A Comparative Study of Radiofrequency Ablation, Microwave Ablation, and Percutaneous Ethanol Injection in Treatment of Hepatocellular Carcinoma—A Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Clinical and Investigational Workup
2.2.1. Treatment Allocation
2.2.2. Procedures
2.2.3. Patient Preparation
2.2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunese, L.; Mercaldo, F.; Santone, A.; Vanoli, G.P. Thermal Ablation Treatment Detection by means of Machine Learning. In Proceedings of the 2021 International Joint Conference on Neural Networks (IJCNN), Shenzhen, China, 18–22 July 2021; IEEE: New York City, NY, USA, 2021; pp. 1–6. [Google Scholar]
- Couri, T.; Pillai, A. Goals and targets for personalized therapy for HCC. Hepatol. Int. 2019, 13, 125–137. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, T.; Wang, Z.G.; Liu, H. Clinical outcome of small hepatocellular carcinoma after different treatments: A meta-analysis. World J. Gastroenterol. 2014, 20, 10174. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, X.; Wang, Z.; Zhong, X.; Tai, S.; Cui, Y. Small nucleolar RNA host gene 1: A new biomarker and therapeutic target for cancers. Pathol.-Res. Pract. 2018, 214, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Kalra, N.; Kang, M.; Duseja, A.K.; Bhatia, A.; Singh, V.; Dhiman, R.K.; Khandelwal, N. Comparison of radiofrequency ablation alone & in combination with percutaneous ethanol injection for management of hepatocellular carcinoma. Indian J. Med. Res. 2017, 146 (Suppl. S2), S30. [Google Scholar]
- Kim, Y.T.; Serrano, B.; Lee, J.K.; Lee, H.; Lee, S.W.; Freeman, C.; Oh, J.-K.; Alemany, L.; Bosch, F.-X.; Bruni, L. Burden of Human papillomavirus (HPV)-related disease and potential impact of HPV vaccines in the Republic of Korea. Papillomavirus Res. 2019, 7, 26–42. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, Y.; He, G.; Yu, M.; Zheng, M.; Liu, L.; Zhou, X. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: A systematic review and meta-analysis. World J. Surg. Oncol. 2017, 15, 126. [Google Scholar] [CrossRef]
