External Validation of Periodontal Screening Using Self-Reports in Dental Settings
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Data
2.2. Participants
2.3. Outcome
2.4. Predictors
2.5. Sample Size
2.6. Statistical Analysis
3. Results
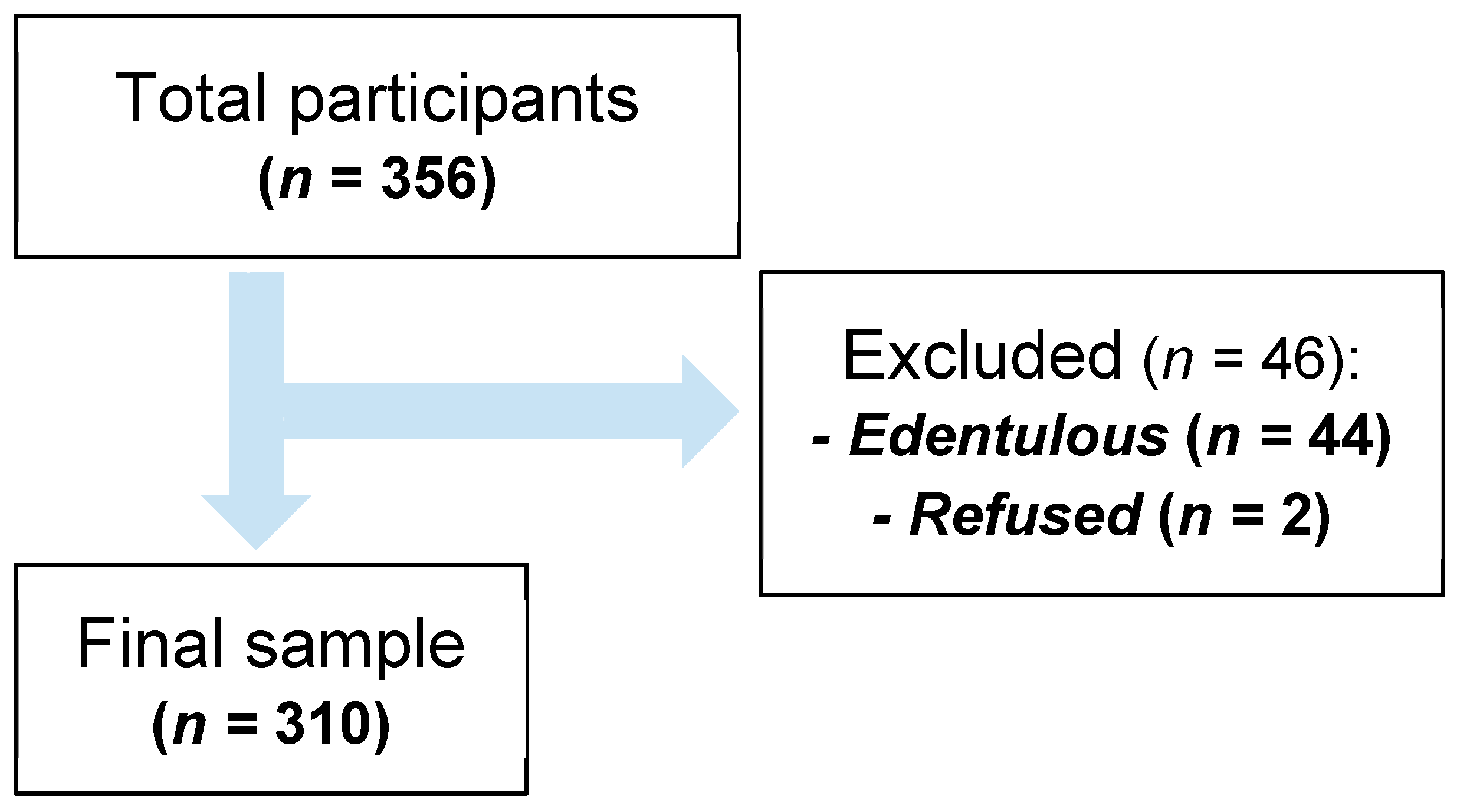
3.1. Participants
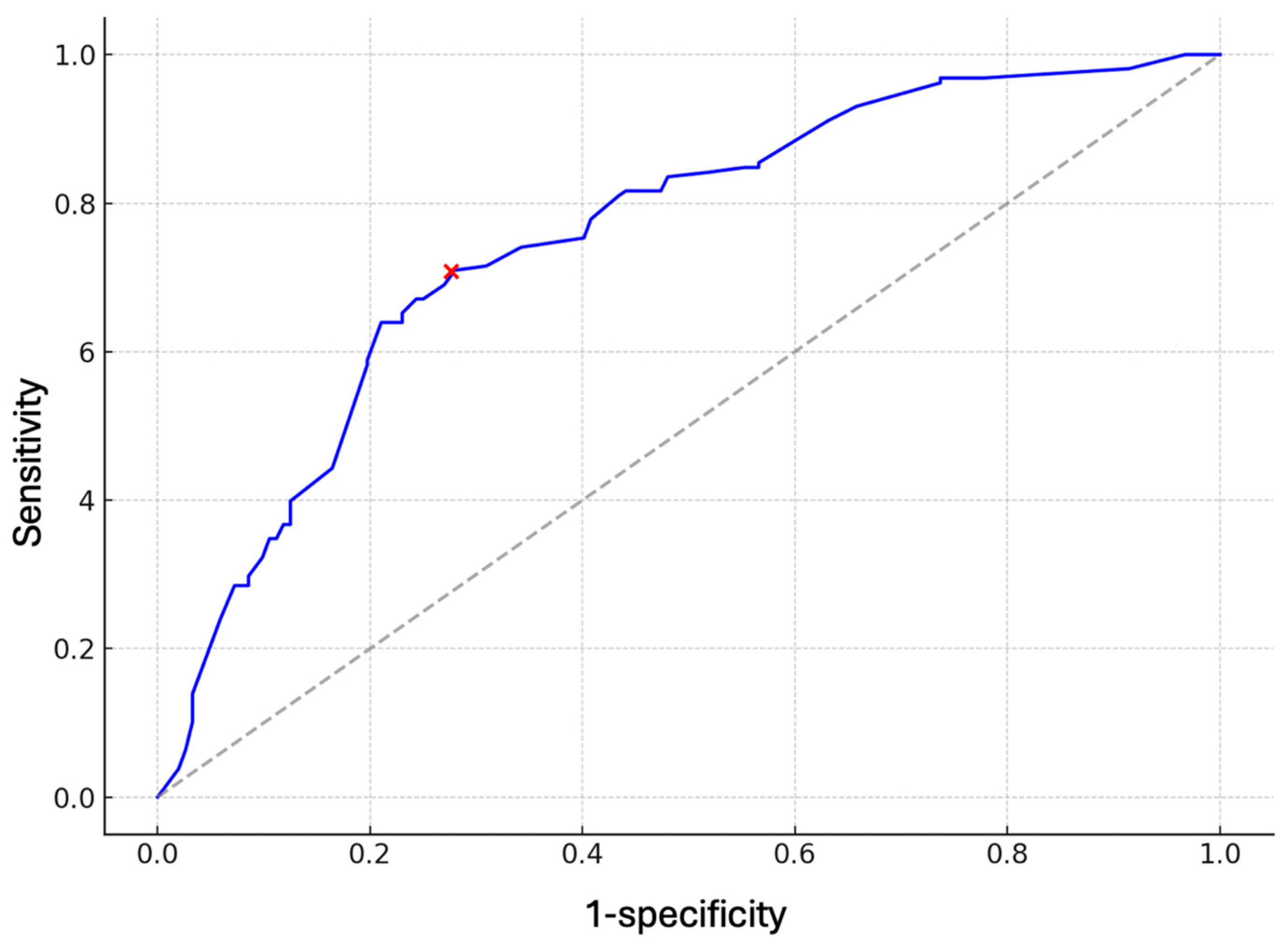
