Applications of Optical Coherence Tomography in Optic Nerve Head Diseases: A Narrative Review
Abstract
1. Introduction
2. OCT in Glaucoma
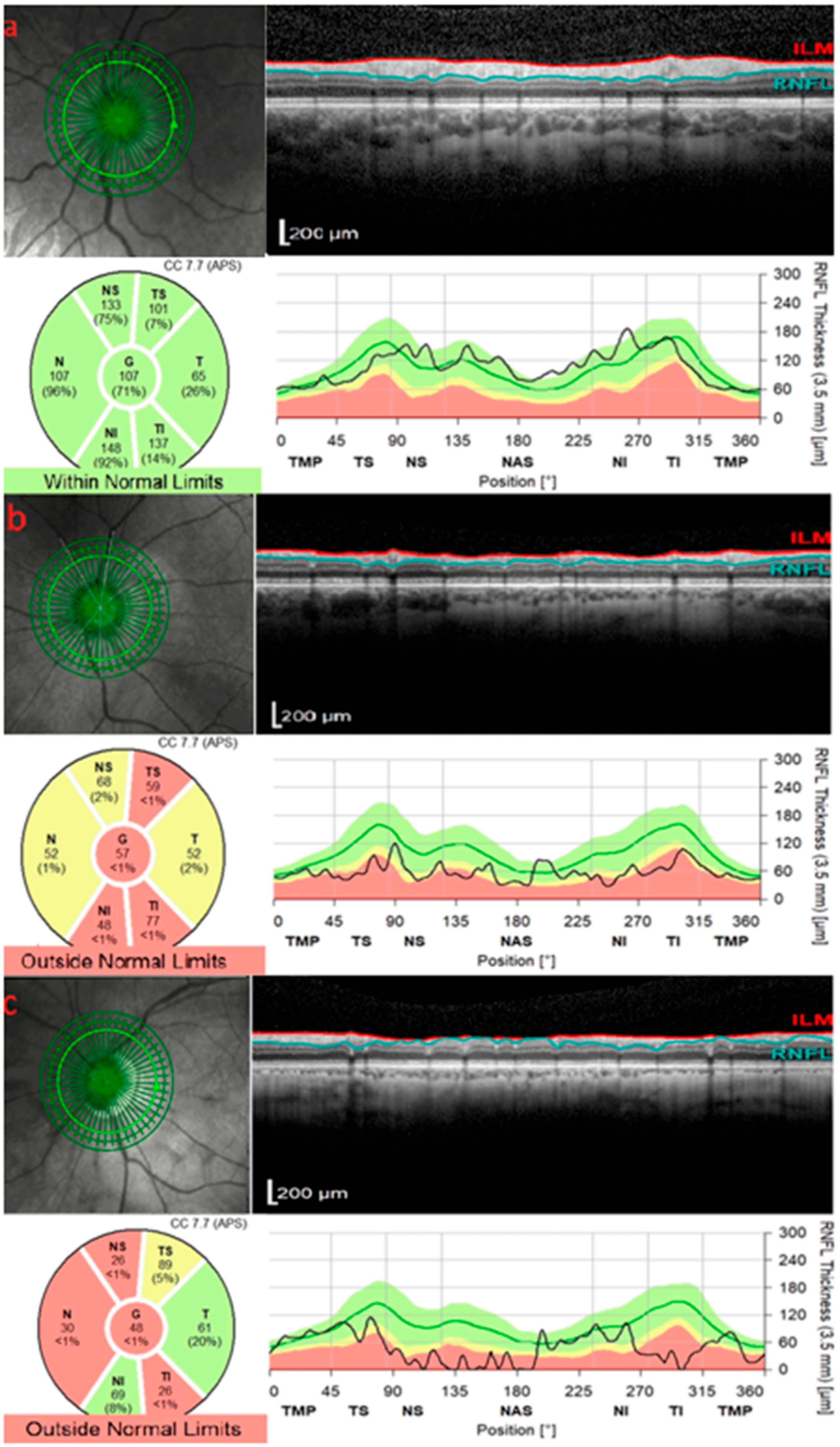
3. OCT in Anterior Ischemic Optic Neuropathy
4. OCT in Inflammatory Optic Neuropathies
5. OCT in Optic Disk Neovascularization
6. OCT in Papilledema and Pseudopapilledema
7. OCT in Optic Pits
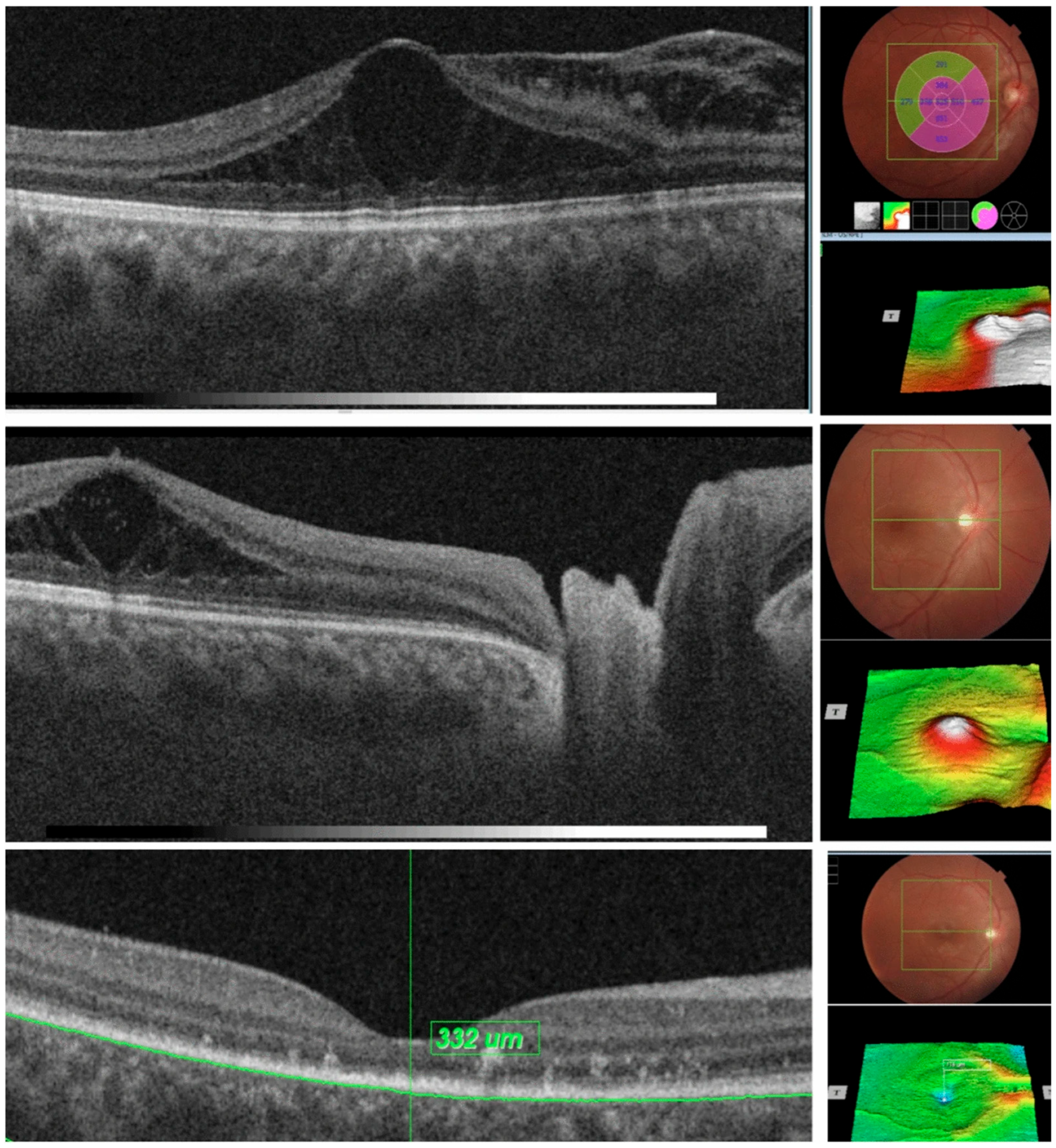
8. OCT in Optic Disc Coloboma
9. OCT in Optic Nerve Head Drusen
10. OCT in Morning Glory Anomaly
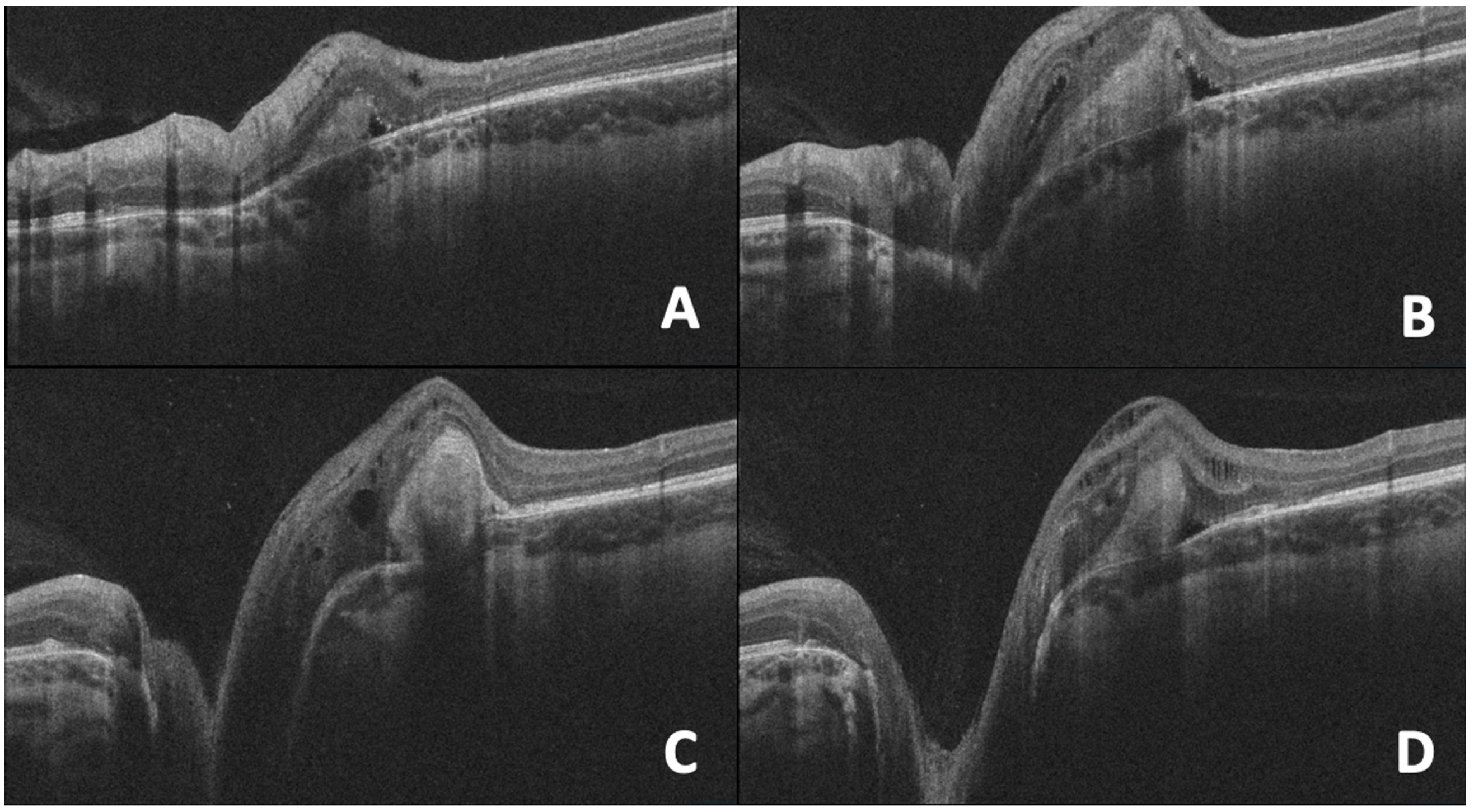
11. Myelinated Nerve Fibers
12. Melanocytoma
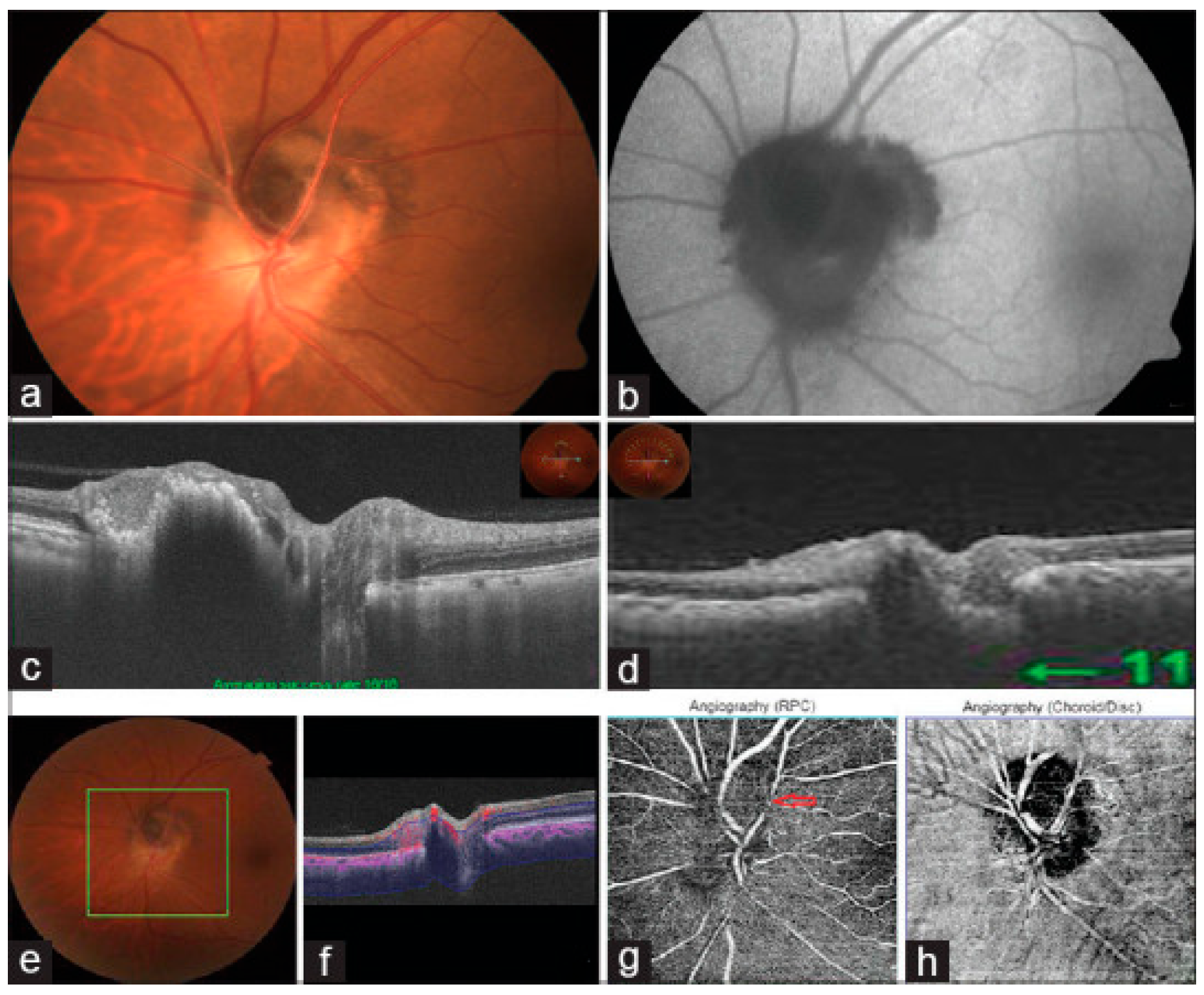
13. Peripapillary and Intrapapillary Pigmentary Structural Optic Nerve Head Changes
13.1. A-Grey Crescent
13.2. B-Peripapillary Atrophy (PPA)
13.3. C-Peripapillary Halo
14. Limitations
15. Future Directions
16. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Downs, J.C.; Girkin, C.A. Lamina cribrosa in glaucoma. Curr. Opin. Ophthalmol. 2017, 28, 113–119. [Google Scholar] [CrossRef]
- Machado, L.F.; Furlanetto, R.L.; Gracitelli, C.P.B. Anatomy and evaluation of the optic nerve head. Arq. Bras. Oftalmol. 2022, 85, 636–643. [Google Scholar] [CrossRef]
- Wang, Y.X.; Panda-Jonas, S.; Jonas, J.B. Optic nerve head anatomy in myopia and glaucoma, including parapapillary zones alpha, beta, gamma and delta: Histology and clinical features. Prog. Retin. Eye Res. 2021, 83, 100933. [Google Scholar] [CrossRef]
- Arnold, A. Vascular supply of the optic nerve head: Implications for optic disc ischaemia. Br. J. Ophthalmol. 2023, 107, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Chidlow, G.; Ebneter, A.; Wood, J.P.; Casson, R.J. The optic nerve head is the site of axonal transport disruption, axonal cytoskeleton damage and putative axonal regeneration failure in a rat model of glaucoma. Acta Neuropathol. 2011, 121, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Geevarghese, A.; Wollstein, G.; Ishikawa, H.; Schuman, J.S. Optical Coherence Tomography and Glaucoma. Annu. Rev. Vis. Sci. 2021, 7, 693–726. [Google Scholar] [CrossRef]
- Gabriele, M.L.; Wollstein, G.; Ishikawa, H.; Kagemann, L.; Xu, J.; Folio, L.S.; Schuman, J.S. Optical coherence tomography: History, current status, and laboratory work. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2425–2436. [Google Scholar] [CrossRef] [PubMed]
- Bhende, M.; Shetty, S.; Parthasarathy, M.K.; Ramya, S. Optical coherence tomography: A guide to interpretation of common macular diseases. Indian. J. Ophthalmol. 2018, 66, 20–35. [Google Scholar] [CrossRef]
- Kashani, A.H.; Chen, C.L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog. Retin. Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef]
- Rodriguez Torres, Y.; Lee, P.; Mihlstin, M.; Tomsak, R.L. Correlation Between Optic Disc Peripapillary Capillary Network and Papilledema Grading in Patients with Idiopathic Intracranial Hypertension: A Study of Optical Coherence Tomography Angiography. J. Neuroophthalmol. 2021, 41, 48–53. [Google Scholar] [CrossRef]
- Kang, J.M.; Tanna, A.P. Glaucoma. Med. Clin. N. Am. 2021, 105, 493–510. [Google Scholar] [CrossRef]
- Wagner, I.V.; Stewart, M.W.; Dorairaj, S.K. Updates on the Diagnosis and Management of Glaucoma. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 618–635. [Google Scholar] [CrossRef]
- Melchior, B.; De Moraes, C.G.; Paula, J.S.; Cioffi, G.A.; Girkin, C.A.; Fazio, M.A.; Weinreb, R.N.; Zangwill, L.M.; Liebmann, J.M. Frequency of Optical Coherence Tomography Testing to Detect Progression in Glaucoma. J. Glaucoma 2022, 31, 854–859. [Google Scholar] [CrossRef]
- Gedde, S.J.; Vinod, K.; Wright, M.M.; Muir, K.W.; Lind, J.T.; Chen, P.P.; Li, T.; Mansberger, S.L.; American Academy of Ophthalmology Preferred Practice Pattern Glaucoma Panel. Primary Open-Angle Glaucoma Preferred Practice Pattern®. Ophthalmology 2021, 128, P71–P150. [Google Scholar] [CrossRef]
- Bussel, I.I.; Wollstein, G.; Schuman, J.S. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br. J. Ophthalmol. 2014, 98 (Suppl. 2), ii15–ii19. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.L.; Babu, J.G.; Addepalli, U.K.; Senthil, S.; Garudadri, C.S. Retinal nerve fiber layer and macular inner retina measurements by spectral domain optical coherence tomograph in Indian eyes with early glaucoma. Eye 2012, 26, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.T.; Zangwill, L.M.; Weinreb, R.N.; Rao, H.L.; Alencar, L.M.; Medeiros, F.A. Structure-function relationships using the Cirrus spectral domain optical coherence tomograph and standard automated perimetry. J. Glaucoma 2012, 21, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Stagg, B.C.; Medeiros, F.A. A Comparison of OCT Parameters in Identifying Glaucoma Damage in Eyes Suspected of Having Glaucoma. Ophthalmol. Glaucoma 2020, 3, 90–96. [Google Scholar] [CrossRef]