- Makarova-Rusher, O.V.; Altekruse, S.F.; McNeel, T.S.; Ulahannan, S.; Duffy, A.G.; Graubard, B.I.; McGlynn, K.A. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 2016, 122, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Nief, C.; Morhard, R.; Chelales, E.; Adrianzen Alvarez, D.; Bourla BS, I.; Lam, C.T.; Sag, A.A.; Crouch, B.T.; Mueller, J.L.; Ramanujam, N. Polymer-assisted intratumoral delivery of ethanol: Preclinical investigation of safety and efficacy in a murine breast cancer model. PLoS ONE 2021, 16, e0234535. [Google Scholar] [CrossRef] [PubMed]
- N’Kontchou, G.; Mahamoudi, A.; Aout, M.; Ganne-Carrié, N.; Grando, V.; Coderc, E.; Vicaut, E.; Trinchet, J.C.; Sellier, N.; Seror, O. Radiofrequency ablation of hepatocellular carcinoma: Long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 2009, 50, 1475–1483. [Google Scholar] [CrossRef]
- O’Leary, C.; Mahler, M.; Soulen, M.C. Curative-intent therapies in localized hepatocellular carcinoma. Curr. Treat. Options Oncol. 2020, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Shiina, S.; Sato, K.; Tateishi, R.; Shimizu, M.; Ohama, H.; Hatanaka, T.; Takawa, M.; Nagamatsu, H.; Imai, Y. Percutaneous ablation for hepatocellular carcinoma: Comparison of various ablation techniques and surgery. Can. J. Gastroenterol. Hepatol. 2018, 2018, 4756147. [Google Scholar] [CrossRef]
- Song, P.; Cai, Y.; Tang, H.; Li, C.; Huang, J. The clinical management of hepatocellular carcinoma worldwide: A concise review and comparison of current guidelines from 2001 to 2017. Biosci. Trends 2017, 11, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Farshid, P.; Naguib, N.N.; Zangos, S.; Bodelle, B.; Paul, J.; Mbalisike, E.C.; Beeres, M.; Nour-Eldin, N.E.A. Ablation therapy of hepatocellular carcinoma: A comparative study between radiofrequency and