Modeling Working Memory in Neurodegeneration: A Focus on EEG Methods
Abstract
1. Introduction
2. Materials and Methods
- -
- Population: Adult participants (≥18 years), including healthy controls (HC), individuals with mild cognitive impairment (MCI), Alzheimer’s disease (AD), frontotemporal dementia (FTD), and other forms of pathological aging.
- -
- Concept: Analysis of EEG-based working memory phenotypes employing machine learning and deep learning methods. Eligible studies reported spectral power measures, coherence, entropy, event-related potentials (ERP), event-related oscillations (ERO), and metrics of functional connectivity.
- -
- Context: Laboratory cognitive paradigms, clinical investigations of patients with neurodegenerative disorders, and experimental studies involving working memory tasks.
- -
- Original empirical studies (randomized controlled trials, cross-sectional, cohort, and longitudinal designs);
- -
- Publications in peer-reviewed journals;
- -
- Availability of EEG features related to working memory;
- -
- Application of classification or modeling approaches.
- -
- Studies lacking EEG data or not involving a working-memory cognitive paradigm;
- -
- Studies involving participants under 18 years of age;
- -
- Publications without accessible full texts.
- -
- “EEG” AND “working memory” AND “neurodegeneration” AND “Alzheimer’s disease”;
- -
- “EEG” AND “working memory” AND “mild cognitive impairment”;
- -
- “event-related oscillations” OR “event-related potentials” AND “cognitive impairment”;
- -
- “machine learning” OR “deep learning” AND “EEG” AND “working memory”;
- -
- “neurodegeneration” AND “EEG phenotypes” AND “connectivity.”
- -
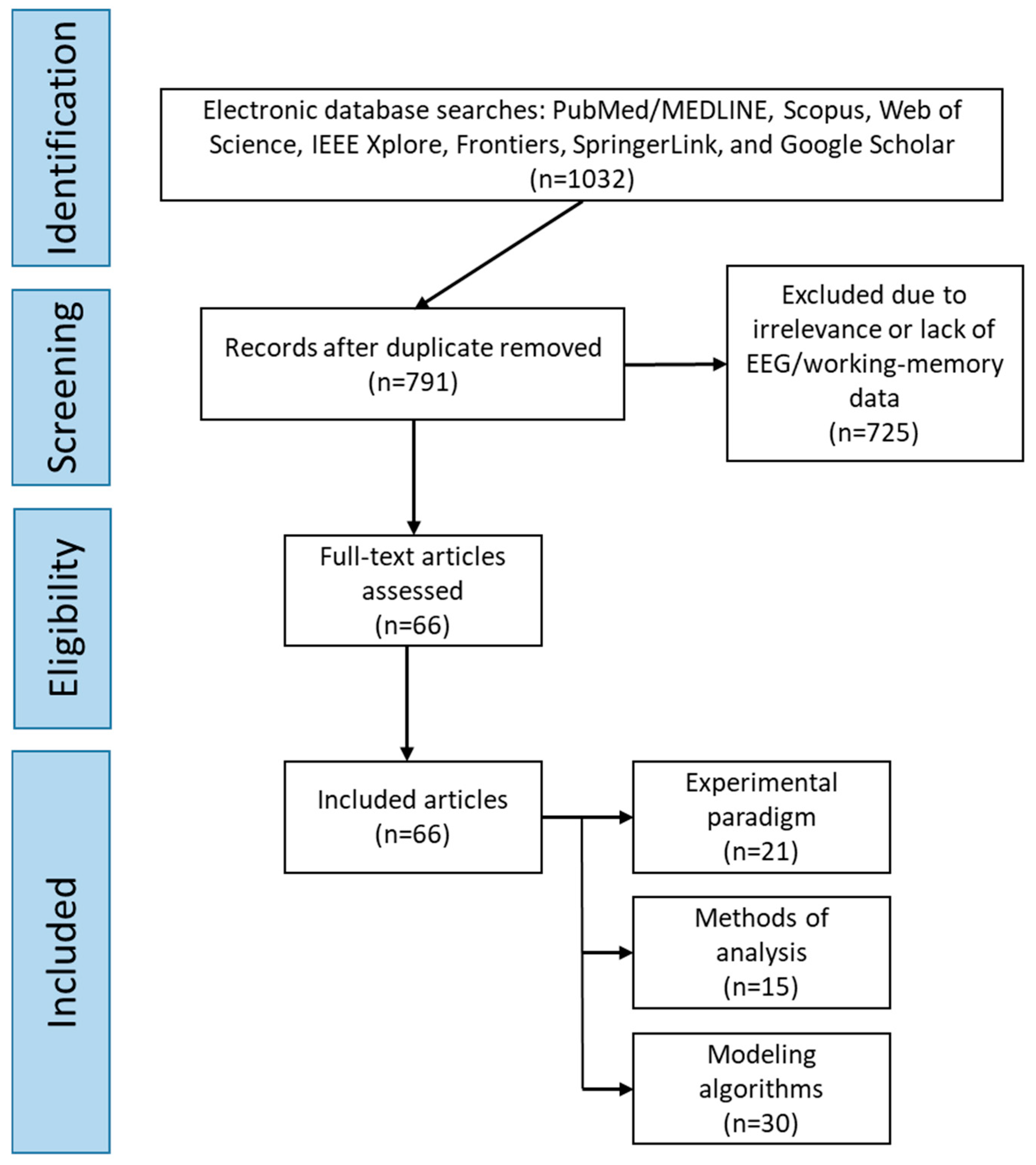
- Total records identified: 1032;
- -
- Duplicates removed: 241;
- -
- Excluded due to irrelevance or lack of EEG/working-memory data: 725;
- -
- Included in the final analysis: 66 studies.
3. Cognitive Impairments and the Role of Working Memory in the Diagnosis of Neurodegenerative Processes
4. Experimental Paradigms of Working Memory
5. EEG Analysis Methods
6. Classification and Modeling Algorithms
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costanzo, M.; Cutrona, C.; Leodori, G.; Malimpensa, L.; D’Antonio, F.; Conte, A.; Belvisi, D. Exploring Easily Accessible Neurophysiological Biomarkers for Predicting Alzheimer’s Disease Progression: A Systematic Review. Alzheimers Res. Ther. 2024, 16, 244. [Google Scholar] [CrossRef]
- Shirolapov, I.V.; Zakharov, A.V.; Smirnova, D.A.; Lyamin, A.V.; Gayduk, A.Y. Correction to: The Role of the Glymphatic Clearance System in the Mechanisms of the Interactions of the Sleep–Waking Cycle and the Development of Neurodegenerative Processes. Neurosci. Behav. Physiol. 2025, 55, 977. [Google Scholar] [CrossRef]
- Abbott, L.F. Lapicque’s Introduction of the Integrate-and-Fire Model Neuron (1907). Brain Res. Bull. 1999, 50, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, A.L.; Huxley, A.F. A Quantitative Description of Membrane Current and Its Application to Conduction and Excitation in Nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef] [PubMed]
