The Potential Roles of IL-1β, IL-6, and RIPK3 in the Pathogenesis of Stevens–Johnson Syndrome/Toxic Epidermal Necrolysis
Abstract
1. Introduction
2. Methods
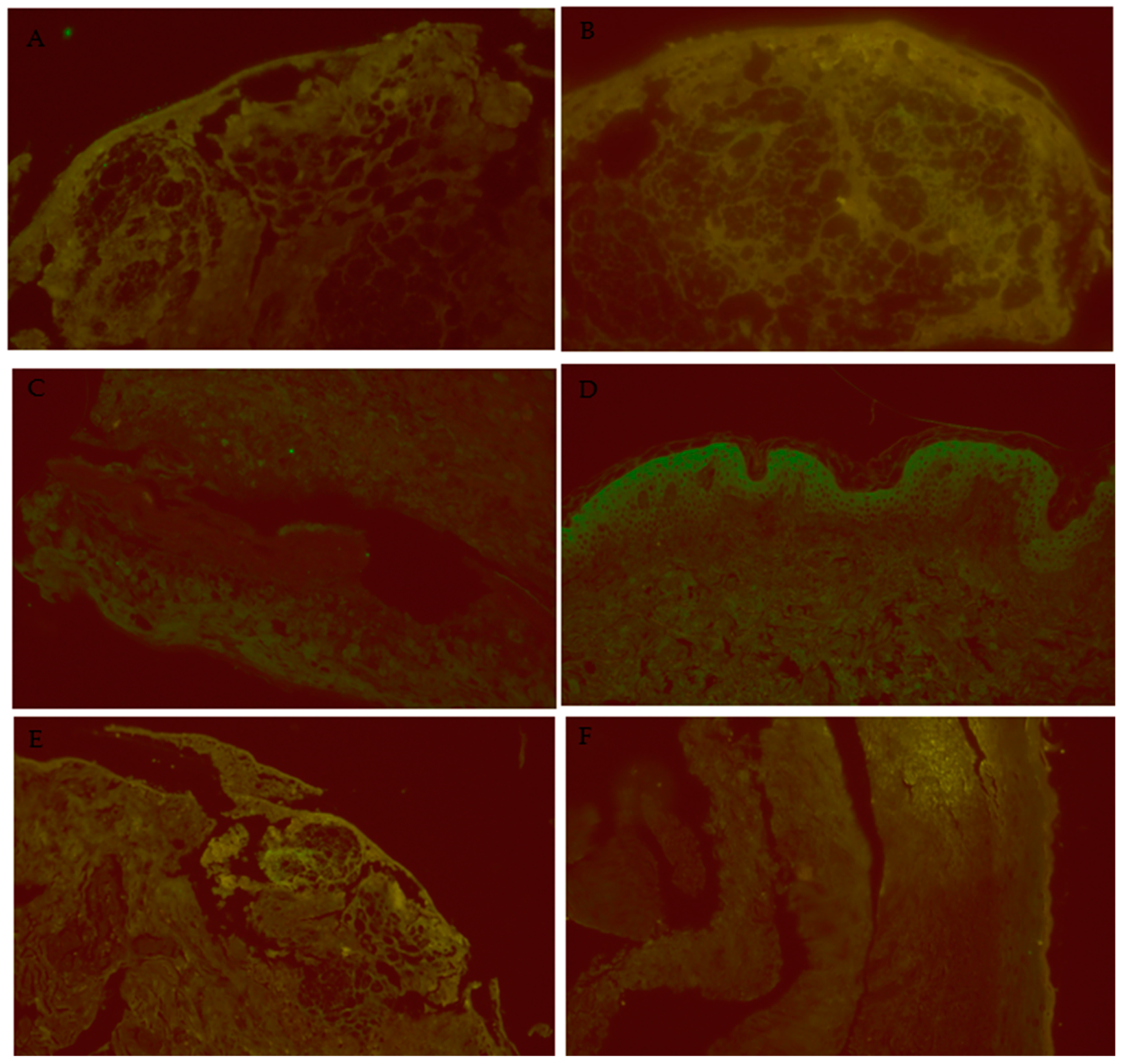
2.1. Experimental Design for Skin Biopsy Analysis
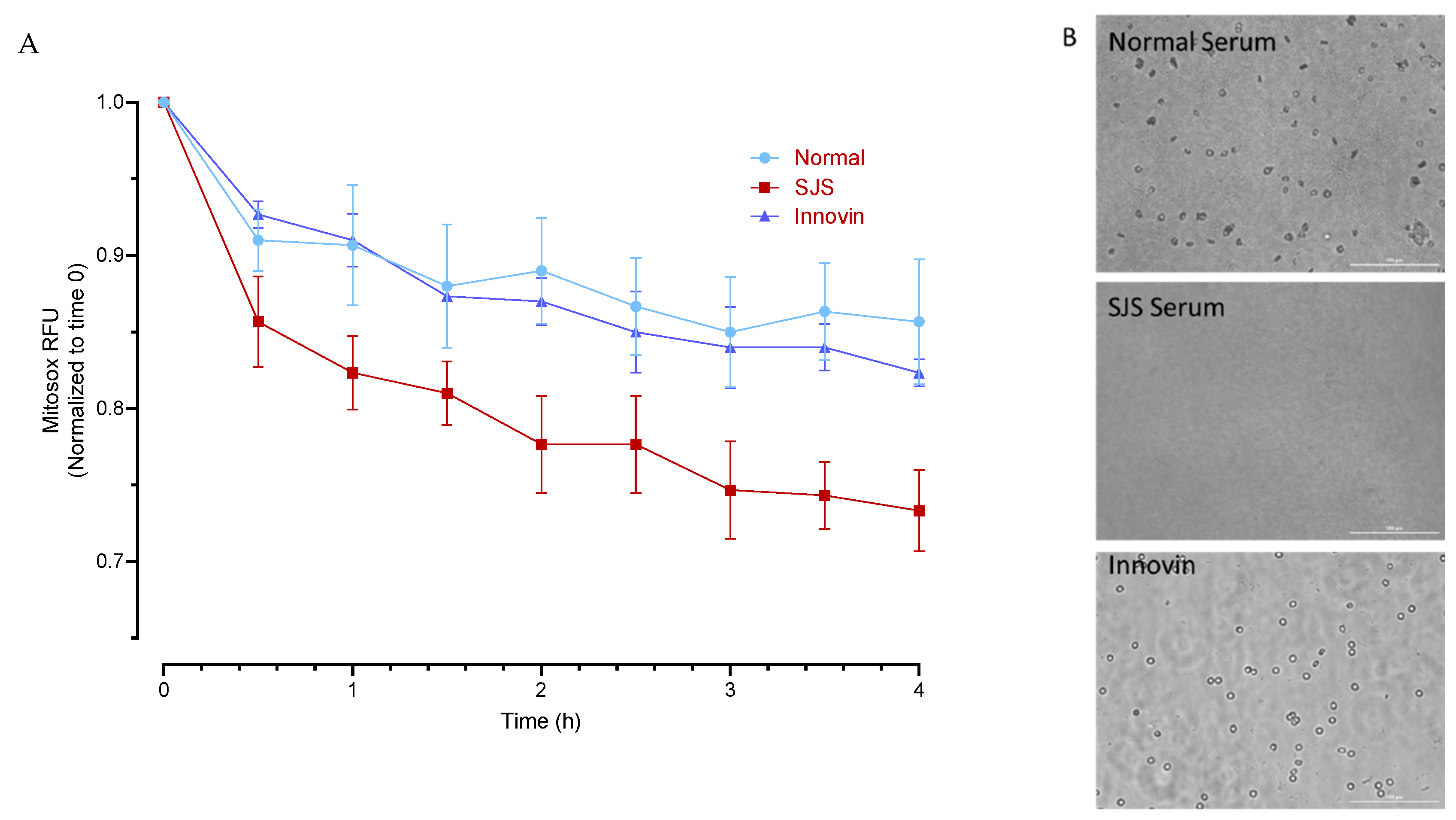
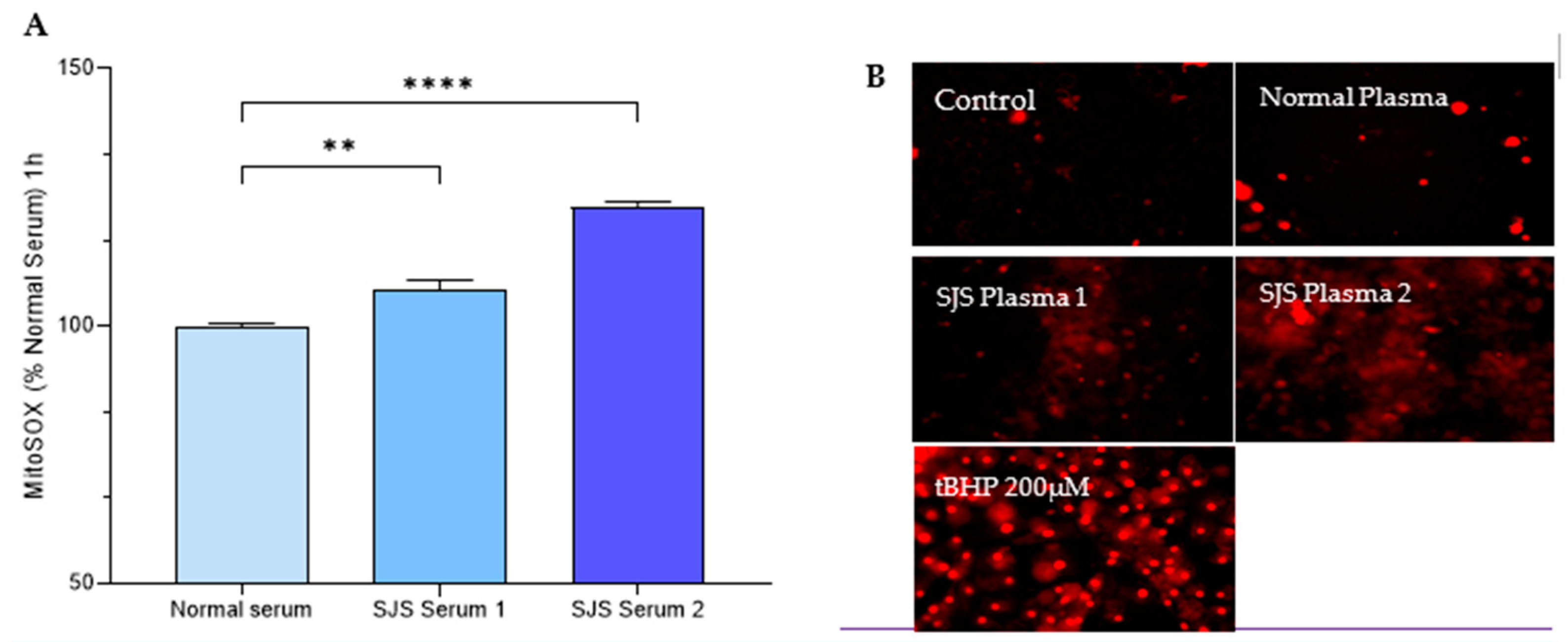
2.2. Experimental Design for Mitochondrial Functioning Assays
2.3. H-CET Cells
3. Results
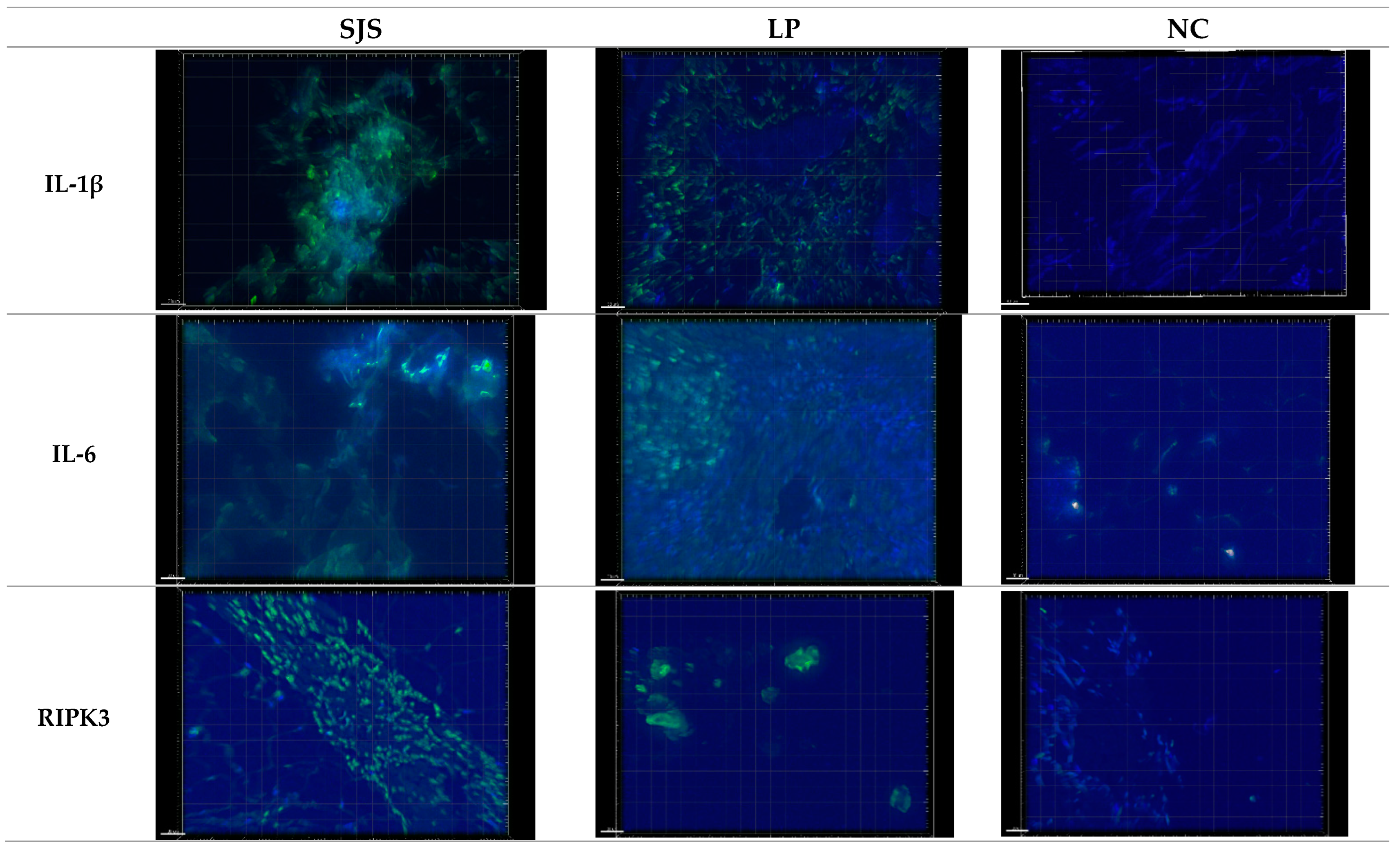
3.1. IL-1β Puncta Count and Intensities in SJS/TEN, Lichen Planus, and Normal Control Tissue Samples
3.2. IL-6 Puncta Count and Intensities in SJS/TEN, Lichen Planus, and Normal Control Tissue Samples
3.3. RIPK3 Puncta Count and Intensities in SJS/TEN, Lichen Planus, and Normal Control Tissue Samples
3.4. Mitochondrial Functioning Assays
4. H-CET
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roujeau, J.C.; Stern, R.S. Severe adverse cutaneous reactions to drugs. N. Engl. J. Med. 1994, 331, 1272–1285. [Google Scholar] [CrossRef]
- Chen, C.-B.; Lee, C.-C.; Wang, C.-W.; Hung, W.-K.; Chung, W.-H. Genetic associations of human leukocyte antigen alleles in cutaneous delayed drug hypersensitivity reactions: An updated review. Dermatol. Sin. 2023, 41, 183–198. [Google Scholar] [CrossRef]
- Chung, W.H.; Hung, S.I.; Hong, H.S.; Hsih, M.S.; Yang, L.C.; Ho, H.C.; Wu, J.Y.; Chen, Y.T. Medical genetics: A marker for Stevens Johnson syndrome. Nature 2004, 428, 486. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-I.; Chung, W.-H.; Liou, L.-B.; Chu, C.-C.; Lin, M.; Huang, H.-P.; Lin, Y.-L.; Lan, J.-L.; Yang, L.-C.; Hong, H.-S.; et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc. Natl. Acad. Sci. USA 2005, 102, 4134–4139. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-I.; Chung, W.-H.; Jee, S.-H.; Chen, W.-C.; Chang, Y.-T.; Lee, W.-R.; Hu, S.-L.; Wu, M.-T.; Chen, G.-S.; Wong, T.-W.; et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet. Genom. 2006, 16, 297–306. [Google Scholar] [CrossRef]
- Roujeau, J.-C. Immune mechanisms in drug allergy. Allergol. Int. 2006, 55, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, O. The Role of NLRP3 Inflammasome in the Pathogenesis of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Med. Res. Arch. 2023, 12, 10–18103. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-H.; Hung, S.-I.; Yang, J.-Y.; Su, S.-C.; Huang, S.-P.; Wei, C.-Y.; Chin, S.-W.; Chiou, C.-C.; Chu, S.-C.; Ho, H.-C.; et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat. Med. 2008, 14, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Nassif, A.; Bensussan, A.; Boumsell, L.; Deniaud, A.; Moslehi, H.; Wolkenstein, P.; Bagot, M.; Roujeau, J.-C. Toxic epidermal necrolysis: Effector cells are drug-specific cytotoxic T cells. J. Allergy Clin. Immunol. 2004, 114, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Chave, T.; Mortimer, N.; Sladden, M.; Hall, A.; Hutchinson, P. Toxic epidermal necrolysis: Current evidence, practical management and future directions. Br. J. Dermatol. 2005, 153, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Till, S.D.; Iqbal, O.; Dharan, A.; Campbell, E.; Bu, P. The roles of IL-33 and TGF-β1 in the pathogenesis of Stevens Johnson syndrome and toxic epidermal necrolysis: Potential biomarkers for disease severity. J. Ophthalmol. Eye Care 2018, 1, 105. [Google Scholar]