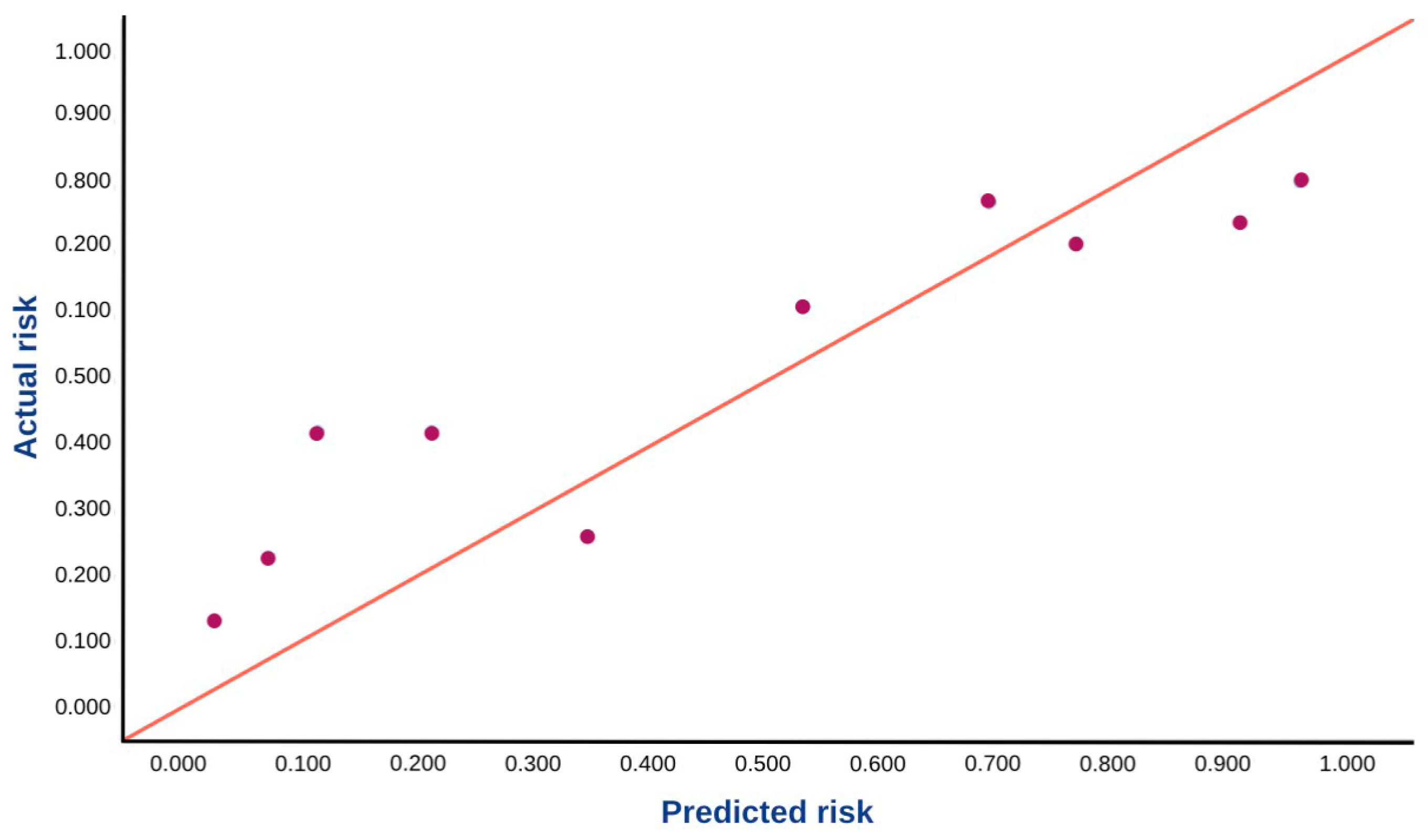
3.2. Model Performance
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trindade, D.; Carvalho, R.; Machado, V.; Chambrone, L.; Mendes, J.J.; Botelho, J. Prevalence of periodontitis in dentate people between 2011 and 2020: A systematic review and meta-analysis of epidemiological studies. J. Clin. Periodontol. 2023, 50, 604–626. [Google Scholar] [CrossRef]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. Global Strategy and Action Plan on Oral Health 2023–2030; World Health Organization: Geneva, Switzerland, 2024; Available online: https://iris.who.int/bitstream/handle/10665/376623/9789240090538-eng.pdf?sequence=1 (accessed on 14 October 2025).