- Rall, W. Electrophysiology of a Dendritic Neuron Model. Biophys. J. 1962, 2, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Rall, W. Core Conductor Theory and Cable Properties of Neurons. Compr. Physiol. 2011, 1, 39–97. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, Y. The Role of Quantitative EEG Biomarkers in Alzheimer’s Disease and Mild Cognitive Impairment: Applications and Insights. Front. Aging Neurosci. 2025, 17, 1522552. [Google Scholar] [CrossRef]
- Tanaka, M.; Yamada, E.; Mori, F. Neurophysiological Markers of Early Cognitive Decline in Older Adults: A Mini-Review of Electroencephalography Studies for Precursors of Dementia. Front. Aging Neurosci. 2024, 16, 1486481. [Google Scholar] [CrossRef]
- Liao, K.; Martin, L.E.; Fakorede, S.; Brooks, W.M.; Burns, J.M.; Devos, H. Machine Learning Based on Event-Related Oscillations of Working Memory Differentiates between Preclinical Alzheimer’s Disease and Normal Aging. Clin. Neurophysiol. 2025, 170, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Trinh, T.-T.; Tsai, C.-F.; Yang, J.-K.; Lee, C.-Y.; Wu, C.-T. A Novel Working Memory Task-Induced EEG Response (WM-TIER) Feature Extraction Framework for Detecting Alzheimer’s Disease and Mild Cognitive Impairment. Biosensors 2025, 15, 289. [Google Scholar] [CrossRef]
- Chetty, C.A.; Bhardwaj, H.; Kumar, G.P.; Devanand, T.; Sekhar, C.S.A.; Aktürk, T.; Kiyi, I.; Yener, G.; Güntekin, B.; Joseph, J.; et al. EEG Biomarkers in Alzheimer’s and Prodromal Alzheimer’s: A Comprehensive Analysis of Spectral and Connectivity Features. Alzheimers Res. Ther. 2024, 16, 236. [Google Scholar] [CrossRef]
- Shirolapov, I.; Zakharov, A.; Smirnova, D.; Khivintseva, E.; Sergeeva, M. Aging Brain, Dementia and Impaired Glymphatic Pathway: Causal Relationships. Psychiatr. Danub. 2023, 35 (Suppl. S2), 236–244. [Google Scholar]
- Tahami Monfared, A.A.; Byrnes, M.J.; White, L.A.; Zhang, Q. The Humanistic and Economic Burden of Alzheimer’s Disease. Neurol. Ther. 2022, 11, 525–551. [Google Scholar] [CrossRef]
- Stopford, C.L.; Thompson, J.C.; Neary, D.; Richardson, A.M.; Snowden, J.S. Working Memory, Attention, and Executive Function in Alzheimer’s Disease and Frontotemporal Dementia. Cortex 2012, 48, 429–446. [Google Scholar] [CrossRef]
- Frolov, N.; Pitsik, E.; Grubov, V.; Badarin, A.; Maksimenko, V.; Zakharov, A.; Kurkin, S.; Hramov, A. Perceptual Integration Compensates for Attention Deficit in Elderly during Repetitive Auditory-Based Sensorimotor Task. Sensors 2023, 23, 6420. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.J.; Abd Hamid, A.I.; Abdullah, J.M. Working Memory from the Psychological and Neurosciences Perspectives: A Review. Front. Psychol. 2018, 9, 401. [Google Scholar] [CrossRef]