- Baniasadi, N.; Paschalis, E.I.; Haghzadeh, M.; Ojha, P.; Elze, T.; Mahd, M.; Chen, T.C. Patterns of Retinal Nerve Fiber Layer Loss in Different Subtypes of Open Angle Glaucoma Using Spectral Domain Optical Coherence Tomography. J. Glaucoma 2016, 25, 865–872. [Google Scholar] [CrossRef]
- Mwanza, J.C.; Durbin, M.K.; Budenz, D.L.; Sayyad, F.E.; Chang, R.T.; Neelakantan, A.; Godfrey, D.G.; Carter, R.; Crandall, A.S. Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: Comparison with nerve fiber layer and optic nerve head. Ophthalmology 2012, 119, 1151–1158. [Google Scholar] [CrossRef]
- Kotowski, J.; Folio, L.S.; Wollstein, G.; Ishikawa, H.; Ling, Y.; Bilonick, R.A.; Kagemann, L.; Schuman, J.S. Glaucoma discrimination of segmented cirrus spectral domain optical coherence tomography (SD-OCT) macular scans. Br. J. Ophthalmol. 2012, 96, 1420–1425. [Google Scholar] [CrossRef]
- Takayama, K.; Hangai, M.; Durbin, M.; Nakano, N.; Morooka, S.; Akagi, T.; Ikeda, H.O.; Yoshimura, N. A novel method to detect local ganglion cell loss in early glaucoma using spectral-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6904–6913. [Google Scholar] [CrossRef]
- Jeoung, J.W.; Choi, Y.J.; Park, K.H.; Kim, D.M. Macular ganglion cell imaging study: Glaucoma diagnostic accuracy of spectral-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4422–4429. [Google Scholar] [CrossRef] [PubMed]
- Oddone, F.; Lucenteforte, E.; Michelessi, M.; Rizzo, S.; Donati, S.; Parravano, M.; Virgili, G. Macular versus Retinal Nerve Fiber Layer Parameters for Diagnosing Manifest Glaucoma: A Systematic Review of Diagnostic Accuracy Studies. Ophthalmology 2016, 123, 939–949. [Google Scholar] [CrossRef]
- Zawadzka, I.; Konopinska, J. From the past to the present, optical coherence tomography in glaucoma: A practical guide to a common disease. F1000Res 2023, 12, 1186. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Biousse, V.; Newman, N.J. Ischemic Optic Neuropathies: Current Concepts. Ann. Indian. Acad. Neurol. 2022, 25, S54–S58. [Google Scholar] [CrossRef]
- Bajpai, V.; Madan, S.; Beri, S. Arteritic anterior ischaemic optic neuropathy: An update. Eur. J. Ophthalmol. 2021, 31, 2818–2827. [Google Scholar] [CrossRef] [PubMed]
- Salvetat, M.L.; Pellegrini, F.; Spadea, L.; Salati, C.; Zeppieri, M. Non-Arteritic Anterior Ischemic Optic Neuropathy (NA-AION): A Comprehensive Overview. Vision 2023, 7, 72. [Google Scholar] [CrossRef]
- Fard, M.A.; Ghahvehchian, H.; Subramanian, P.S. Optical coherence tomography in ischemic optic neuropathy. Ann. Eye Sci. 2020, 5, 6. [Google Scholar] [CrossRef]
- Contreras, I.; Noval, S.; Rebolleda, G.; Munoz-Negrete, F.J. Follow-up of nonarteritic anterior ischemic optic neuropathy with optical coherence tomography. Ophthalmology 2007, 114, 2338–2344. [Google Scholar] [CrossRef]
- Akbari, M.; Abdi, P.; Fard, M.A.; Afzali, M.; Ameri, A.; Yazdani-Abyaneh, A.; Mohammadi, M.; Moghimi, S. Retinal Ganglion Cell Loss Precedes Retinal Nerve Fiber Thinning in Nonarteritic Anterior Ischemic Optic Neuropathy. J. Neuroophthalmol. 2016, 36, 141–146. [Google Scholar] [CrossRef]
- Kupersmith, M.J.; Garvin, M.K.; Wang, J.K.; Durbin, M.; Kardon, R. Retinal ganglion cell layer thinning within one month of presentation for optic neuritis. Mult. Scler. 2016, 22, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Biousse, V.; Newman, N.J. Ischemic Optic Neuropathies. N. Engl. J. Med. 2015, 372, 2428–2436. [Google Scholar] [CrossRef]
- Pellegrini, M.; Giannaccare, G.; Bernabei, F.; Moscardelli, F.; Schiavi, C.; Campos, E.C. Choroidal Vascular Changes in Arteritic and Nonarteritic Anterior Ischemic Optic Neuropathy. Am. J. Ophthalmol. 2019, 205, 43–49. [Google Scholar] [CrossRef]
- Sharma, S.; Ang, M.; Najjar, R.P.; Sng, C.; Cheung, C.Y.; Rukmini, A.V.; Schmetterer, L.; Milea, D. Optical coherence tomography angiography in acute non-arteritic anterior ischaemic optic neuropathy. Br. J. Ophthalmol. 2017, 101, 1045–1051. [Google Scholar] [CrossRef]
- Rebolleda, G.; Diez-Alvarez, L.; Garcia Marin, Y.; de Juan, V.; Munoz-Negrete, F.J. Reduction of Peripapillary Vessel Density by Optical Coherence Tomography Angiography from the Acute to the Atrophic Stage in Non-Arteritic Anterior Ischaemic Optic Neuropathy. Ophthalmologica 2018, 240, 191–199. [Google Scholar] [CrossRef]
- Pierro, L.; Arrigo, A.; Aragona, E.; Cavalleri, M.; Bandello, F. Vessel Density and Vessel Tortuosity Quantitative Analysis of Arteritic and Non-arteritic Anterior Ischemic Optic Neuropathies: An Optical Coherence Tomography Angiography Study. J. Clin. Med. 2020, 9, 1094. [Google Scholar] [CrossRef] [PubMed]
- Costello, F. The afferent visual pathway: Designing a structural-functional paradigm of multiple sclerosis. ISRN Neurol. 2013, 2013, 134858. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; Balcer, L.J.; Calabresi, P.A.; Costello, F.; Frohman, T.C.; Frohman, E.M.; Martinez-Lapiscina, E.H.; Green, A.J.; Kardon, R.; Outteryck, O.; et al. Retinal layer segmentation in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2017, 16, 797–812. [Google Scholar] [CrossRef]
