Inflammatory Signatures and Biological Markers in Platelet-Rich Plasma Therapy for Hair Regrowth: A Comprehensive Narrative Analysis
Abstract
1. Introduction
1.1. The Context of Hair Loss and the Importance of Regenerative Treatments
1.2. Platelet-Rich Plasma (PRP) as a Therapy for Hair Growth
1.3. The Importance of Biological and Inflammatory Markers in PRP Therapy
2. Materials and Methods
- The physiopathological mechanisms through which PRP influences hair follicle regeneration;
- The role of inflammation in the pathogenesis of alopecias and how PRP contributes to its reduction;
- The specific biological markers involved in the response to PRP therapy;
- Management perspectives and strategies for optimizing PRP therapy through the integration of inflammatory markers and complementary technologies.
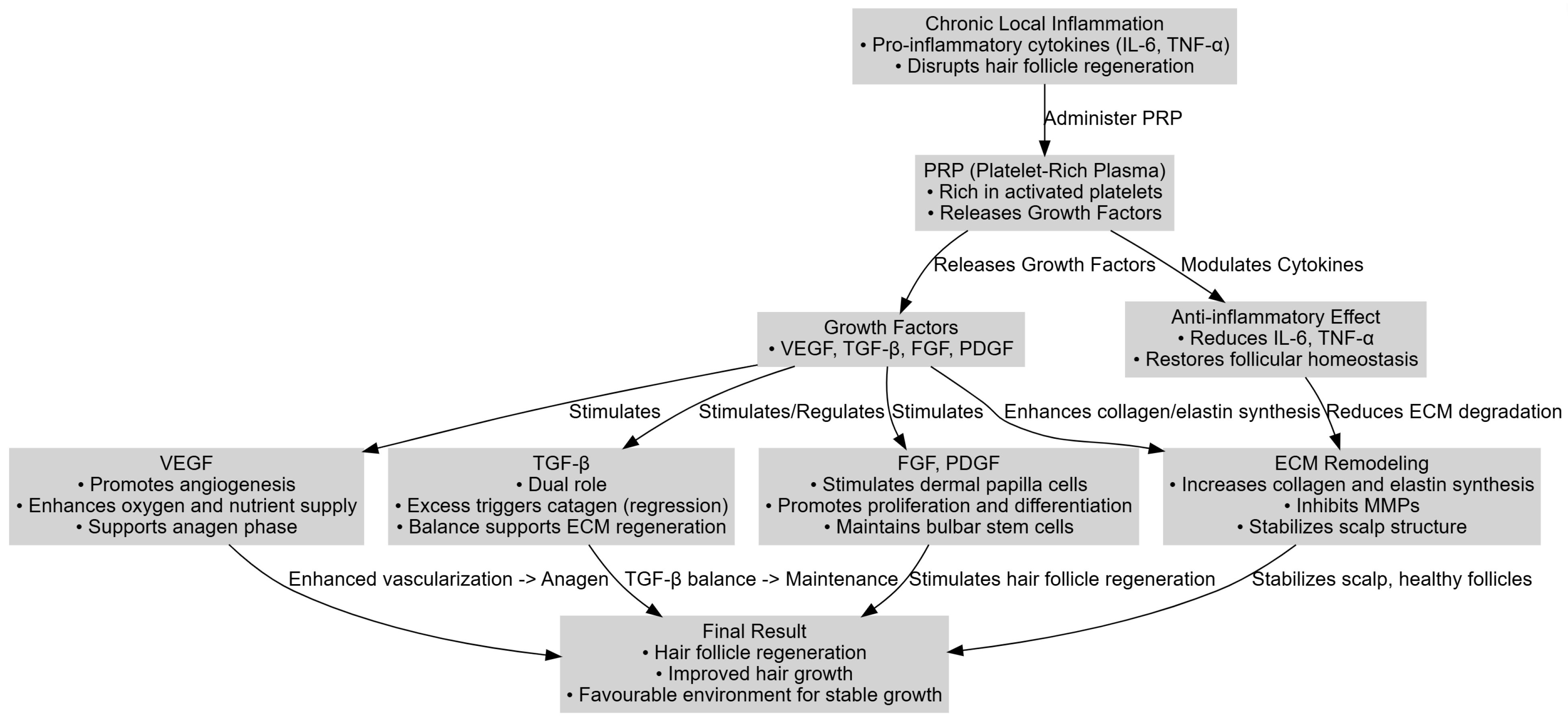
3. Relevant Physiopathological Mechanisms in PRP Therapy and Hair Loss
- Increasing local vascularization through VEGF, thereby sustaining the anagen phase;
- Reducing pro-inflammatory cytokines (IL-6, TNF-α) to restore a balanced immune environment;
- Stimulating the dermal papilla and follicular matrix cells via FGF and PDGF, which supports follicular regeneration.