- Belleville, S.; Chertkow, H.; Gauthier, S. Working Memory and Control of Attention in Persons with Alzheimer’s Disease and Mild Cognitive Impairment. Neuropsychology 2007, 21, 458. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.M. Cognitive Dysfunction in Parkinson’s Disease: The Role of Frontostriatal Circuitry. Neuroscientist 2004, 10, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Kirova, A.M.; Bays, R.B.; Lagalwar, S. Working Memory and Executive Function Decline across Normal Aging, Mild Cognitive Impairment, and Alzheimer’s Disease. Biomed Res. Int. 2015, 2015, 748212. [Google Scholar] [CrossRef]
- Shirolapov, I.V.; Zakharov, A.V.; Bulgakova, S.V.; Khivintseva, E.V.; Sergeeva, M.S.; Romanchuk, N.P.; Pavlova, O.N.; Kazantsev, V.B. Glymphatic Dysfunction in the Pathogenesis of Neurodegenerative Diseases and Pathological Aging. Genes Cells 2023, 18, 309–322. [Google Scholar] [CrossRef]
- Gómez, C.M.; Barriga-Paulino, C.I.; Rodríguez-Martínez, E.I.; Rojas-Benjumea, M.Á.; Arjona, A.; Gómez-González, J. The Neurophysiology of Working Memory Development: From Childhood to Adolescence and Young Adulthood. Rev. Neurosci. 2018, 29, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.A.; Cuevas, K. Using EEG to Study Cognitive Development: Issues and Practices. J. Cogn. Dev. 2012, 13, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Molcho, L.; Maimon, N.B.; Zeimer, T.; Chibotero, O.; Rabinowicz, S.; Armoni, V.; Bar On, N.; Intrator, N.; Sasson, A. Evaluating Cognitive Decline Detection in Aging Populations with Single-Channel EEG Features Based on Two Studies and Meta-Analysis. Sci. Rep. 2025, 15, 25503. [Google Scholar] [CrossRef]
- Dubois, J.; Duffy, J.G.; Field, R.M.; Koch, E.M.; Aghajan, Z.M.; Miller, N.; Perdue, K.L.; Sahagian, G.; Taylor, M. A Functional Neuroimaging Biomarker of Mild Cognitive Impairment Using TD-fNIRS. npj Dement. 2025, 1, 14. [Google Scholar] [CrossRef]
- Goodman, M.S.; Kumar, S.; Zomorrodi, R.; Ghazala, Z.; Cheam, A.S.M.; Barr, M.S.; Daskalakis, Z.J.; Blumberger, D.M.; Fischer, C.; Flint, A.; et al. Theta-Gamma Coupling and Working Memory in Alzheimer’s Dementia and Mild Cognitive Impairment. Front. Aging Neurosci. 2018, 10, 101. [Google Scholar] [CrossRef]
- Fraga, F.J.; Mamani, G.Q.; Johns, E.; Tavares, G.; Falk, T.H.; Phillips, N.A. Early Diagnosis of Mild Cognitive Impairment and Alzheimer’s with Event-Related Potentials and Event-Related Desynchronization in N-Back Working Memory Tasks. Comput. Methods Programs Biomed. 2018, 164, 1–13. [Google Scholar] [CrossRef]
- Kurimoto, R.; Ishii, R.; Canuet, L.; Ikezawa, K.; Iwase, M.; Azechi, M.; Aoki, Y.; Ikeda, S.; Yoshida, T.; Takahashi, H.; et al. Induced Oscillatory Responses during the Sternberg’s Visual Memory Task in Patients with Alzheimer’s Disease and Mild Cognitive Impairment. Neuroimage 2012, 59, 4132–4140. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Schmitt, F.A.; Jicha, G.A.; Munro, N.B.; Zhao, X.; Smith, C.D.; Kryscio, R.J.; Abner, E.L. Memory-Related Frontal Brainwaves Predict Transition to Mild Cognitive Impairment in Healthy Older Individuals Five Years before Diagnosis. J. Alzheimers Dis. 2021, 79, 531–541. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, X.; Guo, Z.; Zhou, X.; He, J.; Jiang, N. Optimizing Electrode Configurations for EEG Mild Cognitive Impairment Detection. Sci. Rep. 2025, 15, 578. [Google Scholar] [CrossRef]