- Su, S.-C.; Mockenhaupt, M.; Wolkenstein, P.; Dunant, A.; Le Gouvello, S.; Chen, C.-B.; Chosidow, O.; Valeyrie-Allanore, L.; Bellon, T.; Sekula, P.; et al. Interleukin-15 Is Associated with severity and mortality in Stevens-Johnson Syndrome/toxic epidermal necrolysis. J. Investig. Dermatol. 2017, 137, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Sadek, M.; Iqbal, O.; Siddiqui, F.; Till, S.; Mazariegos, M.; Campbell, E.; Mudaliar, K.; Speiser, J.; Bontekoe, E.; Kouta, A.; et al. The roles of IL-13, IL-15 and granulysin in the pathogenesis of Stevens-Johnson syndrome/toxic epidermal necrolysis. Clin. Appl. Thromb./Hemost. 2021, 27, 1076029620950831. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L. Current Pharmacogenetic Perspective on Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Front. Pharmacol. 2021, 12, 588063. [Google Scholar] [CrossRef]
- Mockenhaupt, M.; Viboud, C.; Dunant, A.; Naldi, L.; Halevy, S.; Bavinck, J.N.B.; Sidoroff, A.; Schneck, J.; Roujeau, J.-C.; Flahault, A. Stevens-Johnson Syndrome and toxic epidermal necrolysis: Assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J. Investig. Dermatol. 2008, 128, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.S.; Divito, S.J. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Associations, Outcomes, and Pathobiology—Thirty Years of Progress but Still Much to Be Done. J. Investig. Dermatol. 2017, 137, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Correia, O.; Delgado, L.; Barbosa, I.L.; Campilho, F.; Fleming-Torrinha, J. Increased interleukin 10, tumor necrosis factor α, and interleukin 6 levels in blister fluid of toxic epidermal necrolysis. J. Am. Acad. Dermatol. 2002, 47, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Hanin, E.