4. Inflammatory and Biological Markers Associated with Response to PRP
4.1. The Indicators Proposed in the Literature
4.2. Interpretation and Clinical Relevance
4.3. Other Biological Markers
5. The Impact of PRP on Oxidative Stress and Vitamins
6. Clinical and Paraclinical Evaluations of PRP’s Efficacy
6.1. Evaluation Methods
6.1.1. Trichoscopy
6.1.2. Standardized Photography and Objective Measurements
6.1.3. The Use of FotoFinder Technology in Evaluations
6.2. Subjective Parameters
6.2.1. Patient Satisfaction
6.2.2. The Importance of Psychological Counseling and Patient Expectations
7. Perspectives from the Literature and Mechanistic Insights
7.1. A Summary of Key Studies
7.2. Possible Mechanisms of Interaction
7.3. Current Study Limitations and the Path Forward
8. Future Research Directions
8.1. Standardizing the PRP Protocols
8.2. Integrating Inflammatory Markers
8.3. Combining PRP with Other Technologies
9. Discussion
9.1. The Standard PRP Protocols for Hair Regrowth
9.2. PRP as a Promising Therapy and the Influence of Inflammatory Status
9.3. The Use of Inflammatory Markers in Patient Selection
9.4. The Integration of Biological and Growth Markers
9.5. Clarifying the Specificity of Biomarkers
9.6. Expanding on MicroRNAs in PRP: Biological Functions and Biomarker Potential
9.7. PRP-Derived Extracellular Vesicles (EVs) and Exosomes: Functional Significance and Biomarker Potential
9.8. Proteomic Insights and Biomarker Discovery in PRP Therapy for Alopecia
9.9. The Necessity of Standardizing the Protocols and Evaluation Methods
9.10. International Consensus Initiatives in PRP Use
9.11. The Pathophysiology of Alopecia Areata and Androgenetic Alopecia and the Importance of Differential Diagnosis
9.11.1. The Pathophysiology of Alopecia Areata
9.11.2. The Pathophysiology of Androgenetic Alopecia
9.11.3. Differential Diagnosis and Laboratory Screening
9.12. The Limitations and Contraindications of PRP in Alopecia
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aghaei, S.; Saki, N.; Daneshmand, E.; Kardeh, B. Prevalence of psychological disorders in patients with alopecia areata in comparison with normal subjects. ISRN Dermatol. 2014, 2014, 304370. [Google Scholar] [CrossRef]
- Cash, T.; Price, V.; Savin, R. Psychological effects of androgenetic alopecia on women: Comparisons with balding men and with female control subjects. J. Am. Acad. Dermatol. 1993, 29, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Clemmesen, M.E.R.; Gren, S.T.; Frøstrup, A.G.; Thomsen, S.F.; Egeberg, A.; Thein, D. Psychosocial and mental impact of alopecia areata: Analysis of the Danish Skin Cohort. J Eur Acad Dermatol Venereol. 2024, 39, 688–697. [Google Scholar] [CrossRef]
- Saraswat, N.; Shankar, P.; Chopra, A.; Kumar, S.; Mitra, D.; Agarwal, R. Impact of Psychosocial Profile on Alopecia Areata in Pediatric Patients: A Case Control Study from A Tertiary Care Hospital in Eastern Uttar Pradesh. Indian. J. Dermatol. 2020, 65, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Veldi, V.D.K.; Chellaboina, K.; Metta, A.K.; Goel, A.; Angara, S.S.P.; Teja, S.S.; Challa, L.R.; Chinta, V.A.; Dantuluri, R.V.; Ponnada, S.C. Impact of Androgenic Alopecia on Quality of Life Using the Dermatology Life Quality Index (DLQI): A Cross-Sectional Study in a Tertiary Care Hospital. Front. Health Inform. 2024, 13, 345–354. [Google Scholar]
- Ito, T.; Kamei, K.; Yuasa, A.; Matsumoto, F.; Hoshi, Y.; Okada, M.; Noto, S. Health-related quality of life in patients with alopecia areata: Results of a Japanese survey with norm-based comparisons. J. Dermatol. 2022, 49, 584–593. [Google Scholar] [CrossRef]
- Yuan, J.; He, Y.; Wan, H.; Gao, Y. Effectiveness of platelet-rich plasma in treating female hair loss: A systematic review and meta-analysis of randomized controlled trials. Skin. Res. Technol. 2024, 30, e70004. [Google Scholar] [CrossRef]
- Morkuzu, S.; McLennan, A.L.; Kanapathy, M.; Mosahebi, A. Use of Activated Platelet-Rich Plasma (A-PRP) on Alopecia: A Systematic Review and Meta-Analysis. Aesthet Surg J. 2023, 43, NP631–NP649. [Google Scholar] [CrossRef]
- Khatu, S.S.; Yuvraj, E.M.; Neeta, R.G.; Dipali, C.C.; Nitin, B. Platelet-Rich Plasma in Androgenic Alopecia: Myth or an Effective Tool. J. Cutan. Aesthetic Surg. 2014, 7, 107–110. [Google Scholar] [CrossRef]
- Gupta, A.K.; Cole, J.; Deutsch, D.P.; Everts, P.A.; Niedbalski, R.P.; Panchaprateep, R.; Rinaldi, F.; Rose, P.T.; Sinclair, R.; Vogel, J.E.; et al. Platelet-Rich Plasma as a Treatment for Androgenetic Alopecia. Dermatol. Surg. 2019, 45, 1262–1273. [Google Scholar] [CrossRef]
- Rodrigues, B.L.; Montalvão, S.A.L.; Cancela, R.B.B.; Silva, F.A.R.; Urban, A.; Huber, S.C.; Júnior, J.L.R.C.; Lana, J.F.S.D.; Annichinno-Bizzacchi, J.M. Treatment of male pattern alopecia with platelet-rich plasma: A double-blind controlled study with analysis of platelet number and growth factor levels. J. Am. Acad. Dermatol. 2019, 80, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Sabarish, R.; Lavu, V.; Rao, S.R. A Comparison of Platelet Count and Enrichment Percentages in the Platelet Rich Plasma (PRP) Obtained Following Preparation by Three Different Methods. J. Clin. Diagn. Res. 2015, 9, ZC10-2. [Google Scholar] [CrossRef] [PubMed]
- Saqlain, N.; Mazher, N.; Fateen, T.; Siddique, A. Comparison of single and double centrifugation methods for preparation of Platelet-Rich Plasma (PRP). Pak. J. Med. Sci. 2023, 39, 634–637. [Google Scholar] [CrossRef]