microwave ablation. Abdom. Imaging 2015, 40, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Nour-Eldin, N.E.A.; Hammerstingl, R.M.; Panahi, B.; Naguib, N.N. Microwave ablation (MWA): Basics, technique and results in primary and metastatic liver neoplasms–review article. RöFo-Fortschritte Auf Demgebiet Der Röntgenstrahlen Und Der Bildgeb. 2017, 189, 1055–1066. [Google Scholar] [CrossRef]
- Xue, J.; Ni, H.; Wang, F.; Xu, K.; Niu, M. Advances in locoregional therapy for hepatocellular carcinoma combined with immunotherapy and targeted therapy. J. Interv. Med. 2021, 4, 105–113. [Google Scholar] [CrossRef]
- Orci, L.A.; Sanduzzi-Zamparelli, M.; Caballol, B.; Sapena, V.; Colucci, N.; Torres, F.; Bruix, J.; Reig, M.; Toso, C. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: A systematic review, meta-analysis, and meta-regression. Clin. Gastroenterol. Hepatol. 2022, 20, 283–292. e10. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Van Natta, M.L.; Clark, J.; Neuschwander-Tetri, B.A.; Diehl, A.; Dasarathy, S.; Loomba, R.; Chalasani, N.; Kowdley, K.; Hameed, B.; et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N. Engl. J. Med. 2021, 385, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL). EASL clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Lockart, I.; Yeo, M.G.H.; Hajarizadeh, B.; Dore, G.J.; Danta, M. HCC incidence after hepatitis C cure among patients with advanced fibrosisor cirrhosis: Ameta-analysis. Hepatology 2022, 76, 139–154. [Google Scholar] [CrossRef]
- Alonso Lopez, S.; Manzano, M.L.; Gea, F.; Gutiérrez, M.L.; Ahumada, A.M.; Devesa, M.J.; Olveira, A.; Polo, B.A.; Márquez, L.; Fernández, I.; et al. A model Based on noninvasive markers predicts very low hepatocellular carcinoma risk after viral Response in hepatitis C virus advanced fibrosis. Hepatology 2020, 72, 1924–1934. [Google Scholar] [CrossRef]