-K.; Malika, S.; Iqbal, O.; Dharan, A.; Campbell, E.; Bu, P.; Mudaliar, K.; Speiser, J.; Bouchard, C. The potential roles of IL-6 and NKG2C in the pathogenesis of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Clin. Res. Clin. Rep. 2024, 3, 1–7. [Google Scholar]
- Paul, C.; Wolkenstein, P.; Adle, H.; Wechsler, J.; Garchon, H.; Revuz, J.; Roujeau, J. Apoptosis as a mechanism of keratinocyte death in toxic epidermal necrolysis. Br. J. Dermatol. 1996, 134, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dugger, D.L.; Wickliffe, K.E.; Kapoor, N.; de Almagro, M.C.; Vucic, D.; Komuves, L.; Ferrando, R.E.; French, D.M.; Webster, J.; et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 2014, 343, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-W.; Shao, J.; Lin, J.; Zhang, N.; Lu, B.-J.; Lin, S.-C.; Dong, M.-Q.; Han, J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009, 325, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Najjar, M.; Saleh, D.; Zelic, M.; Nogusa, S.; Shah, S.; Tai, A.; Finger, J.N.; Polykratis, A.; Gough, P.J.; Bertin, J.; et al. RIPK1 and RIPK3 Kinases Promote Cell-Death-Independent Inflammation by Toll-like Receptor 4. Immunity 2016, 45, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, W.-J.; Yoon, J.-H.; Ji, J.-H.; Morgan, M.J.; Cho, H.; Kim, Y.C. Upregulated RIP3 expression potentiates MLKL phosphorylation-mediated programmed necrosis in toxic epidermal necrolysis. J. Investig. Dermatol. 2015, 135, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Ma, W.; Zhang, B.; Li, W. NLRP3 Inflammasome: A Promising Therapeutic Target for Drug-Induced Toxicity. Front. Cell Dev. Biol. 2021, 9, 634607. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Girardin, S.E. Mitochondrial ROS fuel the inflammasome. Cell Res. 2011, 21, 558–560. [Google Scholar] [CrossRef]