- Fitzpatrick, J.; Bulsara, M.K.; McCrory, P.R.; Richardson, M.D.; Zheng, M.H. Analysis of Platelet-Rich Plasma Extraction: Variations in Platelet and Blood Components Between 4 Common Commercial Kits. Orthop. J. Sports Med. 2017, 5, 1967116675272. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Saita, Y.; Nishio, H.; Ikeda, H.; Takazawa, Y.; Nagao, M.; Takaku, T.; Komatsu, N.; Kaneko, K. Leukocyte concentration and composition in platelet-rich plasma (PRP) influences the growth factor and protease concentrations. J. Orthop. Sci. 2016, 21, 683–689. [Google Scholar] [CrossRef]
- Dashore, S.; Chouhan, K.; Nanda, S.; Sharma, A. Preparation of Platelet-Rich Plasma: National IADVL PRP Taskforce Recommendations. Indian. Dermatol. Online J. 2021, 12 (Suppl. S1), S12–S23. [Google Scholar] [CrossRef]
- Alves, R.; Grimalt, R. Randomized Placebo-Controlled, Double-Blind, Half-Head Study to Assess the Efficacy of Platelet-Rich Plasma on the Treatment of Androgenetic Alopecia. Dermatol. Surg. 2016, 42, 491–497. [Google Scholar] [CrossRef]
- Dubin, D.P.; Lin, M.J.; Leight, H.M.; Farberg, A.S.; Torbeck, R.L.; Burton, W.B.; Khorasani, H. The effect of platelet-rich plasma on female androgenetic alopecia: A randomized controlled trial. J. Am. Acad. Dermatol. 2020, 83, 1294–1297. [Google Scholar] [CrossRef]
- Gentile, P.; Cole, J.P.; Cole, M.A.; Garcovich, S.; Bielli, A.; Scioli, M.G.; Orlandi, A. Platelet-rich plasma for androgenetic alopecia: A randomized, double-blind study. Stem Cells Cloning. 2017, 10, 1–10. [Google Scholar]
- Rossano, F.; Di Martino, S.; Iodice, L.; Di Paolo, M.; Misso, S.; Tomeo, R.; Marini, A.M.; Brugnone, R.; Marlino, S.; Santorelli, A.; et al. Correlation between individual inflammation genetic profile and platelet rich plasma efficacy in hair follicle regeneration: A pilot study reveals prognostic value of IL-1a polymorphism. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5247–5257. [Google Scholar]
- Zhang, X.; Ji, Y.; Zhou, M.; Zhou, X.; Xie, Y.; Zeng, X.; Shao, F.; Zhang, C. Platelet-Rich Plasma for Androgenetic Alopecia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Cutan. Med. Surg. 2023, 27, 504–508. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, B.; Caulloo, S.; Chen, X.; Li, Y.; Zhang, X. Diffuse alopecia areata is associated with intense inflammatory infiltration and CD8+ T cells in hair loss regions and an increase in serum IgE level. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 709–714. [Google Scholar] [PubMed]
- Harries, M.J.; Meyer, K.C.; Paus, R. Hair loss as a result of cutaneous autoimmunity: Frontiers in the immunopathogenesis of primary cicatricial alopecia. Autoimmun. Rev. 2009, 8, 478–483. [Google Scholar] [CrossRef]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. The Inflammatory Aspect of Male and Female Pattern Hair Loss. J. Inflamm. Res. 2020, 13, 879–881. [Google Scholar] [CrossRef]
- Hadshiew, I.M.; Foitzik, K.; Arck, P.C.; Paus, R. Burden of hair loss: Stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. J. Investig. Dermatol. 2004, 123, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Tejapira, K.; Yongpisarn, T.; Sakpuwadol, N.; Suchonwanit, P. Platelet-rich plasma in alopecia areata and primary cicatricial alopecias: A systematic review. Front. Med. 2022, 9, 1058431. [Google Scholar] [CrossRef]
- Weibrich, G.; Kleis, W.K.; Hafner, G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: Curasan-type PRP kit versus PCCS PRP system. Int. J. Oral. Maxillofac. Implants. 2002, 17, 184–190. [Google Scholar] [PubMed]
- Cole, J.P.; Cole, M.A.; Insalaco, C.; Cervelli, V.; Gentile, P. Alopecia and platelet-derived therapies. Stem Cell Investig. 2017, 4, 88. [Google Scholar] [CrossRef][Green Version]
- Frischbutter, S.; Durek, P.; Witkowski, M.; Angermair, S.; Treskatsch, S.; Maurer, M.; Radbruch, A.; Mashreghi, M.F. Serum TGF-β as a Predictive Biomarker for Severe Disease and Fatality of COVID-19. Eur. J. Immunol. 2023, 53, e2350433. [Google Scholar] [CrossRef]
- Atashi, F.; André-Lévigne, D.; Colin, D.J.; Germain, S.; Pittet-Cuénod, B.; Modarressi, A. Does non-activated platelet-rich plasma (PRP) enhance fat graft outcome? An assessment with 3D CT-scan in mice. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 669–675. [Google Scholar] [CrossRef]
- Foitzik, K.; Lindner, G.; Mueller-Roever, S.; Maurer, M.; Botchkareva, N.; Botchkarev, V.; Handjiski, B.; Metz, M.; Hibino, T.; Soma, T.; et al. Control of murine hair follicle regression (catagen) by TGF-beta1 in vivo. FASEB J. 2000, 14, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Soma, T.; Tsuji, Y.; Hibino, T. Involvement of transforming growth factor-beta2 in catagen induction during the human hair cycle. J. Investig. Dermatol. 2002, 118, 993–997. [Google Scholar] [CrossRef]
- Boswell, S.G.; Schnabel, L.V.; Mohammed, H.O.; Sundman, E.A.; Minas, T.; Fortier, L.A. Increasing Platelet Concentrations in Leukocyte-Reduced Platelet-Rich Plasma Decrease Collagen Gene Synthesis in Tendons. Am. J. Sports Med. 2014, 42, 42–49. [Google Scholar] [CrossRef]
- Kimura-Ueki, M.; Oda, Y.; Oki, J.; Komi-Kuramochi, A.; Honda, E.; Asada, M.; Suzuki, M.; Imamura, T. Hair cycle resting phase is regulated by cyclic epithelial FGF18 signaling. J. Investig. Dermatol. 2012, 132, 1338–1345. [Google Scholar] [CrossRef]
- Goryachkina, V.L.; Ivanova, M.Y.; Tsomartova, D.A.; Kartashkina, N.L.; Kuznetsov, S.L. Regulation of hair follicle cycle. Morfologiia 2014, 146, 83–87. [Google Scholar]