- Vo, T.; Ibrahim, A.K.; Zhuang, H. A Multimodal Multi-Stage Deep Learning Model for the Diagnosis of Alzheimer’s Disease Using EEG Measurements. Neurol. Int. 2025, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.H.; Jeong, Y.H.; Kim, J. Multimodal Multitask Learning for Predicting MCI to AD Conversion Using Stacked Polynomial Attention Network and Adaptive Exponential Decay. Sci. Rep. 2023, 13, 11243. [Google Scholar] [CrossRef]
- Tao, J.X.; Ray, A.; Hawes-Ebersole, S.; Ebersole, J.S. Intracranial EEG Substrates of Scalp EEG Interictal Spikes. Epilepsia 2005, 46, 669–676. [Google Scholar] [CrossRef]
- Beniczky, S.; Schomer, D.L. Electroencephalography: Basic Biophysical and Technological Aspects Important for Clinical Applications. Epileptic Disord. 2020, 22, 697–715. [Google Scholar] [CrossRef] [PubMed]
- Thakor, N.V.; Tong, S. Advances in Quantitative Electroencephalogram Analysis Methods. Annu. Rev. Biomed. Eng. 2004, 6, 453–495. [Google Scholar] [CrossRef] [PubMed]
- Lotte, F.; Bougrain, L.; Cichocki, A.; Clerc, M.; Congedo, M.; Rakotomamonjy, A.; Yger, F. A Review of Classification Algorithms for EEG-Based Brain–Computer Interfaces: A 10 Year Update. J. Neural Eng. 2018, 15, 031005. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Chugh, N. Review of Machine Learning Techniques for EEG Based Brain Computer Interface. Arch. Comput. Methods Eng. 2022, 29, 3001–3020. [Google Scholar] [CrossRef]
- Nardone, R.; Sebastianelli, L.; Versace, V.; Ferrazzoli, D.; Saltuari, L.; Trinka, E. TMS–EEG Co-Registration in Patients with Mild Cognitive Impairment, Alzheimer’s Disease and Other Dementias: A Systematic Review. Brain Sci. 2021, 11, 303. [Google Scholar] [CrossRef]
- Slotnick, S.D. Cognitive Neuroscience of Memory; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Buzsáki, G.; Watson, B.O. Brain Rhythms and Neural Syntax: Implications for Efficient Coding of Cognitive Content and Neuropsychiatric Disease. Dialogues Clin. Neurosci. 2012, 14, 345–367. [Google Scholar] [CrossRef]
- Chapeton, J.I.; Haque, R.; Wittig, J.H.; Inati, S.K.; Zaghloul, K.A. Large-Scale Communication in the Human Brain Is Rhythmically Modulated through Alpha Coherence. Curr. Biol. 2019, 29, 2801–2811. [Google Scholar] [CrossRef]
- Mulert, C. Simultaneous EEG and fMRI: Towards the Characterization of Structure and Dynamics of Brain Networks. Dialogues Clin. Neurosci. 2013, 15, 381–386. [Google Scholar] [CrossRef]
- Jiang, Z.Y. Study on EEG Power and Coherence in Patients with Mild Cognitive Impairment during Working Memory Task. J. Zhejiang Univ. Sci. B 2005, 6, 1213–1219. [Google Scholar] [CrossRef]