- Eke, P.I.; Dye, B. Assessment of self-report measures for predicting population prevalence of periodontitis. J. Periodontol. 2009, 80, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Beck, J.D.; Taylor, G.; Borgnakke, W.; Page, R.; Genco, R. Self-reported Measures for Surveillance of Periodontitis. J. Dent. Res. 2013, 92, 1041–1047. [Google Scholar] [CrossRef]
- Carra, M.C.; Gueguen, A.; Thomas, F.; Pannier, B.; Caligiuri, G.; Steg, P.G.; Zins, M.; Bouchard, P. Self-report assessment of severe periodontitis: Periodontal screening score development. J. Clin. Periodontol. 2018, 45, 818–831. [Google Scholar] [CrossRef]
- Deng, K.; Pelekos, G.; Jin, L.; Tonetti, M.S. Diagnostic accuracy of self-reported measures of periodontal disease: A clinical validation study using the 2017 case definitions. J. Clin. Periodontol. 2021, 48, 1037–1050. [Google Scholar] [CrossRef]
- Iwasaki, M.; Usui, M.; Ariyoshi, W.; Nakashima, K.; Nagai-Yoshioka, Y.; Inoue, M.; Kobayashi, K.; Borgnakke, W.S.; Taylor, G.W.; Nishihara, T. Validation of a self-report questionnaire for periodontitis in a Japanese population. Sci. Rep. 2021, 11, 15078. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.; Lyra, P.; Santos, C.; Proença, L.; Mendes, J.J.; Botelho, J. Self-Reported Measures of Periodontitis in a Portuguese Population: A Validation Study. J. Pers. Med. 2022, 12, 1315. [Google Scholar] [CrossRef]
- Montero, E.; Herrera, D.; Sanz, M.; Dhir, S.; Van Dyke, T.; Sima, C. Development and validation of a predictive model for periodontitis using NHANES 2011–2012 data. J. Clin. Periodontol. 2019, 46, 420–429. [Google Scholar] [CrossRef]
- Nam, S.; Jung, H.; Kang, S.; Inaba, D.; Kwon, H.; Kim, B. Validity of Screening Methods for Periodontitis Using Salivary Hemoglobin Level and Self-Report Questionnaires in People with Disabilities. J. Periodontol. 2015, 86, 536–545. [Google Scholar] [CrossRef]
- Saka-Herrán, C.; Jané-Salas, E.; González-Navarro, B.; Estrugo-Devesa, A.; López-López, J. Validity of a self-reported questionnaire for periodontitis in a Spanish population. J. Periodontol. 2020, 91, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, M.J.L.; Teeuw, W.J.; Bizzarro, S.; Muris, J.; Su, N.; Nicu, E.A.; Nazmi, K.; Bikker, F.J.; Loos, B.G. A rapid, non-invasive tool for periodontitis screening in a medical care setting. BMC Oral Health 2019, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, G.G.; Raittio, E.; Machado, V.; Leite, F.R.M.; Botelho, J. Advancing Universal Oral Health Coverage via Person-Centred Outcomes. Int. Dent. J. 2023, 73, 793–799. [Google Scholar] [CrossRef]
- Verhulst, N.; Overtoom, F.; Gerdes, V.E.A.; Verhulst, M.J.L.; Su, N.; Loos, B.G. External validation of a rapid, non-invasive tool for periodontitis screening in a medical care setting. Clin. Oral. Investig. 2021, 25, 6661–6669. [Google Scholar]