- Costello, F. Evaluating the use of optical coherence tomography in optic neuritis. Mult. Scler. Int. 2011, 2011, 148394. [Google Scholar] [CrossRef]
- Jarius, S.; Paul, F.; Weinshenker, B.G.; Levy, M.; Kim, H.J.; Wildemann, B. Neuromyelitis optica. Nat. Rev. Dis. Primers 2020, 6, 85. [Google Scholar] [CrossRef]
- Bennett, J.L.; de Seze, J.; Lana-Peixoto, M.; Palace, J.; Waldman, A.; Schippling, S.; Tenembaum, S.; Banwell, B.; Greenberg, B.; Levy, M.; et al. Neuromyelitis optica and multiple sclerosis: Seeing differences through optical coherence tomography. Mult. Scler. 2015, 21, 678–688. [Google Scholar] [CrossRef]
- Pisa, M.; Ratti, F.; Vabanesi, M.; Radaelli, M.; Guerrieri, S.; Moiola, L.; Martinelli, V.; Comi, G.; Leocani, L. Subclinical neurodegeneration in multiple sclerosis and neuromyelitis optica spectrum disorder revealed by optical coherence tomography. Mult. Scler. 2020, 26, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Manogaran, P.; Traboulsee, A.L.; Lange, A.P. Longitudinal Study of Retinal Nerve Fiber Layer Thickness and Macular Volume in Patients with Neuromyelitis Optica Spectrum Disorder. J. Neuroophthalmol. 2016, 36, 363–368. [Google Scholar] [CrossRef]
- Ratchford, J.N.; Quigg, M.E.; Conger, A.; Frohman, T.; Frohman, E.; Balcer, L.J.; Calabresi, P.A.; Kerr, D.A. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology 2009, 73, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Abdelhakim, A.; Rasool, N. Neuroretinitis: A review. Curr. Opin. Ophthalmol. 2018, 29, 514–519. [Google Scholar] [CrossRef]
- Habot-Wilner, Z.; Zur, D.; Goldstein, M.; Goldenberg, D.; Shulman, S.; Kesler, A.; Giladi, M.; Neudorfer, M. Macular findings on optical coherence tomography in cat-scratch disease neuroretinitis. Eye 2011, 25, 1064–1068. [Google Scholar] [CrossRef]
- Zatreanu, L.; Sibony, P.A.; Kupersmith, M.J. Optical Coherence Tomography in Neuroretinitis: Epipapillary Infiltrates and Retinal Folds. J. Neuroophthalmol. 2017, 37, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.H.; Melo, G.B.; Zett, C.; Morais, F.B.; Leal, B.C.; Farah, M.E.; Belfort, R., Jr. Optical Coherence Tomography Angiography Findings in Diffuse Unilateral Subacute Neuroretinitis. Ophthalmic Surg. Lasers Imaging Retin. 2020, 51, 76–83. [Google Scholar] [CrossRef]
- Wang, X.N.; Zhou, J.; Cai, X.; Li, T.; Long, D.; Wu, Q. Optical coherence tomography angiography for the detection and evaluation of ptic disc neovascularization: A retrospective, observational study. BMC Ophthalmol. 2022, 22, 125. [Google Scholar] [CrossRef]
- Ishibazawa, A.; Nagaoka, T.; Takahashi, A.; Omae, T.; Tani, T.; Sogawa, K.; Yokota, H.; Yoshida, A. Optical Coherence Tomography Angiography in Diabetic Retinopathy: A Prospective Pilot Study. Am. J. Ophthalmol. 2015, 160, 35–44.e1. [Google Scholar] [CrossRef]
- Rebolleda, G.; Kawasaki, A.; de Juan, V.; Oblanca, N.; Munoz-Negrete, F.J. Optical Coherence Tomography to Differentiate Papilledema from Pseudopapilledema. Curr. Neurol. Neurosci. Rep. 2017, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Sibony, P.A.; Kupersmith, M.J.; Kardon, R.H. Optical Coherence Tomography Neuro-Toolbox for the Diagnosis and Management of Papilledema, Optic Disc Edema, and Pseudopapilledema. J. Neuroophthalmol. 2021, 41, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Biousse, V.; Danesh-Meyer, H.V.; Saindane, A.M.; Lamirel, C.; Newman, N.J. Imaging of the optic nerve: Technological advances and future prospects. Lancet Neurol. 2022, 21, 1135–1150. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Woo, S.J.; Hwang, J.M. Differentiation of optic nerve head drusen and optic disc edema with spectral-domain optical coherence tomography. Ophthalmology 2011, 118, 971–977. [Google Scholar] [CrossRef]
- Sarac, O.; Tasci, Y.Y.; Gurdal, C.; Can, I. Differentiation of optic disc edema from optic nerve head drusen with spectral-domain optical coherence tomography. J. Neuroophthalmol. 2012, 32, 207–211. [Google Scholar] [CrossRef]
- Bassi, S.T.; Mohana, K.P. Optical coherence tomography in papilledema and pseudopapilledema with and without optic nerve head drusen. Indian. J. Ophthalmol. 2014, 62, 1146–1151. [Google Scholar] [CrossRef]
- Johnson, L.N.; Diehl, M.L.; Hamm, C.W.; Sommerville, D.N.; Petroski, G.F. Differentiating optic disc edema from optic nerve head drusen on optical coherence tomography. Arch. Ophthalmol. 2009, 127, 45–49. [Google Scholar] [CrossRef]
- Chen, J.J.; Kardon, R.H. Avoiding Clinical Misinterpretation and Artifacts of Optical Coherence Tomography Analysis of the Optic Nerve, Retinal Nerve Fiber Layer, and Ganglion Cell Layer. J. Neuroophthalmol. 2016, 36, 417–438. [Google Scholar] [CrossRef]
- Spaide, R.F.; Klancnik, J.M., Jr.; Cooney, M.J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015, 133, 45–50. [Google Scholar] [CrossRef]
- Esmaeil, A.; Ali, A.; Almutairi, S.; Alkandari, K.; Behbehani, R.; Alali, A. Congenital optic disc pits and optic disc pit maculopathy: A review. Front. Ophthalmol. 2023, 3, 1222979. [Google Scholar] [CrossRef]