- Bulua, A.C.; Simon, A.; Maddipati, R.; Pelletier, M.; Park, H.; Kim, K.-Y.; Sack, M.N.; Kastner, D.L.; Siegel, R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 2011, 208, 519–533. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, S.S.; Zhao, S.; Yang, Z.; Zhong, C.-Q.; Chen, X.; Cai, Q.; Yang, Z.-H.; Huang, D.; Wu, R.; et al. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat. Commun. 2017, 8, 14329. [Google Scholar] [CrossRef] [PubMed]
- Latz, E. The inflammasomes: Mechanisms of activation and function. Curr. Opin. Immunol. 2010, 22, 28–33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rhodus, N.L.; Cheng, B.; Ondrey, F. Th1/Th2 cytokine ratio in tissue transudates from patients with oral lichen planus. Mediat. Inflamm. 2007, 2007, 19854. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huyen, T.T.; Phuong, P.T.M.; Lan, P.T.; Vinh, N.T.H.; Doanh, L.H. Plasma Levels of Interleukin-1 Beta are Decreased in Patients with Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis at the Time of Hospitalization. Open Access Maced. J. Med. Sci. 2024, 12, 93–97. Available online: https://oamjms.eu/index.php/mjms/article/view/11800 (accessed on 25 July 2024). [CrossRef]
- Koduri, M.A.; Prasad, D.; Upadhyaya, S.; Jaffet, J.; Shanbhag, S.S.; Basu, S.; Singh, V. Differential expression of tear film cytokines in Stevens-Johnson syndrome patients and comparative review of literature. Sci. Rep. 2021, 11, 18433. [Google Scholar] [CrossRef]
