and Drug Administration. Highlights of Prescribing Information: POSLUMA (flotufolastat F 18) Injection; U.S Department of Health and Human Services Food and Drug Administration: Washington, DC, USA, 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216023s000lbl.pdf (accessed on 6 January 2025).
- Obek, C.; Doganca, T.; Argun, O.B.; Kural, A.R. Management of prostate cancer patients during COVID-19 pandemic. Prostate Cancer Prostatic Dis. 2020, 23, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Boughey, J.C.; Snyder, R.A.; Kantor, O.; Zheng, L.; Chawla, A.; Nguyen, T.T.; Hillman, S.L.; Hahn, O.M.; Mandrekar, S.J.; Roland, C.L. Impact of the COVID-19 pandemic on cancer clinical trials. Ann. Surg. Oncol. 2021, 28, 7311–7316. [Google Scholar] [CrossRef]
- Zadeh, S.M.M.; Tajik, F.; Gheytanchi, E.; Kiani, J.; Ghods, R.; Madjd, Z. COVID-19 pandemic impact on screening and diagnosis of prostate cancer: A systematic review. BMJ Support. Palliat. Care 2023, 14, 1–10. [Google Scholar] [CrossRef]
- Wade, J.; Rosario, D.J.; Macefield, R.C.; Avery, K.N.; Salter, C.E.; Goodwin, M.L.; Blazeby, J.M.; Lane, J.A.; Metcalfe, C.; Neal, D.E.; et al. Psychological impact of prostate biopsy: Physical symptoms, anxiety, and depression. J. Clin. Oncol. 2013, 31, 4235–4241. [Google Scholar] [CrossRef]
- European Medicines Agency Heads of Medicines Agency. Guidance on the Management of Clinical Trials During the COVID-19 (Coronavirus) Pandemic (Version 5, 10 February 2022). European Medicines Agency Heads of Medicine Agency European Commission, Belgium 2022. Available online: https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-10/guidanceclinicaltrials_covid19_en.pdf (accessed on 6 January 2025).
- U.S Department of Health and Human Services Food and Drug Administration. Conduct of Clinical Trials of Medical Products During the COVID-19 Public Health Emergency: Guidance for Industry, Investigators, and Institutional Review Boards. March 2020, Updated on August 30, 2021. U.S Department of Health and Human Services Food and Drug Administration: Washington, DC, USA, 2021. Available online: https://public4.pagefreezer.com/content/FDA/02-11-2021T10:11/https://www.fda.gov/media/136238/download (accessed on 6 January 2025).
- Savir-Baruch, B.; Zanoni, L.; Schuster, D.M. Imaging of prostate cancer using fluciclovine. PET Clin. 2017, 12, 145–157. [Google Scholar] [CrossRef]
- Odewole, O.A.; Tade, F.I.; Nieh, P.T.; Savir-Baruch, B.; Jani, A.B.; Master, V.A.; Rossi, P.J.; Halkar, R.K.; Osunkoya, A.O.; Akin-Akintayo, O.; et al. Recurrent prostate cancer detection with anti-3-[18F]FACBC PET/CT: Comparison with CT. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1773–1783. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).