- Jeng-Miller, K.W.; Cestari, D.M.; Gaier, E.D. Congenital anomalies of the optic disc: Insights from optical coherence tomography imaging. Curr. Opin. Ophthalmol. 2017, 28, 579–586. [Google Scholar] [CrossRef]
- Shah, S.D.; Yee, K.K.; Fortun, J.A.; Albini, T. Optic disc pit maculopathy: A review and update on imaging and treatment. Int. Ophthalmol. Clin. 2014, 54, 61–78. [Google Scholar] [CrossRef]
- Prabhu, V.; Mangla, R.; Acharya, I.; Handa, A.; Thadani, A.; Parmar, Y.; Yadav, N.K.; Chhablani, J.; Venkatesh, R. Evaluation of baseline optic disc pit and optic disc coloboma maculopathy features by spectral domain optical coherence tomography. Int. J. Retin. Vitr. 2023, 9, 46. [Google Scholar] [CrossRef]
- Uzel, M.M.; Karacorlu, M. Optic disk pits and optic disk pit maculopathy: A review. Surv. Ophthalmol. 2019, 64, 595–607. [Google Scholar] [CrossRef]
- Hirakata, A.; Inoue, M.; Hiraoka, T.; McCuen, B.W., 2nd. Vitrectomy without laser treatment or gas tamponade for macular detachment associated with an optic disc pit. Ophthalmology 2012, 119, 810–818. [Google Scholar] [CrossRef]
- Margolis, R.; Spaide, R.F. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am. J. Ophthalmol. 2009, 147, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Ohno-Matsui, K.; Hirakata, A.; Inoue, M.; Akiba, M.; Ishibashi, T. Evaluation of congenital optic disc pits and optic disc colobomas by swept-source optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7769–7778. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A.; Shrestha, S.M.; Prasai, G.; Pandit, K.; Bajgai, P.; Agrawal, R.; Gupta, V. Minimally invasive procedure for optic disc pit maculopathy: Vitrectomy with scleral plug and analysis on pattern of resolution. Sci. Rep. 2023, 13, 15724. [Google Scholar] [CrossRef]
- Nakano, Y.; Miki, A.; Honda, S.; Nakamura, M. Polypoidal Choroidal Vasculopathy Associated with Optic Disc Coloboma. Case Rep. Ophthalmol. 2018, 9, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Lingam, G.; Sen, A.C.; Lingam, V.; Bhende, M.; Padhi, T.R.; Xinyi, S. Ocular coloboma-a comprehensive review for the clinician. Eye 2021, 35, 2086–2109. [Google Scholar] [CrossRef]
- Cennamo, G.; Rossi, C.; Ruggiero, P.; de Crecchio, G.; Cennamo, G. Study of the Radial Peripapillary Capillary Network in Congenital Optic Disc Anomalies with Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2017, 176, 1–8. [Google Scholar] [CrossRef]
- Lee, K.M.; Woo, S.J.; Hwang, J.M. Evaluation of congenital excavated optic disc anomalies with spectral-domain and swept-source optical coherence tomography. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Allegrini, D.; Pagano, L.; Ferrara, M.; Borgia, A.; Sorrentino, T.; Montesano, G.; Angi, M.; Romano, M.R. Optic disc drusen: A systematic review: Up-to-date and future perspective. Int. Ophthalmol. 2020, 40, 2119–2127. [Google Scholar] [CrossRef]
- Palmer, E.; Gale, J.; Crowston, J.G.; Wells, A.P. Optic Nerve Head Drusen: An Update. Neuroophthalmology 2018, 42, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Malmqvist, L.; Li, X.Q.; Eckmann, C.L.; Skovgaard, A.M.; Olsen, E.M.; Larsen, M.; Munch, I.C.; Hamann, S. Optic Disc Drusen in Children: The Copenhagen Child Cohort 2000 Eye Study. J. Neuroophthalmol. 2018, 38, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Floyd, M.S.; Katz, B.J.; Digre, K.B. Measurement of the scleral canal using optical coherence tomography in patients with optic nerve drusen. Am. J. Ophthalmol. 2005, 139, 664–669. [Google Scholar] [CrossRef]
- Youn, S.; Loshusan, B.; Armstrong, J.J.; Fraser, J.A.; Hamann, S.; Bursztyn, L. A Comparison of Diagnostic Accuracy of Imaging Modalities to Detect Optic Disc Drusen: The Age of Enhanced Depth Imaging Optical Coherence Tomography. Am. J. Ophthalmol. 2023, 248, 137–144. [Google Scholar] [CrossRef]
- Yi, K.; Mujat, M.; Sun, W.; Burnes, D.; Latina, M.A.; Lin, D.T.; Deschler, D.G.; Rubin, P.A.; Park, B.H.; de Boer, J.F.; et al. Imaging of optic nerve head drusen: Improvements with spectral domain optical coherence tomography. J. Glaucoma 2009, 18, 373–378. [Google Scholar] [CrossRef]
- Silverman, A.L.; Tatham, A.J.; Medeiros, F.A.; Weinreb, R.N. Assessment of optic nerve head drusen using enhanced depth imaging and swept source optical coherence tomography. J. Neuroophthalmol. 2014, 34, 198–205. [Google Scholar] [CrossRef]
- Merchant, K.Y.; Su, D.; Park, S.C.; Qayum, S.; Banik, R.; Liebmann, J.M.; Ritch, R. Enhanced depth imaging optical coherence tomography of optic nerve head drusen. Ophthalmology 2013, 120, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Malmqvist, L.; Bursztyn, L.; Costello, F.; Digre, K.; Fraser, J.A.; Fraser, C.; Katz, B.; Lawlor, M.; Petzold, A.; Sibony, P.; et al. The Optic Disc Drusen Studies Consortium Recommendations for Diagnosis of Optic Disc Drusen Using Optical Coherence Tomography. J. Neuroophthalmol. 2018, 38, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Woo, S.J.; Hwang, J.M. Peripapillary Hyperreflective Ovoid Mass-Like Structures: Is It Optic Disc Drusen or Not? J. Neuroophthalmol. 2018, 38, 567–568. [Google Scholar] [CrossRef]