- Rahmani, W.; Sinha, S.; Biernaskie, J. Immune modulation of hair follicle regeneration. NPJ Regen. Med. 2020, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Jhang, J.F.; Jiang, Y.H.; Lin, T.Y.; Kuo, H.C. The tumor necrosis factor-α level in platelet-rich plasma might be associated with treatment outcome in patients with interstitial cystitis/bladder pain syndrome or recurrent urinary tract infection. Int. J. Mol. Sci. 2023, 25, 163. [Google Scholar] [CrossRef]
- Dejnek, M.; Moreira, H.; Płaczkowska, S.; Barg, E.; Reichert, P.; Królikowska, A. Leukocyte-Rich Platelet-Rich Plasma as an Effective Source of Molecules That Modulate Local Immune and Inflammatory Cell Responses. Oxid. Med. Cell Longev. 2022, 2022, 8059622. [Google Scholar] [CrossRef] [PubMed]
- Textor, J.A.; Tablin, F. Activation of equine platelet-rich plasma: Comparison of methods and characterization of equine autologous thrombin. Vet. Surg. 2012, 41, 784–794. [Google Scholar] [CrossRef]
- Weibrich, G.; Kleis, W.K.; Hitzler, W.E.; Hafner, G. Comparison of the platelet concentrate collection system with the plasma-rich-in-growth-factors kit to produce platelet-rich plasma: A technical report. Int. J. Oral. Maxillofac. Implants 2005, 20, 118–123. [Google Scholar]
- Bernardo, M.M.; Fridman, R. TIMP-2 (tissue inhibitor of metalloproteinase-2) regulates MMP-2 (matrix metalloproteinase-2) activity in the extracellular environment after pro-MMP-2 activation by MT1 (membrane type 1)-MMP. Biochem J. 2003, 374, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Ocklind, A. Effect of latanoprost on the extracellular matrix of the ciliary muscle. A study on cultured cells and tissue sections. Exp. Eye Res. 1998, 67, 179–191. [Google Scholar] [CrossRef]
- Yin, W.; Xu, H.; Sheng, J.; Xu, Z.; Xie, X.; Zhang, C. Comparative evaluation of the effects of platelet-rich plasma formulations on extracellular matrix formation and the NF-κB signaling pathway in human articular chondrocytes. Mol. Med. Rep. 2017, 15, 2940–2948. [Google Scholar] [CrossRef] [PubMed]
- Elias, K.A.; Weiner, R.I.; Mellon, S.H. Effect of extracellular matrix on prolactin secretion and mRNA accumulation in GH3 cells. DNA Cell Biol. 1990, 9, 369–375. [Google Scholar] [CrossRef]
- Kaiser, M.; Abdin, R.; Gaumond, S.I.; Issa, N.; Jimenez, J.J. Treatment of Androgenetic Alopecia: Current Guidance and Unmet Needs. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1387–1406. [Google Scholar] [CrossRef]
- Jin, X.; Wang, K.; Shao, X.; Huang, J. Prognostic implications of the peripheral platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in predicting pathologic complete response after neoadjuvant chemotherapy in breast cancer patients. Gland. Surg. 2022, 11, 1057–1066. [Google Scholar] [CrossRef]
- Graziano, V.; Grassadonia, A.; Iezzi, L.; Vici, P.; Pizzuti, L.; Barba, M.; Quinzii, A.; Camplese, A.; Di Marino, P.; Peri, M.; et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast 2019, 44, 33–38. [Google Scholar] [CrossRef]
- Gentile, P.; Dionisi, L.; Pizzicannella, J.; de Angelis, B.; de Fazio, D.; Garcovich, S. A Randomized Blinded Retrospective Study: The Combined Use of Micro-Needling Technique, Low-Level Laser Therapy and Autologous Non-Activated Platelet-Rich Plasma Improves Hair Re-Growth in Patients with Androgenic Alopecia. Expert Opin. Biol. Ther. 2020, 20, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Zhang, Y.; Yan, H.; Si, H.; Wang, Z.; Dai, G. Neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) and hemoglobin (Hb) predict immunotherapy outcomes in patients with advanced or metastatic gastric cancer. J. Clin. Oncol. 2021, 39, e16075. [Google Scholar] [CrossRef]
- Mousavi Darzikolaee, N.; Rajaeinejad, M.; Farazmand, B.; Ghalehtaki, R.; Jalaeikhoo, H. Role of Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio in Predicting the Response to First Line Chemotherapy in Colorectal Cancer Patients with Synchronous Liver Metastases: A Retrospective Study. Int. J. Cancer Manag. 2021, 14, e113923. [Google Scholar] [CrossRef]
- Ergen, A.S.; Barlas, C.; Dagdelen, M.; Can, G.; Sahinler, I. The prognostic role of the pretreatment peripheral neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio. Ann. Med. Res. 2021, 28, 778–785. [Google Scholar] [CrossRef]
- El-Sharkawy, H.; Kantarci, A.; Deady, J.; Hasturk, H.; Liu, H.; Alshahat, M.; Van Dyke, T.E. Platelet-rich plasma: Growth factors and pro- and anti-inflammatory properties. J. Periodontol. 2007, 78, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.J.; Kim, S.H.; Kim, Y.J.; Kim, M.; Keam, B.; Kim, T.M.; Kim, D.W.; Heo, D.S. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol. Immunother. 2018, 67, 459–470. [Google Scholar] [CrossRef]
- Basher, F.; Saravia, D.; Lopes, G. Prognostic value of systemic inflammatory markers in first- and subsequent-line immunotherapy and durability of response in NSCLC. J. Clin. Oncol. 2021, 39, e21210. [Google Scholar] [CrossRef]
- Ohlendorf, F.; Werner, R.A.; Henkenberens, C.; Ross, T.L.; Christiansen, H.; Bengel, F.M.; Derlin, T. Predictive and Prognostic Impact of Blood-Based Inflammatory Biomarkers in Patients with Gastroenteropancreatic Neuroendocrine Tumors Commencing Peptide Receptor Radionuclide Therapy. Diagnostics 2021, 11, 504. [Google Scholar] [CrossRef] [PubMed]
- Tabara, K.; Kozłowska, M.; Jędrowiak, A.; Bienias, W.; Kaszuba, A. Serum concentrations of selected proinflammatory cytokines in children with alopecia areata. Postep. Dermatol. Alergol. 2019, 36, 63–69. [Google Scholar] [CrossRef]