- Zheng, L.L.; Jiang, Z.Y.; Yu, E.Y. Alpha Spectral Power and Coherence in the Patients with Mild Cognitive Impairment during a Three-Level Working Memory Task. J. Zhejiang Univ. Sci. B 2007, 8, 584–592. [Google Scholar] [CrossRef]
- Al-Qazzaz, N.K.; Ali, S.; Ahmad, S.A.; Escudero, J. Classification Enhancement for Post-Stroke Dementia Using Fuzzy Neighborhood Preserving Analysis with QR-Decomposition. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; pp. 3174–3177. [Google Scholar] [CrossRef]
- Al-Qazzaz, N.K.; Ali, S.H.B.M.; Ahmad, S.A.; Islam, M.S.; Escudero, J. Discrimination of Stroke-Related Mild Cognitive Impairment and Vascular Dementia Using EEG Signal Analysis. Med. Biol. Eng. Comput. 2018, 56, 137–157. [Google Scholar] [CrossRef]
- Al-Qazzaz, N.K.; Ali, S.H.B.M.; Ahmad, S.A. Working Memory Classification Enhancement of EEG Activity in Dementia: A Comparative Study. Al-Khwarizmi Eng. J. 2023, 19, 29–41. [Google Scholar] [CrossRef]
- Trinh, T.-T.; Tsai, C.-F.; Hsiao, Y.-T.; Lee, C.-Y.; Wu, C.-T.; Liu, Y.-H. Identifying Individuals with Mild Cognitive Impairment Using Working Memory-Induced Intra-Subject Variability of Resting-State EEGs. Front. Comput. Neurosci. 2021, 15, 700467. [Google Scholar] [CrossRef]
- Khatun, S.; Morshed, B.I.; Bidelman, G.M. A Single-Channel EEG-Based Approach to Detect Mild Cognitive Impairment via Speech-Evoked Brain Responses. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1063–1070. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, H.; Chen, Y.; Liu, C.; Zhang, Y.; Zhang, Z.; Jing, R.; Guo, L.; Li, D.; Chu, W.; et al. Small–world network and neuroscience. Brain X 2025, 3, e70025. [Google Scholar] [CrossRef]
- Abdulrasul, H.; Brice, H.; Jasińska, K.K. Developmental timing of adversity and neural network organization: An fNIRS study of the impact of refugee displacement. Dev. Cogn. Neurosci. 2025, 73, 101532. [Google Scholar] [CrossRef]
- Sanz-Arigita, E.J.; Schoonheim, M.M.; Damoiseaux, J.S.; Rombouts, S.A.R.B.; Maris, E.; Barkhof, F.; Scheltens, P.; Stam, C.J. Loss of ‘small-world’ networks in Alzheimer’s disease: Graph analysis of FMRI resting-state functional connectivity. PLoS ONE 2010, 5, e13788. [Google Scholar] [CrossRef] [PubMed]
- Stam, C.V.; Van Straaten, E.C.W. The Organization of Physiological Brain Networks. Clin. Neurophysiol. 2012, 123, 1067–1087. [Google Scholar] [CrossRef] [PubMed]
- Reijneveld, J.C.; Ponten, S.C.; Berendse, H.W.; Stam, C.J. The Application of Graph Theoretical Analysis to Complex Networks in the Brain. Clin. Neurophysiol. 2007, 118, 2317–2331. [Google Scholar] [CrossRef]
- Bellucci, A.; Navarria, L.; Zaltieri, M.; Missale, C.; Spano, P. Alpha-Synuclein Synaptic Pathology and Its Implications in the Development of Novel Therapeutic Approaches to Cure Parkinson’s Disease. Brain Res. 2012, 1432, 95–113. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Rastogi, R.; Shim, K. Efficient Algorithms for Mining Outliers from Large Data Sets. In Proceedings of the 2000 ACM SIGMOD International Conference on Management of Data, Dallas, TX, USA, 15–18 May 2000; pp. 427–438. [Google Scholar] [CrossRef]