- Traber, G.L.; Weber, K.P.; Sabah, M.; Keane, P.A.; Plant, G.T. Enhanced Depth Imaging Optical Coherence Tomography of Optic Nerve Head Drusen: A Comparison of Cases with and without Visual Field Loss. Ophthalmology 2017, 124, 66–73. [Google Scholar] [CrossRef]
- Li, B.; Li, H.; Huang, Q.; Zheng, Y. Peripapillary hyper-reflective ovoid mass-like structures (PHOMS): Clinical significance, associations, and prognostic implications in ophthalmic conditions. Front. Neurol. 2023, 14, 1190279. [Google Scholar] [CrossRef]
- Heath Jeffery, R.C.; Chen, F.K. Peripapillary hyperreflective ovoid mass-like structures: Multimodal imaging—A review. Clin. Exp. Ophthalmol. 2023, 51, 67–80. [Google Scholar] [CrossRef]
- Bhagat, D.; Garcia, A.; Ying, G.S.; Liu, G.T.; Avery, R.A. Comparison of Ultrasound Characteristics of Peripapillary Hyperreflective Ovoid Mass-Like Structures and Optic Disc Drusen in Children. J. Neuroophthalmol. 2025, 45, 209–214. [Google Scholar] [CrossRef]
- Ghassibi, M.P.; Chien, J.L.; Abumasmah, R.K.; Liebmann, J.M.; Ritch, R.; Park, S.C. Optic Nerve Head Drusen Prevalence and Associated Factors in Clinically Normal Subjects Measured Using Optical Coherence Tomography. Ophthalmology 2017, 124, 320–325. [Google Scholar] [CrossRef]
- Gaier, E.D.; Rizzo, J.F., 3rd; Miller, J.B.; Cestari, D.M. Focal Capillary Dropout Associated With Optic Disc Drusen Using Optical Coherence Tomographic Angiography. J. Neuroophthalmol. 2017, 37, 405–410. [Google Scholar] [CrossRef]
- Cennamo, G.; Tebaldi, S.; Amoroso, F.; Arvanitis, D.; Breve, M.; Cennamo, G. Optical Coherence Tomography Angiography in Optic Nerve Drusen. Ophthalmic Res. 2018, 59, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, K.M.; Pasol, J.; Rosa, P.R.; Lam, B.L. Differentiating mild papilledema and buried optic nerve head drusen using spectral domain optical coherence tomography. Ophthalmology 2014, 121, 959–963. [Google Scholar] [CrossRef]
- Flores-Rodriguez, P.; Gili, P.; Martin-Rios, M.D. Sensitivity and specificity of time-domain and spectral-domain optical coherence tomography in differentiating optic nerve head drusen and optic disc oedema. Ophthalmic Physiol. Opt. 2012, 32, 213–221. [Google Scholar] [CrossRef]
- Yan, Y.; Liao, Y.J. Updates on ophthalmic imaging features of optic disc drusen, papilledema, and optic disc edema. Curr. Opin. Neurol. 2021, 34, 108–115. [Google Scholar] [CrossRef]
- Costello, F.; Malmqvist, L.; Hamann, S. The Role of Optical Coherence Tomography in Differentiating Optic Disc Drusen from Optic Disc Edema. Asia Pac. J. Ophthalmol. 2018, 7, 271–279. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, K.M.; Hwang, J.M.; Yang, H.K.; Woo, S.J. Morphologic Features of Buried Optic Disc Drusen on En Face Optical Coherence Tomography and Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2020, 213, 125–133. [Google Scholar] [CrossRef]
- Chang, S.; Gregory-Roberts, E.; Chen, R. Retinal detachment associated with optic disc colobomas and morning glory syndrome. Eye 2012, 26, 494–500. [Google Scholar] [CrossRef]
- Inoue, M. Retinal complications associated with congenital optic disc anomalies determined by swept source optical coherence tomography. Taiwan J. Ophthalmol. 2016, 6, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Harasymowycz, P.; Chevrette, L.; Decarie, J.C.; Hanna, N.; Aroichane, M.; Jacob, J.L.; Milot, J.; Homsy, M. Morning glory syndrome: Clinical, computerized tomographic, and ultrasonographic findings. J. Pediatr. Ophthalmol. Strabismus 2005, 42, 290–295. [Google Scholar] [CrossRef]
- Taskintuna, I.; Oz, O.; Teke, M.Y.; Kocak, H.; Firat, E. Morning glory syndrome: Association with moyamoya disease, midline cranial defects, central nervous system anomalies, and persistent hyaloid artery remnant. Retina 2003, 23, 400–402. [Google Scholar] [CrossRef]
- Wu, Y.K.; Wu, T.E.; Peng, P.H.; Cheng, C.K. Quantitative optical coherence tomography findings in a 4-year-old boy with typical morning glory disk anomaly. J. AAPOS 2008, 12, 621–622. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, G.; de Crecchio, G.; Iaccarino, G.; Forte, R.; Cennamo, G. Evaluation of morning glory syndrome with spectral optical coherence tomography and echography. Ophthalmology 2010, 117, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T.; Mitamura, Y.; Katome, T.; Nagasato, D.; Tabuchi, H. Swept-source optical coherence tomographic findings in morning glory syndrome. Retina 2014, 34, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Baksh, B.S.; Silverman, J.I.M.; Dumitrescu, A.V. Morning Glory Disc Anomaly. EyeRounds.org, The University of Iowa. 22 January 2024. Available online: https://EyeRounds.org/cases/354-Morning-Glory-Disc-Anomaly.htm#gsc.tab=0 (accessed on 1 October 2025).