- Mouawad, R.; Rixe, O.; Meric, J.B.; Khayat, D.; Soubrane, C. Serum interleukin-6 concentrations as predictive factor of time to progression in metastatic malignant melanoma patients treated by biochemotherapy: A retrospective study. Cytokines Cell Mol. Ther. 2002, 7, 151–156. [Google Scholar] [CrossRef]
- Christensen, R.E.; Anvery, N.; Dirr, M.A. Regenerative therapies in alopecia areata. J. Cosmet. Dermatol. 2022, 21, 5250–5251. [Google Scholar] [CrossRef]
- Irmak, G.; Demirtaş, T.T.; Gümüşderelioğlu, M. Sustained release of growth factors from photoactivated platelet rich plasma (PRP). Eur. J. Pharm. Biopharm. 2020, 148, 67–76. [Google Scholar] [CrossRef]
- Pakfar, A.; Irani, S.; Hanaee-Ahvaz, H. Expressions of pathologic markers in PRP based chondrogenic differentiation of human adipose derived stem cells. Tissue Cell. 2017, 49, 122–130. [Google Scholar] [CrossRef]
- Du, F.; Li, J.; Zhang, S.; Zeng, X.; Nie, J.; Li, Z. Oxidative stress in hair follicle development and hair growth: Signalling pathways, intervening mechanisms and potential of natural antioxidants. J. Cell Mol. Med. 2024, 28, e18486. [Google Scholar] [CrossRef] [PubMed]
- Behling, C.S.; Andrade, A.S.; Putti, J.S.; Mahl, C.D.; Hackenhaar, F.S.; da Silva, A.C.; e Silva, M.N.; Salomon, T.B.; Dos Santos, C.E.; Dias, J.F.; et al. Treatment of oxidative stress in brain of ovariectomized rats with omega-3 and lipoic acid. Mol. Nutr. Food Res. 2015, 59, 2547–2555. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Q. Effect of nutrition intervention on antioxidant capacity and lipid peroxide in patients with bone marrow transplantation. Di Yi Jun Yi Da Xue Xue Bao 2002, 22, 530–532. [Google Scholar]
- Dandapat, J.; Chainy, G.B.; Rao, K.J. Dietary vitamin-E modulates antioxidant defence system in giant freshwater prawn, Macrobrachium rosenbergii. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2000, 127, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, K.N.; Caliskan, E.; Caglar, B. Platelet rich plasma application by dermapen microneedling and intradermal point-by-point injection methods, and their comparison with clinical findings and trichoscan in patients with androgenetic alopecia. Dermatol. Ther. 2022, 35, e15182. [Google Scholar] [CrossRef]
- Gentile, P.; Cole, J.P.; Cole, M.A.; Garcovich, S.; Bielli, A.; Scioli, M.G.; Orlandi, A.; Insalaco, C.; Cervelli, V. Evaluation of Not-Activated and Activated PRP in Hair Loss Treatment: Role of Growth Factor and Cytokine Concentrations Obtained by Different Collection Systems. Int. J. Mol. Sci. 2017, 18, 408. [Google Scholar] [CrossRef]
- Dhurat, R.; Saraogi, P. Hair evaluation methods: Merits and demerits. Int. J. Trichol. 2009, 1, 108–119. [Google Scholar] [CrossRef]
- Arthur, E.; Takwale, A.; Farrant, P.; Holmes, S.; Harries, M.; Rowe, S.; Benton, N.; Wells, S. Hair Loss Photography; Institute of Medical Illustrators: London, UK, 2024; Available online: https://www.imi.org.uk/wp-content/uploads/2024/06/NG_HairLoss_1_0_S.pdf (accessed on 15 March 2025).
- Verma, K.; Tegta, G.R.; Verma, G.; Gupta, M.; Negi, A.; Sharma, R. A Study to Compare the Efficacy of Platelet-rich Plasma and Minoxidil Therapy for the Treatment of Androgenetic Alopecia. Int. J. Trichol. 2019, 11, 68–79. [Google Scholar] [CrossRef]
- Kim, M.; Kang, S.; Lee, B.D. Evaluation of Automated Measurement of Hair Density Using Deep Neural Networks. Sensors 2022, 22, 650. [Google Scholar] [CrossRef]
- Gassmueller, J.; Rowold, E.; Frase, T.; Hughes-Formella, B. Validation of TrichoScan technology as a fully-automated tool for evaluation of hair growth parameters. Eur. J. Dermatol. 2009, 19, 224–231. [Google Scholar] [CrossRef]
- Lee, M.S.; Kossard, S.; Wilkinson, B.; Doyle, J.A. Quantification of hair follicle parameters using computer image analysis: A comparison of androgenetic alopecia with normal scalp biopsies. Australas. J. Dermatol. 1995, 36, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Gudobba, C.; Mane, T.; Bayramova, A.; Rodriguez, N.; Castelo-Soccio, L.; Ogunleye, T.A.; Taylor, S.C.; Cotsarelis, G.; Bernardis, E. Automating Hair Loss Labels for Universally Scoring Alopecia From Images: Rethinking Alopecia Scores. JAMA Dermatol. 2023, 159, 143–150. [Google Scholar] [CrossRef]
- Chernyshov, P.V.; Tomas-Aragones, L.; Finlay, A.Y.; Manolache, L.; Marron, S.E.; Sampogna, F.; Spillekom-van Koulil, S.; Pustisek, N.; Suru, A.; Evers, A.W.M.; et al. Quality of life measurement in alopecia areata. Position statement of the European Academy of Dermatology and Venereology Task Force on Quality of Life and Patient Oriented Outcomes. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1614–1621. [Google Scholar] [CrossRef]
- Nasimi, M.; Ghandi, N.; Torabzade, L.; Shakoei, S. Alopecia Areata-Quality of Life Index Questionnaire (Reliability and Validity of the Persian Version) in Comparison to Dermatology Life Quality Index. Int. J. Trichol. 2020, 12, 227–233. [Google Scholar]
- Yücesoy, S.N.; Uzunçakmak, T.K.; Selçukoğlu, Ö.; Aşkın, Ö.; Ak, T.; Özdil Eser, A.; Turan, Ş.; Serdaroğlu, S. Evaluation of quality of life scores and family impact scales in pediatric patients with alopecia areata: A cross-sectional cohort study. Int. J. Dermatol. 2024, 63, 1414–1420. [Google Scholar] [CrossRef]