- Shimpi, N.; McRoy, S.; Zhao, H.; Wu, M.; Acharya, A. Development of a periodontitis risk assessment model for primary care providers in an interdisciplinary setting. Technol. Health Care 2020, 28, 143–154. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): The TRIPOD Statement. Ann. Intern. Med. 2015, 162, 55–63. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Proença, L.; Alves, R.; Cavacas, M.A.; Amaro, L.; Mendes, J.J. Study of Periodontal Health in Almada-Seixal (SoPHiAS): A cross-sectional study in the Lisbon Metropolitan Area. Sci. Rep. 2019, 9, 15538. [Google Scholar] [CrossRef]
- Ainamo, J.; Barmes, D.; Beagrie, G.; Cutress, T.; Martin, J.; Sardo-Infirri, J. Development of the World Health Organization (WHO) community periodontal index of treatment needs (CPITN). Int. Dent. J. 1982, 32, 281–291. [Google Scholar]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 1. [Google Scholar] [CrossRef]
- Kapellas, K.; Ali, A.; Jamieson, L.M. Modelling the Validity of Periodontal Disease Screening Questions in a Nondental Clinical Setting. Int. Dent. J. 2021, 71, 407–413. [Google Scholar] [CrossRef]
- Swami, N.; Corti, C.; Curigliano, G.; Celi, L.A.; Dee, E.C. Exploring biases in predictive modelling across diverse populations. Lancet Healthy Longev. 2022, 3, e88. [Google Scholar] [CrossRef]
- Nijland, N.; Su, N.; Gerdes, V.E.A.; Loos, B.G. Attempts to Modify Periodontal Screening Models Based on a Self-Reported Oral Health Questionnaire in the Medical Care Setting. J. Clin. Periodontol. 2025, 52, 387–398. [Google Scholar] [CrossRef]
- Varela-Centelles, P.; Diz-Iglesias, P.; Estany-Gestal, A.; Seoane-Romero, J.M.; Bugarín-González, R.; Seoane, J. Periodontitis Awareness Amongst the General Public: A Critical Systematic Review to Identify Gaps of Knowledge. J. Periodontol. 2016, 87, 403–415. [Google Scholar] [CrossRef]
- Jönsson, B.; Öhrn, K.; Oscarson, N.; Lindberg, P. The effectiveness of an individually tailored oral health educational programme on oral hygiene behaviour in patients with periodontal disease: A blinded randomized-controlled clinical trial (one-year follow-up). J. Clin. Periodontol. 2009, 36, 1025–1034. [Google Scholar] [CrossRef]
- Romano, F.; Perotto, S.; Bianco, L.; Parducci, F.; Mariani, G.M.; Aimetti, M. Self-Perception of Periodontal Health and Associated Factors: A Cross-Sectional Population-Based Study. Int. J. Environ. Res. Public Health 2020, 17, 2758. [Google Scholar] [CrossRef] [PubMed]
- Özçaka, Ö.; Becerik, S.; Bıçakcı, N.; Kiyak, A.H. Periodontal disease and systemic diseases in an older population. Arch. Gerontol. Geriatr. 2014, 59, 474–479. [Google Scholar] [CrossRef]
- Marques, M.D.; Tei, A. Prevalence and determinants of periodontal disease in Portuguese adults: Results from a multifactorial approach. Acta Odontol. Scand. 2000, 58, 201–206. [Google Scholar] [CrossRef]