- Ramkumar, H.L.; Verma, R.; Ferreyra, H.A.; Robbins, S.L. Myelinated Retinal Nerve Fiber Layer (RNFL): A Comprehensive Review. Int. Ophthalmol. Clin. 2018, 58, 147–156. [Google Scholar] [CrossRef]
- Tarabishy, A.B.; Alexandrou, T.J.; Traboulsi, E.I. Syndrome of myelinated retinal nerve fibers, myopia, and amblyopia: A review. Surv. Ophthalmol. 2007, 52, 588–596. [Google Scholar] [CrossRef]
- Rao, R.; Turkoglu, E.B.; Say, E.A.T.; Shields, C.L. Clinical Features, Imaging, and Natural History of Myelinated Retinal Nerve Fiber Layer. Retina 2019, 39, 1125–1132. [Google Scholar] [CrossRef]
- Shields, J.A.; Demirci, H.; Mashayekhi, A.; Eagle, R.C., Jr.; Shields, C.L. Melanocytoma of the optic disk: A review. Indian. J. Ophthalmol. 2019, 67, 1949–1958. [Google Scholar] [CrossRef]
- Finger, P.T.; Natesh, S.; Milman, T. Optical coherence tomography: Pathology correlation of optic disc melanocytoma. Ophthalmology 2010, 117, 114–119. [Google Scholar] [CrossRef]
- Apinyawasisuk, S.; McCannel, T.; Arnold, A.C. Clinical and Spectral-Domain Optical Coherence Tomography Appearance of Optic Disc Melanocytoma: A New Classification and Differentiation from Pigmented Choroidal Lesions. Ocul. Oncol. Pathol. 2017, 3, 142–148. [Google Scholar] [CrossRef]
- Takkar, B.; Molla, K.; Venkatesh, P. Swept-source optical coherence tomography of an optic disc melanocytoma: The importance of the hyperreflective foci. Indian J. Ophthalmol. 2018, 66, 140–142. [Google Scholar] [CrossRef]
- Kikuchi, I.; Kase, S.; Hashimoto, Y.; Hirooka, K.; Ishida, S. Involvement of circulatory disturbance in optic disk melanocytoma with visual dysfunction. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Raval, V.; Reddy, R.; Kaliki, S.; Das, T.; Singh, A.D. Optic nerve head melanocytoma: Optical coherence tomography/angiography features. Indian J. Ophthalmol. 2021, 69, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Shields, M.B. Gray crescent in the optic nerve head. Am. J. Ophthalmol. 1980, 89, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, O.; Damji, K.F.; Jonasson, F.; Arnarsson, A.; Eysteinsson, T.; Sasaki, H.; Sasaki, K. Epidemiology of the optic nerve grey crescent in the Reykjavik Eye Study. Br. J. Ophthalmol. 2005, 89, 36–39. [Google Scholar] [CrossRef]
- Sayed, M.S.; Margolis, M.; Chen, J.L.; Gregori, G.; Lee, R.K. Shields Gray Crescents Masquerading as Glaucomatous Cupping of the Optic Nerve Head. Ophthalmol. Glaucoma 2018, 1, 99–107. [Google Scholar] [CrossRef]
- Jonas, J.B. Optic nerve grey crescent. Br. J. Ophthalmol. 2005, 89, 3. [Google Scholar] [CrossRef]
- Davies, I.J.; Muir, K.W.; Halabis, J.A.; Stinnett, S.S.; Allingham, R.R.; Shields, M.B. Gray Optic Disc Crescent: Evaluation of Anatomic Correlate by Spectral-Domain OCT. Ophthalmol. Glaucoma 2019, 2, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.A.; Sharpe, G.P.; Vianna, J.R.; Nicolela, M.T.; Chauhan, B.C. Anatomical Features of Gray Crescent. JAMA Ophthalmol. 2018, 136, 1419–1420. [Google Scholar] [CrossRef]
- Reis, A.S.; Sharpe, G.P.; Yang, H.; Nicolela, M.T.; Burgoyne, C.F.; Chauhan, B.C. Optic disc margin anatomy in patients with glaucoma and normal controls with spectral domain optical coherence tomography. Ophthalmology 2012, 119, 738–747. [Google Scholar] [CrossRef]
- Chauhan, B.C.; O’Leary, N.; AlMobarak, F.A.; Reis, A.S.C.; Yang, H.; Sharpe, G.P.; Hutchison, D.M.; Nicolela, M.T.; Burgoyne, C.F. Enhanced detection of open-angle glaucoma with an anatomically accurate optical coherence tomography-derived neuroretinal rim parameter. Ophthalmology 2013, 120, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Rayat, J.; Damji, K.F. Optic nerve gray crescent can confound neuroretinal rim interpretation: Review of the literature. Can. J. Ophthalmol. 2014, 49, 238–242. [Google Scholar] [CrossRef]
- See, J.L.; Nicolela, M.T.; Chauhan, B.C. Rates of neuroretinal rim and peripapillary atrophy area change: A comparative study of glaucoma patients and normal controls. Ophthalmology 2009, 116, 840–847. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Wang, S.; Wang, Y.; Wang, Y.; Jonas, J.B. Characteristics of highly myopic eyes: The Beijing Eye Study. Ophthalmology 2007, 114, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B. Clinical implications of peripapillary atrophy in glaucoma. Curr. Opin. Ophthalmol. 2005, 16, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Jonas, S.B.; Jonas, R.A.; Holbach, L.; Dai, Y.; Sun, X.; Panda-Jonas, S. Parapapillary atrophy: Histological gamma zone and delta zone. PLoS ONE 2012, 7, e47237. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Weinreb, R.N.; Zangwill, L.M.; Suh, M.H. Characteristics of Focal Gamma Zone Parapapillary Atrophy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 17. [Google Scholar] [CrossRef]
- Manjunath, V.; Shah, H.; Fujimoto, J.G.; Duker, J.S. Analysis of peripapillary atrophy using spectral domain optical coherence tomography. Ophthalmology 2011, 118, 531–536. [Google Scholar] [CrossRef]
- Teng, C.C.; De Moraes, C.G.; Prata, T.S.; Liebmann, C.A.; Tello, C.; Ritch, R.; Liebmann, J.M. The region of largest beta-zone parapapillary atrophy area predicts the location of most rapid visual field progression. Ophthalmology 2011, 118, 2409–2413. [Google Scholar] [CrossRef]
- Khreish, M.; Schuman, J.S.; Lee, T.; Ghassabi, Z.; Zambrano, R.; Hu, J.; Ishikawa, H.; Wollstein, G.; Lavinsky, F. Peripapillary Atrophy Area as an Indicator of Glaucomatous Structural and Functional Progression. Transl. Vis. Sci. Technol. 2024, 13, 1. [Google Scholar] [CrossRef]