- Dolte, K.S.; Girman, C.J.; Hartmaier, S.; Roberts, J.; Bergfeld, W.; Waldstreicher, J. Development of a health-related quality of life questionnaire for women with androgenetic alopecia. Clin. Exp. Dermatol. 2000, 25, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Meyers, A.; Jin, A.; Kwiecien, G.J.; Gatherwright, J.; Khetarpal, S.; Zins, J.E. Platelet-Rich Plasma for Treatment of Hair Loss Improves Patient-Reported Quality of Life. Aesthet. Surg. J. 2023, 47, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, M.; Siotos, C.; Gasteratos, K.C.; Spyropoulou, G.A.; Gentile, P. Autologous Platelet-Rich Plasma Treatment for Androgenic Alopecia: A Systematic Review and Meta-Analysis of Clinical Trials. Plast. Reconstr. Surg. 2023, 151, 739e–747e. [Google Scholar]
- Gentile, P.; Garcovich, S. Autologous activated platelet-rich plasma (AA-PRP) and non-activated (A-PRP) in hair growth: A retrospective, blinded, randomized evaluation in androgenetic alopecia. Expert. Opin. Biol. Ther. 2020, 20, 327–337. [Google Scholar] [CrossRef]
- Sasaki, G.H. The Effects of Lower vs. Higher Cell Number of Platelet-Rich Plasma (PRP) on Hair Density and Diameter in Androgenetic Alopecia (AGA): A Randomized, Double-Blinded, Placebo, Parallel-Group Half-Scalp IRB-Approved Study. Aesthet. Surg. J. 2021, 41, NP1659–NP1672. [Google Scholar] [CrossRef]
- Shah, K.B.; Shah, A.N.; Solanki, R.B.; Raval, R.C. A Comparative Study of Microneedling with Platelet-rich Plasma Plus Topical Minoxidil (5%) and Topical Minoxidil (5%) Alone in Androgenetic Alopecia. Int. J. Trichol. 2017, 9, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Huang, J.; Li, M.; Jian, J.; Shi, W. Complete Blood Collection-based Systemic Inflammation Biomarkers as a Severity Biomarker in Alopecia Areata: A Cross-sectional Study. Acta Derm. Venereol. 2024, 104, adv40971. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, C.; He, W.; Wang, D. Study on the Value of Blood Biomarkers NLR and PLR in the Clinical Diagnosis of Influenza A Virus Infection in Children. Clin. Lab. 2021, 67, 2540–2547. [Google Scholar] [CrossRef]
- Wu, L.; Zou, S.; Wang, C.; Hu, Y.; Wang, G.; Zhang, Z. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in Chinese Han population from Chaoshan region in South China. BMC Cardiovasc. Disord. 2019, 19, 125. [Google Scholar] [CrossRef] [PubMed]
- Rybalka, Y.; Malyk, S. Dynamics of the cytological picture of the ras process in patients with chronic ranes in application of prp-therapy. Clin. Anat. Oper. Surg. 2018, 17, 2. [Google Scholar]
- Bao, L.; Zong, H.; Fang, S.; Zheng, L.; Li, Y. Randomized trial of electrodynamic microneedling combined with 5% minoxidil topical solution for treating androgenetic alopecia in Chinese males and molecular mechanistic study of the involvement of the Wnt/β-catenin signaling pathway. J. Dermatol. Treat. 2022, 33, 483–493. [Google Scholar] [CrossRef]
- Pakhomova, E.E.; Smirnova, I.O. Comparative Evaluation of the Clinical Efficacy of PRP-Therapy, Minoxidil, and Their Combination with Immunohistochemical Study of the Dynamics of Cell Proliferation in the Treatment of Men with Androgenetic Alopecia. Int. J. Mol. Sci. 2020, 21, 6516. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, C.; Qu, Q.; Zhang, C.; Wang, J.; Fan, Z.; Miao, Y.; Hu, Z. The effectiveness of combination therapies for androgenetic alopecia: A systematic review and meta-analysis. Dermatol. Ther. 2020, 33, e13741. [Google Scholar] [CrossRef]
- Kushida, S.; Kakudo, N.; Morimoto, N.; Hara, T.; Ogawa, T.; Mitsui, T.; Kusumoto, K. Platelet and growth factor concentrations in activated platelet-rich plasma: A comparison of seven commercial separation systems. J. Artif. Organs 2014, 17, 186–192. [Google Scholar] [CrossRef]
- Castillo, T.N.; Pouliot, M.A.; Kim, H.J.; Dragoo, J.L. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am. J. Sports Med. 2011, 39, 266–271. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, W.; Park, K.U.; Roh, Y.H. Comparison of the Cellular Composition and Cytokine-Release Kinetics of Various Platelet-Rich Plasma Preparations. Am. J. Sports Med. 2015, 43, 3062–3070. [Google Scholar] [CrossRef] [PubMed]
- Karakaş, D.Ö.; Dandin, Ö.; Müftüoğlu, T.; Tihan, D.; Bal, A.S.; Yıldırım, Ş. Effect of platelet-rich plasma on postoperative peritoneal inflammation and adhesions. Arch. Med. Sci. 2020, 17, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Llovera, G.; Liesz, A. The next step in translational research: Lessons learned from the first preclinical randomized controlled trial. J. Neurochem. 2016, 139 (Suppl. S2), 271–279. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Kranenbarg, E.K.; Hermans, J.; van de Velde, C.J.; van Krieken, J.H. Pathology data in the central databases of multicenter randomized trials need to be based on pathology reports and controlled by trained quality managers. J. Clin. Oncol. 2000, 18, 1771–1779. [Google Scholar] [CrossRef]
- Landoni, G.; Pieri, M.; Young, P.J.; Bellomo, R. Why do multicenter randomized controlled trials not confirm the positive findings of single center randomized controlled trials in acute care? Minerva Anestesiol. 2019, 85, 194–200. [Google Scholar] [CrossRef]
- Rudez, G.; Janssen, N.A.; Kilinc, E.; Leebeek, F.W.; Gerlofs-Nijland, M.E.; Spronk, H.M.; ten Cate, H.; Cassee, F.R.; de Maat, M.P. Effects of ambient air pollution on hemostasis and inflammation. Environ. Health Perspect. 2009, 117, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Meir, A.Y.; Yun, H.; Hu, J.; Li, J.; Liu, J.; Bever, A.; Ratanatharathorn, A.; Song, M.; Heather Eliassen, A.; Chibnik, L.; et al. Cross omics risk scores of inflammation markers are associated with all-cause mortality: The Canadian Longitudinal Study on Aging. medRxiv 2024. preprint. [Google Scholar]