- Semmler, G.; Meyer, E.L.; Kozbial, K.; Schwabl, P.; Hametner-Schreil, S.; Zanetto, A.; Bauer, D.; Chromy, D.; Simbrunner, B.; Scheiner, B.; et al. HCC risk stratification after cure of hepatitis C in patients with compensated advanced chronic liver disease. J. Hepatol. 2022, 76, 812–821. [Google Scholar] [CrossRef]
- Pons, M.; Rodríguez-Tajes, S.; Esteban, J.I.; Mariño, Z.; Vargas, V.; Lens, S.; Buti, M.; Augustin, S.; Forns, X.; Mínguez, B.; et al. Non-invasive prediction of liver-related events in patients with HCV-associated compensated advanced chronic liver disease after oral antivirals. J. Hepatol. 2020, 72, 472–480. [Google Scholar] [CrossRef]
- Zhu, Z.X.; Liao, M.H.; Wang, X.X.; Huang, J.W. Radiofrequency ablation with or without ethanol injection for hepatocellular carcinoma: A systematic review and metaanalysis. Minerva Med. 2016, 107, 381–391. [Google Scholar]
- Dajti, E.; Marasco, G.; Ravaioli, F.; Colecchia, L.; Ferrarese, A.; Festi, D.; Colecchia, A. Risk of hepatocellular carcinoma after HCV eradication: Determining the role of portal hypertension by measuring spleen stiffness. JHEP Rep. 2021, 3, 100289. [Google Scholar] [CrossRef]
- Ibrahim, E.; Abdel-Samiee, M.; Youssef, M.I.; El-Shazly, H.; AEl-Gendy, A.; Sakr, A.A.; Elwazzan, D.; Nassar, M.R.; Elshormilisy, A.A.; Madkour, A.; et al. Variceal recurrence 4 years post endoscopic band ligation in hepatitis C patients who achieved sustained virological response with oral direct-acting antiviral therapy. J. Viral Hepat. 2021, 28, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Ronot, M.; Fouque, O.; Esvan, M.; Lebigot, J.; Aube, C.; Vilgrain, V. Comparison of the accuracy of AASLD and LI-RADS criteria for the non-invasive diagnosis of HCC smaller than 3 cm. J. Hepatol. 2018, 68, 715–723. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, S.; Tovoli, F.; Barbera, M.A.; Garuti, F.; Palloni, A.; Frega, G.; Garajovà, I.; Rizzo, A.; Trevisani, F.; Brandi, G. Metronomic capecitabine vs. best supportive care in Child-Pugh B hepatocellular carcinoma: A proof of concept. Sci. Rep. 2018, 8, 9997. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Cheng, Z.; Wu, P.; Sheng, Y.; Qu, X.; Lu, W.; Zhao, C.; Qian, G. Clinical outcome of medium-sized hepatocellular carcinoma treated with microwave ablation. World J Gastroenterol. 2015, 21, 2997–3004. [Google Scholar] [CrossRef]
- Rizzo, A.; Brandi, G. Biochemical predictors of response to immune checkpoint inhibitors in unresectable hepatocellular carcinoma. Cancer Treat. Res. Commun. 2021, 27, 100328. [Google Scholar] [CrossRef]
- Rossi, S.; Fornari, F.; Buscarini, L. Percutaneous ultrasound-guided radiofrequency electrocautery for the treatment of small hepatocellular carcinoma. J. IntervRadiol. 1993, 8, 97–103. [Google Scholar]
- Lu, D.-E.; Cheng, S.-W.; Lin, Y.-S.; Tu, M.-W.; Lee, C.-H.; Chen, C.; Chen, K.-H. Combination of radiofrequency ablation and percutaneous ethanol injection versus radiofrequency ablation alone for hepatocellular carcinoma: A systematic review and meta-analysis. Ann. Hepatol. 2022, 27, 100729. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, K.; Lin, S.M.; Mi, D.H.; Cao, N.; Wen, Z.Z.; Li, Z.-X. Radiofrequency ablation combined with percutaneous ethanol injection for hepatocellular carcinoma: A systematic review and meta-analysis. Int. J. Hyperth. 2017, 33, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
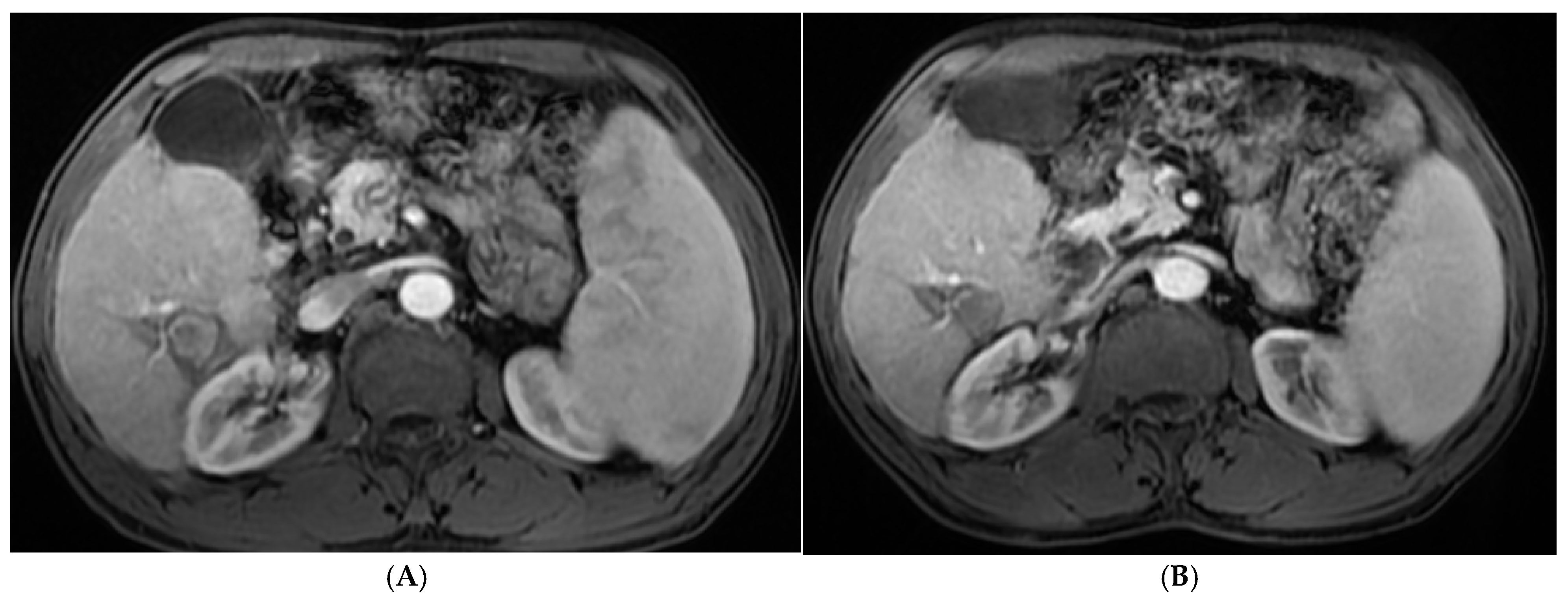
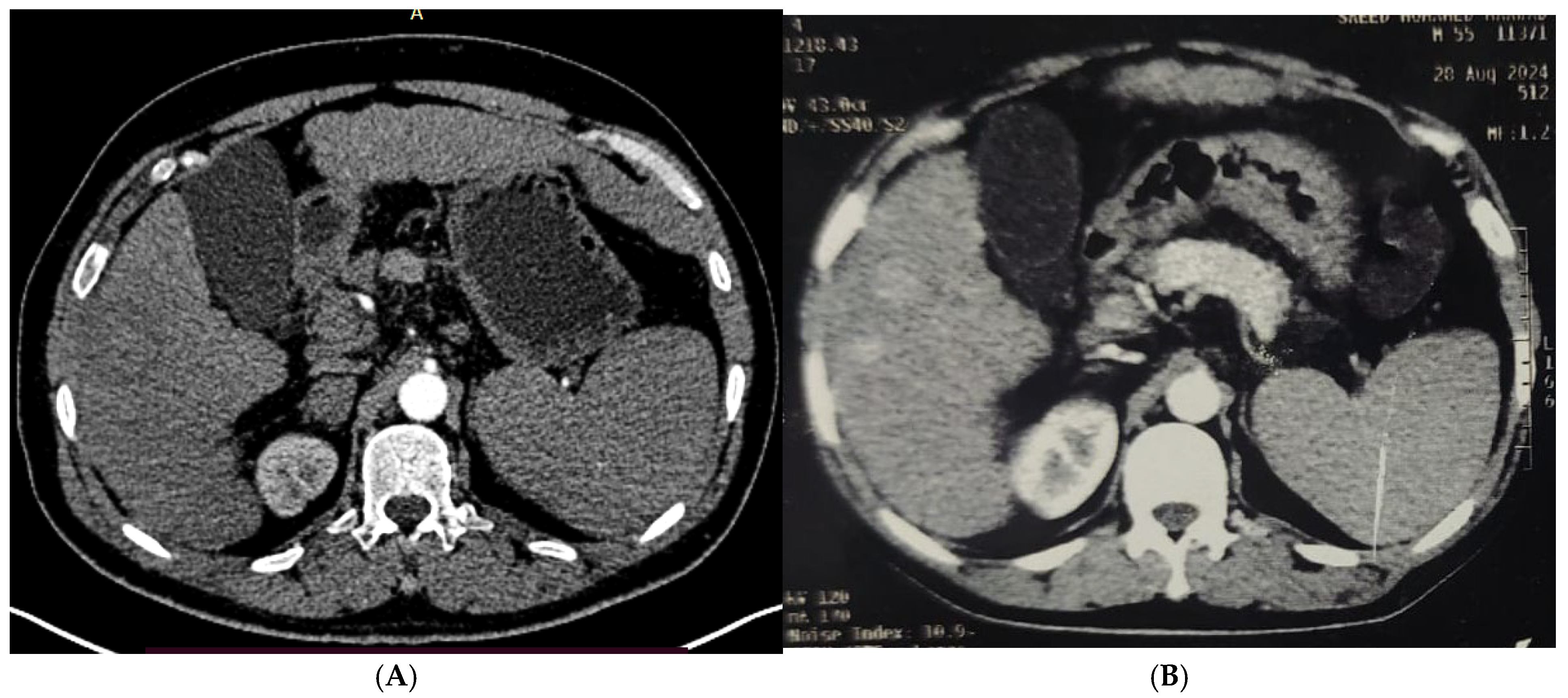
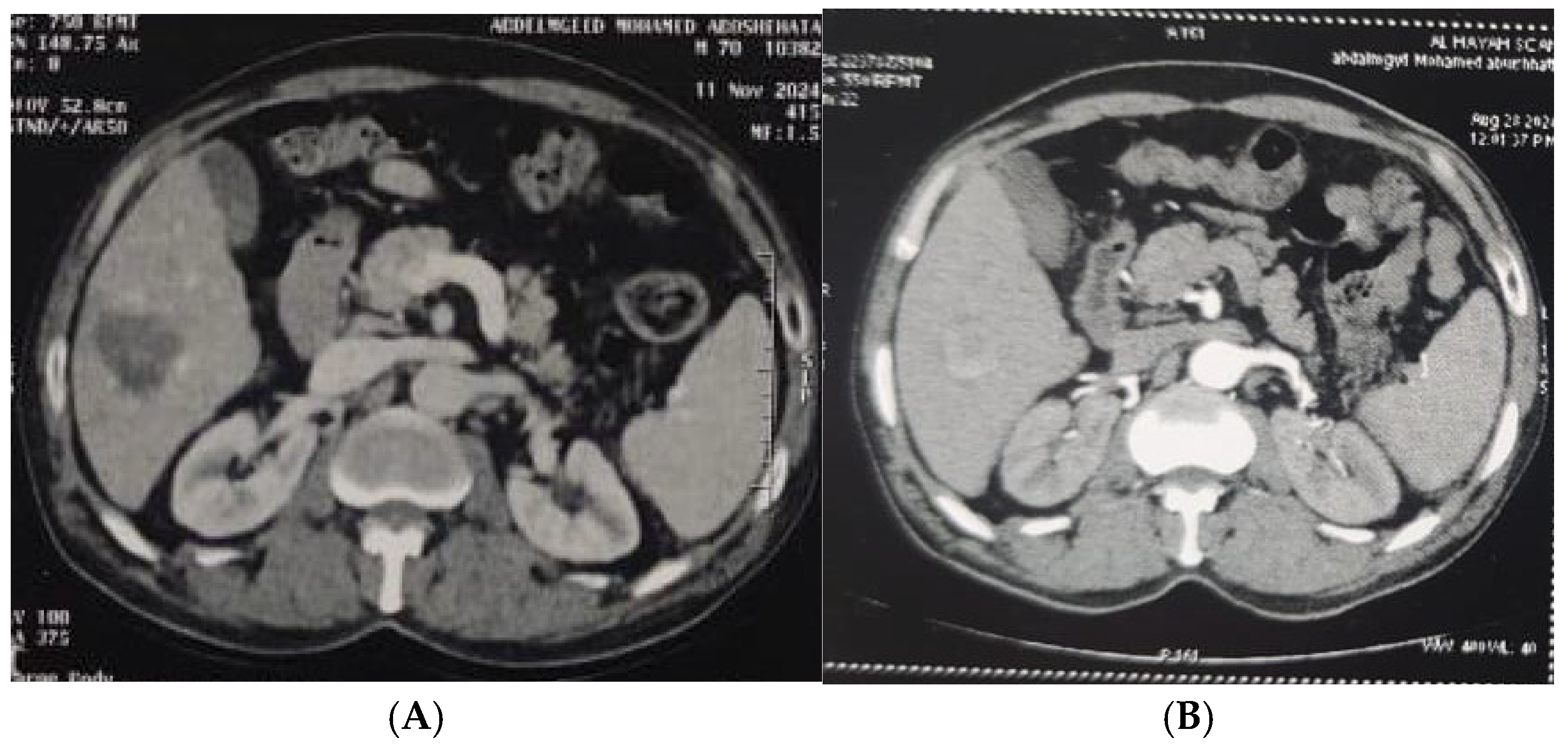

| Demographic Data | RFA (n = 50) | MW (n = 50) | PEI (n = 50) | RFA&PEI (n = 50) | MW& PEI (n = 50) | Test of Sig. (p) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Gender | |||||||||||
| Male | 38 | 76.0 | 37 | 74.0 | 42 | 84.0 | 39 | 78.0 | 38 | 76.0 | χ2 = 1.454 (0.835) |
| Female | 12 | 24.0 | 13 | 26.0 | 8 | 16.0 | 11 | 22.0 | 12 | 24.0 | |
| History of hypertension | |||||||||||
| Negative | 37 | 74.0 | 38 | 76.0 | 39 | 78.0 | 36 | 72.0 | 40 | 80.0 | 1.096(0.895) |