- Alcaraz, J.; Labbé, M.; Landete, M. Support Vector Machine with Feature Selection: A Multiobjective Approach. Expert Syst. Appl. 2022, 204, 117485. [Google Scholar] [CrossRef]
- Sridhar, S.; Romney, A.; Manian, V. A Deep Neural Network for Working Memory Load Prediction from EEG Ensemble Empirical Mode Decomposition. Information 2023, 14, 473. [Google Scholar] [CrossRef]
- Ho, T.K.K.; Jeon, Y.; Na, E.; Ullah, Z.; Kim, B.C.; Lee, K.H.; Song, J.; Gwak, J. DeepADNet: A CNN-LSTM Model for the Multi-Class Classification of Alzheimer’s Disease Using Multichannel EEG. Alzheimers Dement. 2021, 17, e057573. [Google Scholar] [CrossRef]
- Xia, W.; Zhang, R.; Zhang, X.; Usman, M. A Novel Method for Diagnosing Alzheimer’s Disease Using Deep Pyramid CNN Based on EEG Signals. Heliyon 2023, 9, e14858. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.J.; Escudero, J.; A Parra, M.; Scally, B.; Anghinah, R.; De Araújo, A.V.L.; Basile, L.F.; Abasolo, D. Deep Learning of Resting-State Electroencephalogram Signals for Three-Class Classification of Alzheimer’s Disease, Mild Cognitive Impairment and Healthy Ageing. J. Neural Eng. 2021, 18, 046087. [Google Scholar] [CrossRef]
- Duan, F.; Huang, Z.; Sun, Z.; Zhang, Y.; Zhao, Q.; Cichocki, A.; Yang, Z.; Sole-Casals, J. Topological Network Analysis of Early Alzheimer’s Disease Based on Resting-State EEG. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Ajra, Z.; Xu, B.; Dray, G.; Montmain, J.; Perrey, S. Using Shallow Neural Networks with Functional Connectivity from EEG Signals for Early Diagnosis of Alzheimer’s and Frontotemporal Dementia. Front. Neurol. 2023, 14, 1270405. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Park, S.; Park, U.; Kang, S.W.; Kang, S.Y. Fatigue in Parkinson’s Disease Is Due to Decreased Efficiency of the Frontal Network: Quantitative EEG Analysis. J. Mov. Disord. 2024, 17, 304. [Google Scholar] [CrossRef]
- Bar-On, M.; Baharav, S.; Katzir, Z.; Mirelman, A.; Sosnik, R.; Maidan, I. Task-Related Reorganization of Cognitive Network in Parkinson’s Disease Using Electrophysiology. Mov. Disord. 2023, 38, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Long-Term Potentiation at CA3–CA1 Hippocampal Synapses with Special Emphasis on Aging, Disease, and Stress. Front. Aging Neurosci. 2011, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Varoquaux, G. Cross-validation failure: Small sample sizes lead to large error bars. Neuroimage 2018, 180, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Poldrack, R.A.; Huckins, G.; Varoquaux, G. Establishment of best practices for evidence for prediction: A review. JAMA Psychiatry 2020, 77, 534–540. [Google Scholar] [CrossRef]
- Varma, S.; Simon, R. Bias in error estimation when using cross-validation for model selection. BMC Bioinform. 2006, 23, 91. [Google Scholar] [CrossRef]
- Toga, A.W.; Thompson, P.M. Mapping brain asymmetry. Nat. Rev. Neurosci. 2004, 4, 37–48. [Google Scholar] [CrossRef]