- Bondarenko, V.V. PRP application in dermatology: Review of current approaches. Med. Alphabet 2021, 9, 55–58. (In Russian) [Google Scholar] [CrossRef]
- Hausauer, A.K.; Humphrey, S. The Physician’s Guide to Platelet-Rich Plasma in Dermatologic Surgery Part I: Definitions, Mechanisms of Action, and Technical Specifications. Dermatol. Surg. 2020, 46, 348–357. [Google Scholar] [CrossRef]
- Xiong, Y.; Gong, C.; Peng, X.; Liu, X.; Su, X.; Tao, X.; Li, Y.; Wen, Y.; Li, W. Efficacy and safety of platelet-rich plasma injections for the treatment of osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Front. Med. (Lausanne) 2023, 10, 1204144. [Google Scholar] [CrossRef]
- Dong, Q.; Zhao, F.; Li, Y.; Song, F.; Li, E.; Gao, L.; Xin, Y.; Shen, G.; Ren, D.; Wang, M.; et al. The correlation between systemic inflammatory markers and efficiency for advanced gastric cancer patients treated with ICIs combined with chemotherapy. Immunology 2024, 172, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Rebuzzi, S.E.; Signori, A.; Banna, G.L.; Maruzzo, M.; De Giorgi, U.; Pedrazzoli, P.; Sbrana, A.; Zucali, P.A.; Masini, C.; Naglieri, E.; et al. Inflammatory indices and clinical factors in metastatic renal cell carcinoma patients treated with nivolumab: The development of a novel prognostic score (Meet-URO 15 study). Ther. Adv. Med. Oncol. 2021, 13, 17588359211019642. [Google Scholar] [CrossRef]
- Kedia, S.; Virmani, S.; KVuyyuru, S.; Kumar, P.; Kante, B.; Sahu, P.; Kaushal, K.; Farooqui, M.; Singh, M.; Verma, M.; et al. Faecal microbiota transplantation with anti-inflammatory diet (FMT-AID) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: A randomised controlled trial. Gut 2022, 71, 2401–2413. [Google Scholar] [CrossRef] [PubMed]
- Sköldstam, L.; Hagfors, L.; Johansson, G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 208–214. [Google Scholar] [CrossRef]
- Budania, A.; Mandal, S.; Agrawal, A.; Lahoria, U.; Pathania, Y.S. A split scalp study to evaluate the effects of platelet rich plasma prepared by two different methods in androgenetic alopecia. Australas. J. Dermatol. 2023, 64, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Sarsik, S.M.; Heba, S.E.-A. Reversal of steroid-induced atrophy and leucoderma in a teenage girl by serial alternative saline and platelet-rich plasma injections. Dermatol. Ther. 2021, 34, e14869. [Google Scholar] [CrossRef]
- Legiawati, L.; Yusharyahya, S.N.; Bernadette, I.; Novianto, E.; Priyanto, M.H.; Gliselda, K.C.; Iriyanty, S.; Mutiara, R. Comparing Single-spin Versus Double-spin Platelet-rich Plasma (PRP) Centrifugation Methods on Thrombocyte Count and Clinical Improvement of Androgenetic Alopecia: A Preliminary, Randomized, Double-blind Clinical Trial. J. Clin. Aesthet. Dermatol. 2023, 16, 39–44. [Google Scholar]
- Bhatia, A.; Ramya, B.S.; Biligi, D.S.; Panchakshari Prasanna, B.K. Comparison of different methods of centrifugation for preparation of platelet-rich plasma (PRP). Indian J. Pathol. Oncol. 2016, 3, 535. [Google Scholar] [CrossRef]
- Ragab, S.E.M.; Nassar, S.O.; Morad, H.A.; Hegab, D.S. Platelet-rich plasma in alopecia areata: Intradermal injection versus topical application with transepidermal delivery via either fractional carbon dioxide laser or microneedling. Acta Dermatovenerol. Alp. Pannonica Adriat. 2020, 29, 169–173. [Google Scholar] [CrossRef]
- Yepuri, V.; Mysore, V. Platelet-Rich Plasma with Microneedling in Androgenetic Alopecia: Study of Efficacy of the Treatment and the Number of Sessions Required. J. Cutan. Aesthetic Surg. 2021, 14, 184–190. [Google Scholar] [CrossRef]
- Muhammad, S.A.; Fatima, N.; Paracha, R.Z.; Ali, A.; Chen, J.Y. A systematic simulation-based meta-analytical framework for prediction of physiological biomarkers in alopecia. J. Biol. Res. 2019, 26, 2. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C., Jr.; Anderson, J.L.; Cannon, R.O., 3rd; Fadl, Y.Y.; Koenig, W.; Libby, P.; Lipshultz, S.E.; Mensah, G.A.; Ridker, P.M.; Rosenson, R. AHA CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: Report from the clinical practice discussion group. Circulation 2004, 110, e550–e553. [Google Scholar]
- Lyle, S.; Christofidou-Solomidou, M.; Liu, Y.; Elder, D.E.; Albelda, S.; Cotsarelis, G. Human hair follicle bulge cells are biochemically distinct and possess an epithelial stem cell phenotype. J. Investig. Dermatol. Symp. Proc. 1999, 4, 296–301. [Google Scholar] [CrossRef]
- Rittié, L.; Stoll, S.W.; Kang, S.; Voorhees, J.J.; Fisher, G.J. Hedgehog signaling maintains hair follicle stem cell phenotype in young and aged human skin. Aging Cell 2009, 8, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Trempus, C.S.; Morris, R.J.; Bortner, C.D.; Cotsarelis, G.; Faircloth, R.S.; Reece, J.M.; Tennant, R.W. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Investig. Dermatol. 2003, 120, 501–511. [Google Scholar]
- Harries, M.J.; Meyer, K.; Chaudhry, I.; EKloepper, J.; Poblet, E.; Griffiths, C.E.; Paus, R. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle’s epithelial stem cell niche. J. Pathol. 2013, 231, 236–247. [Google Scholar] [CrossRef]
- Whiting, D.A. Histopathologic features of alopecia areata: A new look. Arch. Dermatol. 2003, 139, 1555–1559. [Google Scholar] [CrossRef]