- Teng, C.C.; De Moraes, C.G.; Prata, T.S.; Tello, C.; Ritch, R.; Liebmann, J.M. Beta-Zone parapapillary atrophy and the velocity of glaucoma progression. Ophthalmology 2010, 117, 909–915. [Google Scholar] [CrossRef]
- Savatovsky, E.; Mwanza, J.C.; Budenz, D.L.; Feuer, W.J.; Vandenbroucke, R.; Schiffman, J.C.; Anderson, D.R.; Ocular Hypertension Treatment, S. Longitudinal changes in peripapillary atrophy in the ocular hypertension treatment study: A case-control assessment. Ophthalmology 2015, 122, 79–86. [Google Scholar] [CrossRef]
- Rath, E.Z.; Rehany, U.; Linn, S.; Rumelt, S. Correlation between optic disc atrophy and aetiology: Anterior ischaemic optic neuropathy vs optic neuritis. Eye 2003, 17, 1019–1024. [Google Scholar] [CrossRef]
- Brasil, O.F.; Brasil, M.V.; Japiassu, R.M.; Biancardi, A.L.; Souza, D.D.; Oliveira, R.C.; Moraes, H.V., Jr. Fundus changes evaluation in degenerative myopia]. Arq. Bras. Oftalmol. 2006, 69, 203–206. [Google Scholar] [CrossRef]
- Chui, T.Y.; Zhong, Z.; Burns, S.A. The relationship between peripapillary crescent and axial length: Implications for differential eye growth. Vis. Res. 2011, 51, 2132–2138. [Google Scholar] [CrossRef]
- Primrose, J. Early signs of the glaucomatous disc. Br. J. Ophthalmol. 1971, 55, 820–825. [Google Scholar] [CrossRef]
- Nonaka, A.; Hangai, M.; Akagi, T.; Mori, S.; Nukada, M.; Nakano, N.; Yoshimura, N. Biometric features of peripapillary atrophy beta in eyes with high myopia. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6706–6713. [Google Scholar] [CrossRef] [PubMed]
- Khodeiry, M.M.; Liu, X.; Sayed, M.S.; Goldhardt, R.; Gregori, G.; Albini, T.A.; Lee, R.K. Peripapillary Halo in Inflammatory Papillitis of Birdshot Chorioretinopathy. Clin. Ophthalmol. 2021, 15, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Warner, C.V.; Syc, S.B.; Stankiewicz, A.M.; Hiremath, G.; Farrell, S.K.; Crainiceanu, C.M.; Conger, A.; Frohman, T.C.; Bisker, E.R.; Balcer, L.J.; et al. The impact of utilizing different optical coherence tomography devices for clinical purposes and in multiple sclerosis trials. PLoS ONE 2011, 6, e22947. [Google Scholar] [CrossRef]
- Giani, A.; Cigada, M.; Choudhry, N.; Deiro, A.P.; Oldani, M.; Pellegrini, M.; Invernizzi, A.; Duca, P.; Miller, J.W.; Staurenghi, G. Reproducibility of retinal thickness measurements on normal and pathologic eyes by different optical coherence tomography instruments. Am. J. Ophthalmol. 2010, 150, 815–824. [Google Scholar] [CrossRef]
- Llanas, S.; Linderman, R.E.; Chen, F.K.; Carroll, J. Assessing the Use of Incorrectly Scaled Optical Coherence Tomography Angiography Images in Peer-Reviewed Studies: A Systematic Review. JAMA Ophthalmol. 2020, 138, 86–94. [Google Scholar] [CrossRef]
- Somfai, G.M.; Salinas, H.M.; Puliafito, C.A.; Fernandez, D.C. Evaluation of potential image acquisition pitfalls during optical coherence tomography and their influence on retinal image segmentation. J. Biomed. Opt. 2007, 12, 041209. [Google Scholar] [CrossRef]
- Ray, R.; Stinnett, S.S.; Jaffe, G.J. Evaluation of image artifact produced by optical coherence tomography of retinal pathology. Am. J. Ophthalmol. 2005, 139, 18–29. [Google Scholar] [CrossRef]
- Li, A.; Thompson, A.C.; Asrani, S. Impact of Artifacts From Optical Coherence Tomography Retinal Nerve Fiber Layer and Macula Scans on Detection of Glaucoma Progression. Am. J. Ophthalmol. 2021, 221, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.; Essaid, L.; Alder, B.D.; Santiago-Turla, C. Artifacts in spectral-domain optical coherence tomography measurements in glaucoma. JAMA Ophthalmol. 2014, 132, 396–402. [Google Scholar] [CrossRef]
- Tariq, Y.M.; Samarawickrama, C.; Pai, A.; Burlutsky, G.; Mitchell, P. Impact of ethnicity on the correlation of retinal parameters with axial length. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4977–4982. [Google Scholar] [CrossRef]
- Mwanza, J.C.; Durbin, M.K.; Budenz, D.L.; Girkin, C.A.; Leung, C.K.; Liebmann, J.M.; Peace, J.H.; Werner, J.S.; Wollstein, G.; Cirrus, O.C.T.N.D.S.G. Profile and predictors of normal ganglion cell-inner plexiform layer thickness measured with frequency-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7872–7879. [Google Scholar] [CrossRef]
- Knight, O.J.; Girkin, C.A.; Budenz, D.L.; Durbin, M.K.; Feuer, W.J.; Cirrus, O.C.T.N.D.S.G. Effect of race, age, and axial length on optic nerve head parameters and retinal nerve fiber layer thickness measured by Cirrus HD-OCT. Arch. Ophthalmol. 2012, 130, 312–318. [Google Scholar] [CrossRef]
- Szanto, D.; Wang, J.K.; Woods, B.; Garvin, M.K.; Johnson, B.A.; Kardon, R.H.; Linton, E.F.; Kupersmith, M.J. Deep Learning Differentiates Papilledema, NAION, and Healthy Eyes with Unsegmented 3D OCT Volumes. Am. J. Ophthalmol. 2025, 277, 249–259. [Google Scholar] [CrossRef]
- An, G.; Omodaka, K.; Hashimoto, K.; Tsuda, S.; Shiga, Y.; Takada, N.; Kikawa, T.; Yokota, H.; Akiba, M.; Nakazawa, T. Glaucoma Diagnosis with Machine Learning Based on Optical Coherence Tomography and Color Fundus Images. J. Healthc. Eng. 2019, 2019, 4061313. [Google Scholar] [CrossRef]
- Balaskas, K.; Glinton, S.; Keenan, T.D.L.; Faes, L.; Liefers, B.; Zhang, G.; Pontikos, N.; Struyven, R.; Wagner, S.K.; McKeown, A.; et al. Prediction of visual function from automatically quantified optical coherence tomography biomarkers in patients with geographic atrophy using machine learning. Sci. Rep. 2022, 12, 15565. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.L.W.; Airody, A.; Sivaprasad, S.; Gale, R.P. Optical coherence tomography imaging biomarkers associated with neovascular age-related macular degeneration: A systematic review. Eye 2023, 37, 2438–2453. [Google Scholar] [CrossRef]