- Tjoa, E.; Guan, C. A survey on explainable artificial intelligence (xai): Toward medical xai. IEEE Trans. Neural Netw. Learn. Syst. 2020, 32, 4793–4813. [Google Scholar] [CrossRef]
| Authors | Preprocessing Method | Feature Characteristics | Architecture/Algorithm | Sample Size | Classification Task | Accuracy |
|---|---|---|---|---|---|---|
| Sridhar et al. [57] | EEMD (Ensemble Empirical Mode Decomposition) | Intramodal Functions (IMFs) | DNN | 18 HC | HC vs. MCI | 97.62% |
| Thi Kieu Khanh Ho [58] | ERSP extraction (event-related spectral perturbation) | Time–frequency patterns (delta–theta–alpha ranges) | CNN + LSTM (hybrid architecture) | Oddball: 63 (23 HC, 17 aAD, 23 pAD); N-back: 36 (13 HC, 11 aAD, 12 pAD) | Neurodegeneration stage classification | Oddball: 71.95% ± 0.019 (raw), 75.95% ± 0.017 (oversampled); N-back: 69.40% ± 0.003 (raw), 73.70% ± 0.010 (oversampled) |
| Wei Xia [59] | Overlapping sliding windows to augment the one-dimensional EEG | Temporal signal segments; sample augmentation | Deep Pyramid CNN (DPCNN) | 100 (49 AD, 37 MCI, 14 HC) | HC, MCI, AD | 97.10%; F1: 97.11% |
| Cameron J Huggins [60] | Artifact cleaning; CWT (Morse mother wavelet) | Time–frequency maps (0–600), topographic images according to 10–20 system; final dataset: 16,197 images | AlexNet | 141 (52 AD, 37 MCI, 52 HA) | AD vs. MCI vs. HA (healthy ageing) | 98.9% |
| Feng Duan [61] | Frequency-domain analysis (θ, low α, high α); functional connectivity | Global metrics (network resilience), connectivity metrics, node versatility; LOFC bands | ResNet-18 | Datasets: MCI and mild AD (exact n not specified) | HC vs. MCI; HC vs. mild AD | MCI: 93.42% (avg.), up to 98.33% (best); mild AD: 98.54% (avg.), up to 100% (best) |
| Zaineb Ajra [62] | Signal cleaning; extraction of spectral–temporal features and functional connectivity (multiple thresholds) | Functional connectivity | Shallow NN | 88 participants (36 AD, 23 FTD, 29 HC) | AD vs. FTD vs. HC | 94.54% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komarova, Y.; Zakharov, A.; Sergeeva, M.; Romanchuk, N.; Vladimirova, T.; Shirolapov, I. Modeling Working Memory in Neurodegeneration: A Focus on EEG Methods. Diagnostics 2025, 15, 2992. https://doi.org/10.3390/diagnostics15232992
Komarova Y, Zakharov A, Sergeeva M, Romanchuk N, Vladimirova T, Shirolapov I. Modeling Working Memory in Neurodegeneration: A Focus on EEG Methods. Diagnostics. 2025; 15(23):2992. https://doi.org/10.3390/diagnostics15232992
Chicago/Turabian StyleKomarova, Yuliya, Alexander Zakharov, Mariya Sergeeva, Natalia Romanchuk, Tatyana Vladimirova, and Igor Shirolapov. 2025. "Modeling Working Memory in Neurodegeneration: A Focus on EEG Methods" Diagnostics 15, no. 23: 2992. https://doi.org/10.3390/diagnostics15232992
APA StyleKomarova, Y., Zakharov, A., Sergeeva, M., Romanchuk, N., Vladimirova, T., & Shirolapov, I. (2025). Modeling Working Memory in Neurodegeneration: A Focus on EEG Methods. Diagnostics, 15(23), 2992. https://doi.org/10.3390/diagnostics15232992