| Positive | 13 | 26.0 | 12 | 24.0 | 11 | 22.0 | 14 | 28.0 | 10 | 20.0 | |
| History of diabetes mellitus | |||||||||||
| Negative | 36 | 72.0 | 34 | 68.0 | 34 | 68.0 | 37 | 74.0 | 35 | 70.0 | 0.653 (0.957) |
| Positive | 14 | 28.0 | 16 | 32.0 | 16 | 32.0 | 13 | 26.0 | 15 | 30.0 | |
| Bilharziasis | |||||||||||
| Negative | 29a | 58.0 | 25 | 50.0 | 31 | 62.0 | 39 | 78.0 | 39 | 78.0 | 13.680 * (0.008 *) |
| Positive | 21a | 42.0 | 25 | 50.0 | 19 | 38.0 | 11 | 22.0 | 11 | 22.0 | |
| HBV | |||||||||||
| Negative | 47 | 94.0 | 46 | 92.0 | 49 | 98.0 | 44 | 88.0 | 48 | 96.0 | 4.564 (MCp = 0.343) |
| Positive | 3 | 6.0 | 4 | 8.0 | 1 | 2.0 | 6 | 12.0 | 2 | 4.0 | |
| HCV | |||||||||||
| Negative | 9a | 18.0 | 21b | 42.0 | 10a | 20.0 | 20b | 40.0 | 12ab | 24.0 | 12.601 * (0.013 *) |
| Positive | 41a | 82.0 | 29b | 58.0 | 40a | 80.0 | 30b | 60.0 | 38ab | 76.0 | |
| Liver cirrhosis | |||||||||||
| No | 1 | 2.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 317.809 * (<0.001 *) |
| Yes | 49 | 98.0 | 50 | 100.0 | 50 | 100.0 | 50 | 100.0 | 50 | 100.0 | |
| Performance status | |||||||||||
| 0 | 49 | 98.0 | 48 | 96.0 | 46 | 92.0 | 47 | 94.0 | 47 | 94.0 | 2.196 (0.821) |
| 1 | 1 | 2.0 | 2 | 4.0 | 4 | 8.0 | 3 | 6.0 | 3 | 6.0 | |
| Child–Turcotte–Pugh score (CTP) | |||||||||||
| A5 | 21 | 42.0 | 16 | 32.0 | 21 | 42.0 | 14 | 28.0 | 14 | 28.0 | χ2 = 6.269 (0.617) |
| A6 | 20 | 40.0 | 26 | 52.0 | 20 | 40.0 | 28 | 56.0 | 28 | 56.0 | |
| B7 | 9 | 18.0 | 8 | 16.0 | 9 | 18.0 | 8 | 16.0 | 8 | 16.0 | |
| Age | |||||||||||
| Min.–Max. | 33.0–74.0 | 40.0–73.0 | 48.0–78.0 | 48.0–75.0 | 45.0–70.0 | F = 0.341 (0.850) | |||||
| Mean ± SD. | 59.72 ± 8.12 | 60.22 ± 7.23 | 60.10 ± 7.32 | 60.30 ± 7.24 | 61.34 ± 6.53 | ||||||
| Median | 62.50 | 60.0 | 61.50 | 62.0 | 64.0 | ||||||
| RFA (n = 50) | MW (n = 50) | PEI (n = 50) | RFA&PEI (n = 50) | MW&PEI (n = 50) | H (p) | ||
|---|---|---|---|---|---|---|---|