- Nurjanah, T.; Patel, M.; Mar, J.; Holden, D.; Barrett, S.C.; Yannuzzi, N.A. Expanding Application of Optical Coherence Tomography Beyond the Clinic: A Narrative Review. Diagnostics 2025, 15, 1140. [Google Scholar] [CrossRef]
- Foust, J.; McCloud, M.; Narawane, A.; Trout, R.M.; Chen, X.; Dhalla, A.H.; Li, J.D.; Viehland, C.; Draelos, M.; Vajzovic, L.; et al. New Directions for Ophthalmic OCT—Handhelds, Surgery, and Robotics. Transl. Vis. Sci. Technol. 2025, 14, 14. [Google Scholar] [CrossRef]
- Ong, J.; Zarnegar, A.; Corradetti, G.; Singh, S.R.; Chhablani, J. Advances in Optical Coherence Tomography Imaging Technology and Techniques for Choroidal and Retinal Disorders. J. Clin. Med. 2022, 11, 5139. [Google Scholar] [CrossRef]
- Bunod, R.; Lubrano, M.; Pirovano, A.; Chotard, G.; Brasnu, E.; Berlemont, S.; Labbe, A.; Augstburger, E.; Baudouin, C. A Deep Learning System Using Optical Coherence Tomography Angiography to Detect Glaucoma and Anterior Ischemic Optic Neuropathy. J. Clin. Med. 2023, 12, 507. [Google Scholar] [CrossRef]
- Hormel, T.T.; Hwang, T.S.; Bailey, S.T.; Wilson, D.J.; Huang, D.; Jia, Y. Artificial intelligence in OCT angiography. Prog. Retin. Eye Res. 2021, 85, 100965. [Google Scholar] [CrossRef]
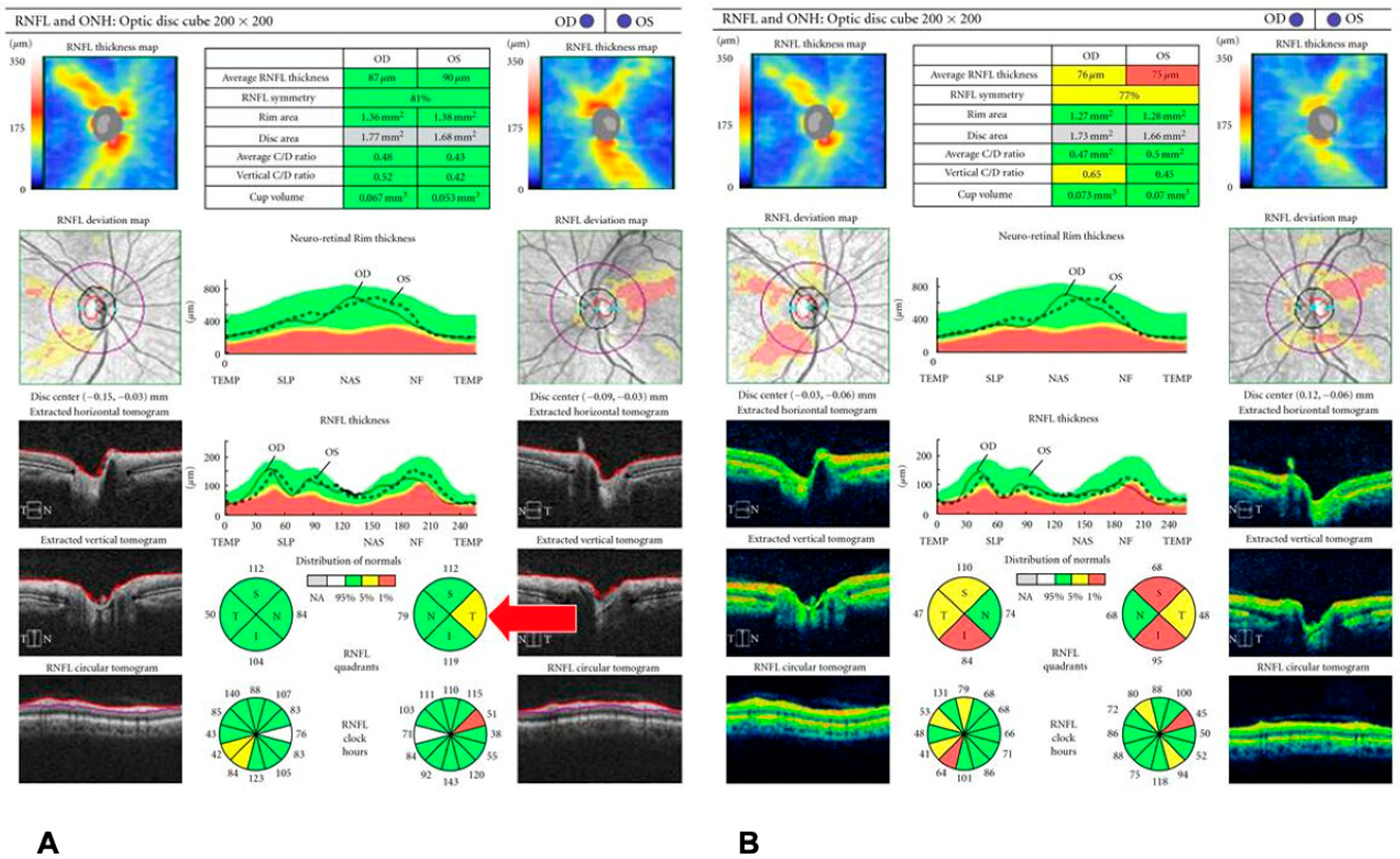
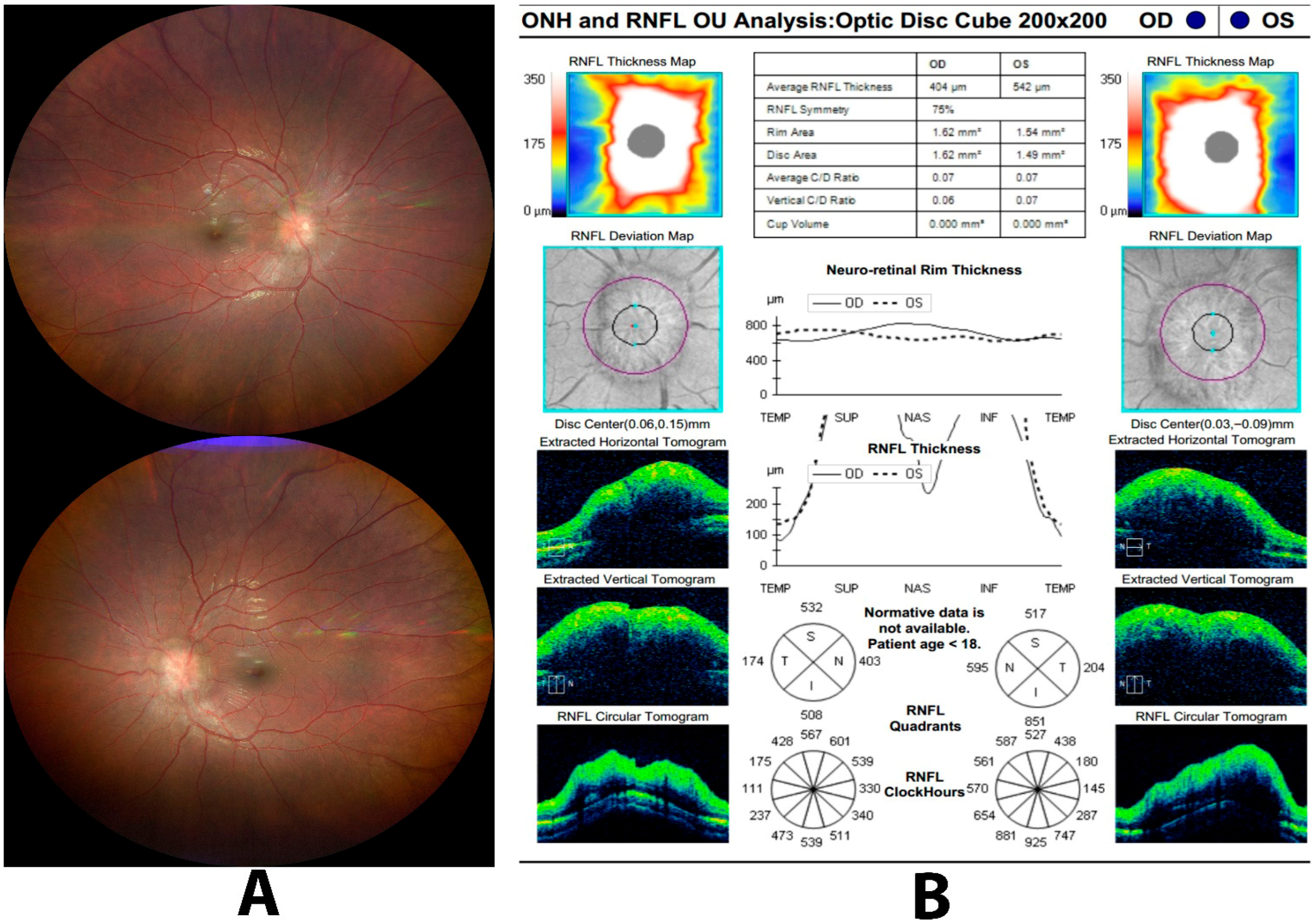
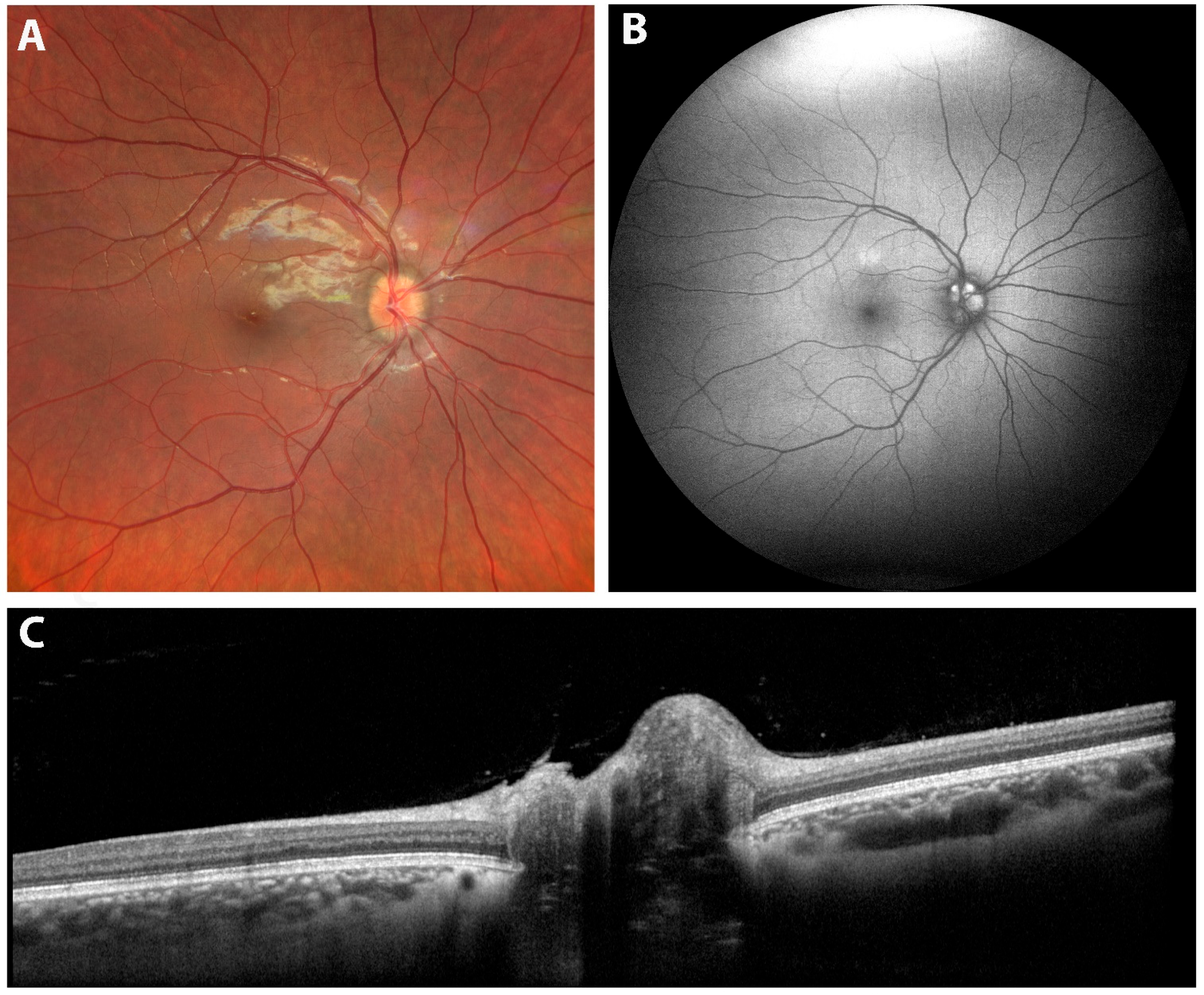
| Condition | Key OCT Findings | Key Learning Point |
|---|---|---|
| 1. Glaucoma | RNFL thinning (especially inferior/superior quadrants); GCIPL loss | OCT and OCT-A provide objective assessment of structural and vascular damage. RNFL and GCIPL analyses enable early detection and progression monitoring, while reduced peripapillary capillary density correlates with disease severity. |
| 2. AION (NAION/AAION) | Acute: RNFL thickening. Chronic: RNFL thinning and GCIPL loss | Sequential OCT changes reflect ischemic damage, acute swelling followed by RNFL thinning and GCIPL loss. OCT-A shows greater vessel density and tortuosity loss in AAION than NAION, reflecting more severe microvascular damage. |
| 3. Optic Neuritis (MS/NMO) | RNFL thickening acutely → Early GCIPL loss | OCT complements MRI by detecting chronic neuroaxonal loss and subclinical progression in MS-related optic neuritis, which MRI may miss. |
| 4. Neuroretinitis | Subretinal and intraretinal fluid; macular star formation | OCT detects early neuroretinitis changes before macular star formation, improving diagnostic accuracy. |
| 5. Papilledema | Diffuse peripapillary RNFL thickening in all quadrants with optic nerve head elevation on B-scan. | RNFL thickening, especially in the nasal quadrant, supports true optic disc edema; OCT-A vessel density correlates with disease severity in IIH. |
| 6. Pseudopapilledema | Normal or only mildly increased average RNFL thickness without diffuse quadrant thickening or true disc elevation. | OCT helps differentiate pseudopapilledema from papilledema, but interpretation must consider refractive error, pediatric norms, and device variability. |
| 7. Optic Pit | Deep excavation of the optic disc with lamina cribrosa defect, herniation of nerve tissue, and associated serous macular detachment or intraretinal schisis. | Advanced OCT (EDI and SS-OCT) enables full visualization of pit morphology and fluid dynamics, improving diagnosis and monitoring of OP maculopathy. |
| 8. Optic Nerve Head Drusen | Signal-poor core with hyperreflective margins above the lamina cribrosa; best visualized on EDI-OCT. | EDI-OCT is the gold standard for drusen detection, distinguishing ONHD from papilledema and revealing detailed drusen morphology. |
| 9. Morning Glory Anomaly | Large, funnel-shaped excavation of the optic disc with peripapillary gliosis, subretinal fluid, and abnormal communication with the subarachnoid space. | OCT reveals characteristic disc excavation and associated retinal detachment, aiding differentiation from other congenital optic nerve anomalies. |
| 10. Myelinated Nerve Fibers | Localized RNFL thickening with hyperreflectivity and shadowing of underlying layers. | OCT confirms myelination within the RNFL and helps differentiate it from true optic disc edema. |
| 11. Melanocytoma | Dome-shaped hyperreflective lesion with posterior shadowing and overlying retinal disorganization; SS-OCT may show choroidal invasion. | OCT and OCT-A help differentiate melanocytoma from malignant lesions by revealing its structural depth, intrinsic vascularity, and benign features. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodeiry, M.M.; Colvin, E.; Ayoubi, M.; Mendoza, X.; Kostic, M. Applications of Optical Coherence Tomography in Optic Nerve Head Diseases: A Narrative Review. Diagnostics 2025, 15, 3001. https://doi.org/10.3390/diagnostics15233001
Khodeiry MM, Colvin E, Ayoubi M, Mendoza X, Kostic M. Applications of Optical Coherence Tomography in Optic Nerve Head Diseases: A Narrative Review. Diagnostics. 2025; 15(23):3001. https://doi.org/10.3390/diagnostics15233001
Chicago/Turabian StyleKhodeiry, Mohamed M., Elizabeth Colvin, Mohammad Ayoubi, Ximena Mendoza, and Maja Kostic. 2025. "Applications of Optical Coherence Tomography in Optic Nerve Head Diseases: A Narrative Review" Diagnostics 15, no. 23: 3001. https://doi.org/10.3390/diagnostics15233001
APA StyleKhodeiry, M. M., Colvin, E., Ayoubi, M., Mendoza, X., & Kostic, M. (2025). Applications of Optical Coherence Tomography in Optic Nerve Head Diseases: A Narrative Review. Diagnostics, 15(23), 3001. https://doi.org/10.3390/diagnostics15233001