- Gupta, A.K.; Bamimore, M. Platelet-Rich Plasma Monotherapies for Androgenetic Alopecia: A Network Meta-Analysis and Meta-Regression Study. J. Drugs Dermatol. 2022, 21, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Shumez, H.; Prasad, P.V.S.; Kavirarsan, P.K.; Deepika, R. Intralesional Platelet Rich Plasma vs. Intralesional Triamcinolone in the Treatment of Alopecia Areata: A Comparative Study. Int. J. Med. Res. Health Sci. 2014, 4, 3–17. [Google Scholar] [CrossRef]
- Ametati, H.; Malik, D.A.; Gunawan, I.; Rahayu, M.; Kadarhadi, E. Platelet-Rich Plasma (PRP) As a new approach and promising therapy in patients with Alopecia areata. Medica Hosp. J. Clin. Med. 2023, 10, 118–123. [Google Scholar] [CrossRef]
- Suh, S.; Park, M.; Babadjouni, A.; Atanaskova Mesinkovska, N. Evaluating Anti-Inflammatory Potential of Platelet-Rich Plasma in Scarring Alopecia: A Systematic Review. J. Drugs Dermatol. 2024, 23, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. The role of miR-126 in embryonic angiogenesis, adult vascular homeostasis, and vascular repair and its alterations in atherosclerotic disease. J. Mol. Cell Cardiol. 2016, 97, 47–55. [Google Scholar] [CrossRef]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell. 2008, 15, 261–271. [Google Scholar] [CrossRef]
- Babian, S.; Salehpour, S.; Nazari, L.; Ghorbanmehr, N. The expression level of mir-21-3p in platelet-rich plasma: A potential effective factor and predictive biomarker in recurrent implantation failure. Mol. Reprod. Dev. 2022, 89, 498–505. [Google Scholar] [CrossRef] [PubMed]
- de Boer, H.C.; van Solingen, C.; Prins, J.; Duijs, J.M.; Huisman, M.V.; Rabelink, T.J.; van Zonneveld, A.J. Abstract 487: Aspirin Treatment Hampers the Use of Plasma MicroRNA-126 as Biomarker for the Progression of Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 3451–3457. [Google Scholar] [CrossRef]
- Moon, P.G.; Lee, J.E.; You, S.; Kim, T.K.; Cho, J.H.; Kim, I.S.; Kwon, T.H.; Kim, Y.B.; Park, S.H.; Hwang, D.; et al. Proteomic analysis of urinary exosomes from patients of early IgA nephropathy and thin basement membrane nephropathy. Proteomics 2019, 19, e1900168. [Google Scholar]
- Bellingham, S.A.; Guo, B.B.; Coleman, B.M.; Hill, A.F. Exosomes: Vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front. Physiol. 2017, 8, 63. [Google Scholar] [CrossRef]
- Zhang, L.J.; Hu, Y.X.; Huang, R.Z.; Xu, Y.Y.; Dong, S.H.; Guo, F.H.; Guo, J.J.; Qiu, J.J.; Cao, Z.Y.; Wei, L.J.; et al. Intraplatelet miRNA-126 regulates thrombosis and its reduction contributes to platelet inhibition. Cardiovasc. Res. 2024, 120, 1622–1635. [Google Scholar] [CrossRef]
- Anitua, E.; Pino, A.; Azkargorta, M.; Elortza, F.; Prado, R. High-Throughput ProteomicAnalysis of Human DermalFibroblast Response to DifferentBlood Derivatives: AutologousTopical Serum Derived from PlasmaRich in Growth Factors (PRGF)Versus Leukocyte- and Platelet-RichPlasma (L-PRP). Biomolecules 2022, 12, 1002. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; McKenzie, M.B.; Rahman, M.M.; Cleland, H. Allogeneic Platelet-Rich. Plasma: Is. It Safe and Effective for Wound Repair? Eur. Surg. Res. 2021, 62, 1–9. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Huang, A.W.; Fan, J.J.; Wei, K.; Jin, D.; Chen, B.; Li, D.; Bi, L.; Wang, J.; Pei, G. The Potential Use of Allogeneic Platelet-Rich Plasma for Large Bone Defect Treatment: Immunogenicity and Defect Healing Efficacy. Cell Transplant. 2013, 22, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.; Alsousou, J. The state of the art and future of PRP therapy. Platelets 2021, 32, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Lü, M.; Pei, G.; Bi, L. A comparative study of effects of autologous versus allogeneic platelet-rich plasma on the proliferation and differentiation of bone marrow stromal stem cells in rabbits. Chin. J. Orthop. Trauma 2013, 15, 698–703. [Google Scholar]
- Ciccarese, G.; Facciorusso, A.; Mastrolonardo, M.; Herzum, A.; Parodi, A.; Drago, F. Atypical Manifestations of Syphilis: A 10-Year Retrospective Study. J. Clin. Med. 2024, 13, 1603. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrapcea, A.; Pisoschi, C.G.; Ciupeanu-Calugaru, E.D.; Traşcă, E.-T.; Tutunaru, C.V.; Rădulescu, P.-M.; Rădulescu, D. Inflammatory Signatures and Biological Markers in Platelet-Rich Plasma Therapy for Hair Regrowth: A Comprehensive Narrative Analysis. Diagnostics 2025, 15, 1123. https://doi.org/10.3390/diagnostics15091123
Vrapcea A, Pisoschi CG, Ciupeanu-Calugaru ED, Traşcă E-T, Tutunaru CV, Rădulescu P-M, Rădulescu D. Inflammatory Signatures and Biological Markers in Platelet-Rich Plasma Therapy for Hair Regrowth: A Comprehensive Narrative Analysis. Diagnostics. 2025; 15(9):1123. https://doi.org/10.3390/diagnostics15091123
Chicago/Turabian StyleVrapcea, Adelina, Cătălina Gabriela Pisoschi, Eleonora Daniela Ciupeanu-Calugaru, Emil-Tiberius Traşcă, Cristina Violeta Tutunaru, Patricia-Mihaela Rădulescu, and Dumitru Rădulescu. 2025. "Inflammatory Signatures and Biological Markers in Platelet-Rich Plasma Therapy for Hair Regrowth: A Comprehensive Narrative Analysis" Diagnostics 15, no. 9: 1123. https://doi.org/10.3390/diagnostics15091123
APA StyleVrapcea, A., Pisoschi, C. G., Ciupeanu-Calugaru, E. D., Traşcă, E.-T., Tutunaru, C. V., Rădulescu, P.-M., & Rădulescu, D. (2025). Inflammatory Signatures and Biological Markers in Platelet-Rich Plasma Therapy for Hair Regrowth: A Comprehensive Narrative Analysis. Diagnostics, 15(9), 1123. https://doi.org/10.3390/diagnostics15091123