| Largest tumor diameter | |||||||
| Min.–Max. | 2.0–3.50 | 3.0–5.0 | 1.0–2.50 | 3.0–4.50 | 4.0–5.0 | 155.389 * (<0.001 *) | |
| Mean ± SD. | 2.70 ± 0.56 | 3.73 ± 0.53 | 1.82 ± 0.54 | 3.59 ± 0.41 | 4.50 ± 0.47 | ||
| Median | 2.80 | 3.50 | 2.0 | 3.50 | 4.0 | ||
| Significance between groups | p1 < 0.001 *, p2 = 0.002 *, p3 < 0.001 *, p4 < 0.001 *, p5 < 0.001 *, p6 = 0.570, p7 = 0.013 *, p8 < 0.001 *, p9 < 0.001 *, p10 = 0.002 * | ||||||
| No. of Sessions | RFA (n = 50) | MWA (n = 50) | PEI (n = 50) | RFA&PEI (n = 50) | MW&PEI (n = 50) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| One session | 44 | 88.0% | 50 | 100% | 0 | 0.0% | 50 | 100% | 50 | 100% |
| Two sessions | 6 | 12.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Four sessions | 0 | 0.0% | 0 | 0.0% | 46 | 92.0% | 0 | 0.0% | 0 | 0.0% |
| Six sessions | 0 | 0.0% | 0 | 0.0% | 4 | 8.0% | 0 | 0.0% | 0 | 0.0% |
| RFA (n = 50) | MW (n = 50) | PEI (n = 50) | RFA&PEI (n = 50) | MW& PEI (n = 50) | χ2(p) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Incomplete response | 4 | 8.0 | 3 | 6.0 | 5 | 10.0 | 1 | 2.0 | 0 | 0.0 | 33.837 * (<0.001 *) |
| Complete response | 46 | 92.0 | 47 | 94.0 | 45 | 90.0 | 49 | 98.0 | 50 | 100.0 | |
| RFA (n = 50) | MW (n = 50) | PEI (n = 50) | RFA&PEI (n = 50) | MW& PEI (n = 50) | χ2(p) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| No recurrence | 41 | 78.0 | 44 | 88.0 | 34 | 60 | 46 | 92.0 | 48 | 96.0 | 33.837 * (<0.001 *) |
| Local recurrence Distant recurrence | 4 3 | 8.0 6.0 | 4 2 | 8.0 4.0 | 10 6 | 20.0 8.0 | 2 1 | 4.0 2.0 | 02 | 0.0 4.0 | |
| Local and distal recurrence | 2 | 4.0 | 0 | 0.0 | 0 | 0.0 | 1 | 2.0 | 0 | 0.0 | |
| Recurrence Time/Months | RFA (n = 50) | MW (n = 50) | PEI (n = 50) | RFA&PEI (n = 50) | MW&PEI (n = 50) | χ2(p) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| (n = 9) | (n = 6) | (n = 16) | (n = 4) | (n = 2) | 11.563 (MC p = 0.365) | ||||||
| 6 moths | 1 | 11.1 | 0 | 0.0 | 6 | 37.5 | 0 | 0.0 | 0 | 0.0 | |