- Zimmermann, S.; Sekula, P.; Venhoff, M.; Motschall, E.; Knaus, J.; Schumacher, M.; Mockenhaupt, M. Systemic Immunomodulating Therapies for Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review and Meta-analysis. JAMA Dermatol. 2017, 153, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Panayotova-Dimitrova, D.; Feoktistova, M.; Leverkus, M. RIPping the Skin Apart: Necroptosis Signaling in Toxic Epidermal Necrolysis. J. Investig. Dermatol. 2015, 135, 1940–1943. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sooranahalli, C.; Rao, V.R.; Zelman, B.; Shekhar, M.; Komurlu Keceli, S.; Bouchard, C.; Iqbal, O. The Potential Roles of IL-1β, IL-6, and RIPK3 in the Pathogenesis of Stevens–Johnson Syndrome/Toxic Epidermal Necrolysis. Diagnostics 2025, 15, 290. https://doi.org/10.3390/diagnostics15030290
Sooranahalli C, Rao VR, Zelman B, Shekhar M, Komurlu Keceli S, Bouchard C, Iqbal O. The Potential Roles of IL-1β, IL-6, and RIPK3 in the Pathogenesis of Stevens–Johnson Syndrome/Toxic Epidermal Necrolysis. Diagnostics. 2025; 15(3):290. https://doi.org/10.3390/diagnostics15030290
Chicago/Turabian StyleSooranahalli, Chandana, Vidhya R. Rao, Brandon Zelman, Mallika Shekhar, Sevnur Komurlu Keceli, Charles Bouchard, and Omer Iqbal. 2025. "The Potential Roles of IL-1β, IL-6, and RIPK3 in the Pathogenesis of Stevens–Johnson Syndrome/Toxic Epidermal Necrolysis" Diagnostics 15, no. 3: 290. https://doi.org/10.3390/diagnostics15030290
APA StyleSooranahalli, C., Rao, V. R., Zelman, B., Shekhar, M., Komurlu Keceli, S., Bouchard, C., & Iqbal, O. (2025). The Potential Roles of IL-1β, IL-6, and RIPK3 in the Pathogenesis of Stevens–Johnson Syndrome/Toxic Epidermal Necrolysis. Diagnostics, 15(3), 290. https://doi.org/10.3390/diagnostics15030290