- Petersen, P.E.; Ogawa, H. Strengthening the Prevention of Periodontal Disease: The WHO Approach. J. Periodontol. 2005, 76, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Gelskey, S.C.; Young, T.K.; Singer, D.L. Factors associated with adult periodontitis in a dental teaching clinic population. Community Dent. Oral Epidemiol. 1998, 26, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Dye, B.A.; Wei, L.; Thornton-Evans, G.O.; Genco, R.J. Prevalence of Periodontitis in Adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef]
- Savage, A.; Eaton, K.A.; Moles, D.R.; Needleman, I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J. Clin. Periodontol. 2009, 36, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Borgnakke, W.S.; Genco, R.J. Recent Epidemiologic Trends in Periodontitis in the USA. Periodontology 2000 2020, 82, 257–267. [Google Scholar] [CrossRef]
- Baelum, V.; Papapanou, P.N. CPITN and the epidemiology of periodontal disease Commentary. Community Dent. Oral Epidemiol. 1996, 24, 367–368. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 310) | Severe PD (n = 158) | Non-Severe PD (n = 152) | p-Value |
|---|---|---|---|---|
| Age, mean (SD) (years) | 52.8 (16.3) | 58.5 (12.0) | 46.9 (18.0) | <0.0001 |
| Sex, % (n) | ||||
| Female | 52.3 (162) | 44.9 (71) | 59.9 (91) | 0.0118 |
| Male | 47.7 (148) | 55.1 (87) | 40.1 (61) | |
| Smoking, % (n) | ||||
| Never | 51.0 (158) | 44.3 (70) | 57.9 (88) | 0.0552 |
| Former | 21.3 (66) | 24.7 (39) | 17.8 (27) | |
| Active | 27.7 (86) | 31.0 (49) | 24.3 (37) | |
| Employment status, % (n) | ||||
| Student | 12.6% (39) | 4.4 (7) | 21.1 (32) | <0.0001 |
| Employed | 59.7% (185) | 61.4 (97) | 57.9 (88) | |
| Unemployed | 7.4% (23) | 8.9 (14) | 5.9 (9) | |
| Retired | 20.3% (63) | 25.3 (40) | 15.1 (23) | |
| Education, % (n) | ||||
| Elementary | 24.8% (77) | 31.6 (50) | 17.8 (27) | 0.0066 |
| Middle | 43.9% (136) | 43.7 (69) | 44.1 (67) | |
| Higher | 31.3% (97) | 24.7 (39) | 38.2 (58) | |
| Number of remaining teeth | 20.4 (5.7) | 20.5 (5.6) | 22.4 (5.6) | 0.0007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viana, J.; Machado, V.; Lyra, P.; Proença, L.; Su, N.; Mendes, J.J.; Botelho, J. External Validation of Periodontal Screening Using Self-Reports in Dental Settings. Diagnostics 2025, 15, 3015. https://doi.org/10.3390/diagnostics15233015
Viana J, Machado V, Lyra P, Proença L, Su N, Mendes JJ, Botelho J. External Validation of Periodontal Screening Using Self-Reports in Dental Settings. Diagnostics. 2025; 15(23):3015. https://doi.org/10.3390/diagnostics15233015
Chicago/Turabian StyleViana, João, Vanessa Machado, Patrícia Lyra, Luís Proença, Naichuan Su, José João Mendes, and João Botelho. 2025. "External Validation of Periodontal Screening Using Self-Reports in Dental Settings" Diagnostics 15, no. 23: 3015. https://doi.org/10.3390/diagnostics15233015
APA StyleViana, J., Machado, V., Lyra, P., Proença, L., Su, N., Mendes, J. J., & Botelho, J. (2025). External Validation of Periodontal Screening Using Self-Reports in Dental Settings. Diagnostics, 15(23), 3015. https://doi.org/10.3390/diagnostics15233015