| 12 months | 6 | 66.7 | 4 | 66.6 | 4 | 25.0 | 2 | 50.0 | 0 | 0.0 | |
| 18 months | 2 | 22.2 | 1 | 16.7 | 6 | 37.5 | 1 | 25.0 | 1 | 50.0 | |
| 24 months | 0 | 0.0 | 1 | 16.7 | 0 | 0.0 | 1 | 25.0 | 1 | 50.0 | |
| Min.–Max. | Mean ± SD. | Median | ||
|---|---|---|---|---|
| Survival time (in years) | 0.6–5.0 | 2.71 ± 1.06 | 2.50 | |
| Survival time | No. | % | ||
| 1 year | 231 | 92.4 | ||
| 3 years | 125 | 66.5 | ||
| 5 years | 96 | 38.4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Samiee, M.; Elkazaz, R.R.; Omar, H.; Salama, N.M.; Gomaa, A.I.; Rady, M.A.; Waked, I. A Comparative Study of Radiofrequency Ablation, Microwave Ablation, and Percutaneous Ethanol Injection in Treatment of Hepatocellular Carcinoma—A Single-Center Experience. Diagnostics 2025, 15, 3027. https://doi.org/10.3390/diagnostics15233027
Abdel-Samiee M, Elkazaz RR, Omar H, Salama NM, Gomaa AI, Rady MA, Waked I. A Comparative Study of Radiofrequency Ablation, Microwave Ablation, and Percutaneous Ethanol Injection in Treatment of Hepatocellular Carcinoma—A Single-Center Experience. Diagnostics. 2025; 15(23):3027. https://doi.org/10.3390/diagnostics15233027
Chicago/Turabian StyleAbdel-Samiee, Mohamed, Reham Reda Elkazaz, Hazem Omar, Nada Mohsen Salama, Asmaa Ibrahim Gomaa, Mohamed Akl Rady, and Imam Waked. 2025. "A Comparative Study of Radiofrequency Ablation, Microwave Ablation, and Percutaneous Ethanol Injection in Treatment of Hepatocellular Carcinoma—A Single-Center Experience" Diagnostics 15, no. 23: 3027. https://doi.org/10.3390/diagnostics15233027
APA StyleAbdel-Samiee, M., Elkazaz, R. R., Omar, H., Salama, N. M., Gomaa, A. I., Rady, M. A., & Waked, I. (2025). A Comparative Study of Radiofrequency Ablation, Microwave Ablation, and Percutaneous Ethanol Injection in Treatment of Hepatocellular Carcinoma—A Single-Center Experience. Diagnostics, 15(23), 3027. https://doi.org/10.3390/diagnostics15233027






