Diagnostic Advances and Public Health Challenges for Monkeypox Virus: Clade-Specific Insight and the Urgent Need for Rapid Testing in Africa
Abstract
1. Introduction
2. Virology and Transmission
3. Epidemiology
4. Clinical Presentation
5. Diagnostic Testing Approaches
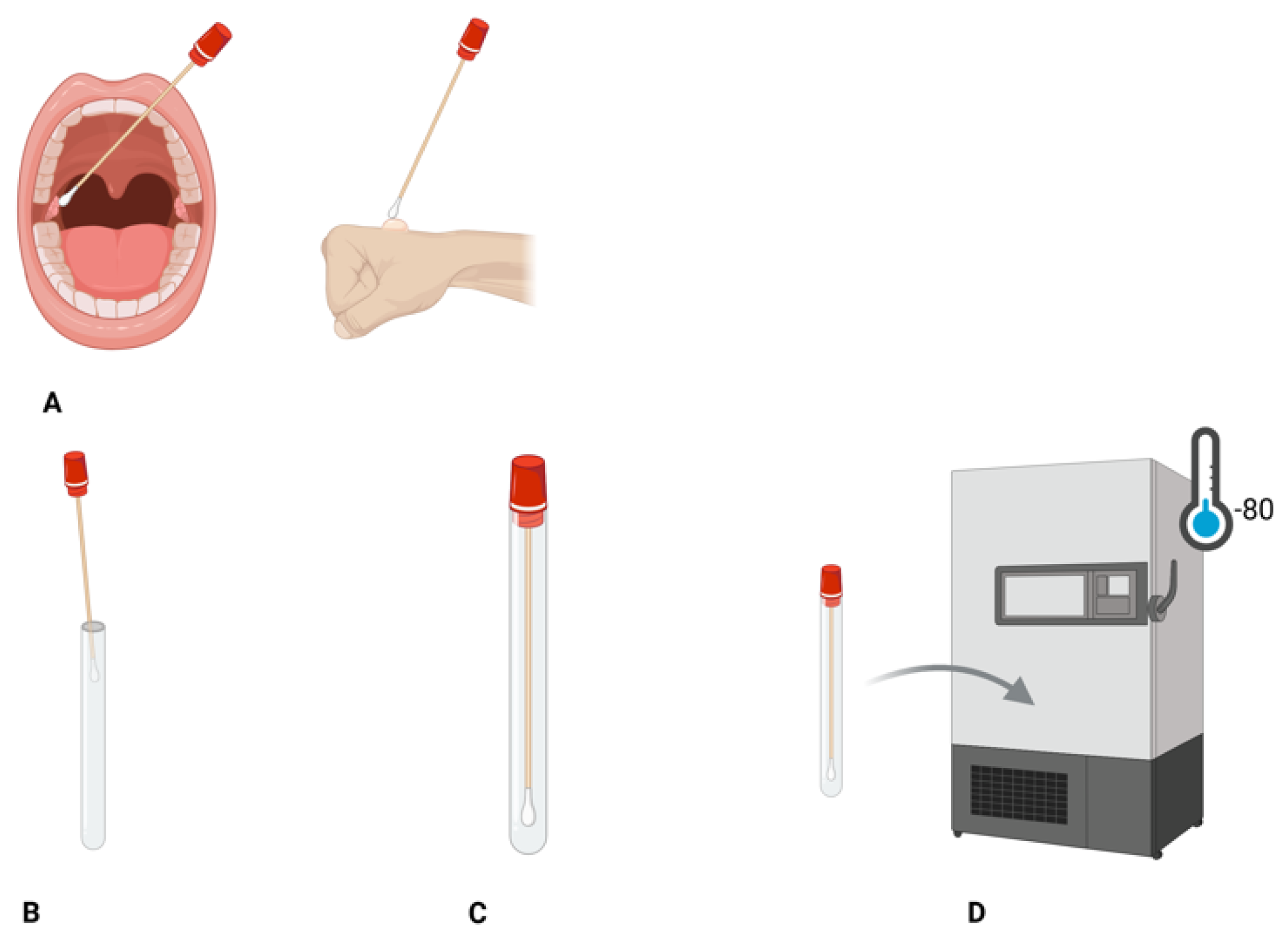
5.1. Sample Collection and Storage
5.2. Real-Time Polymerase Chain Reaction (RT-PCR)
5.3. Molecular Point-of-Care Testing (mPOC)
5.3.1. Serological Testing Method
Enzyme-Linked Immunosorbent Assay
Lateral Flow Assay
5.3.2. Loop-Mediated Isothermal Amplification (LAMP)
5.3.3. Loop-Mediated Isothermal Amplification and Lateral Flow Assay (LAMP-LFA)
5.3.4. Recombinase Polymerase Amplification (RPA)
5.4. CRISPR/CAS-Based Technology
5.5. Viral Culture
5.6. Whole Genome Sequencing
6. Diagnostics Limitations
6.1. Limited Testing Coverage
6.2. Gaps in POC and Rapid Diagnostics
6.3. Reliance on Imported Kits and Medications
6.4. Lack of Clade Multiplexing
6.5. Limited Laboratory Facilities and Discontinuation of the Smallpox Vaccine
7. Conclusions and Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harapan, H.; Ophinni, Y.; Megawati, D.; Frediansyah, A.; Mamada, S.S.; Salampe, M.; Bin Emran, T.; Winardi, W.; Fathima, R.; Sirinam, S.; et al. Monkeypox: A Comprehensive Review. Viruses 2022, 14, 2155. [Google Scholar] [CrossRef]
- Olawade, D.B.; Wada, O.Z.; Fidelis, S.C.; Oluwole, O.S.; Alisi, C.S.; Orimabuyaku, N.F.; Clement David-Olawade, A. Strengthening Africa’s Response to Mpox (Monkeypox): Insights from Historical Outbreaks and the Present Global Spread. Sci. One Health 2024, 3, 100085. [Google Scholar] [CrossRef]
- Okoh, M.P.; Nwachukwu, K.C. A Virulent Disease Called Monkeypox: A Case Report ofCountries in Africa Where The Disease Is Endemic. Indo Glob. J. Pharm. Sci. 2022, 12, 237–244. [Google Scholar] [CrossRef]
- Ben-Enukora, C.; Oyero, O.; Okorie, N.; Odiboh, O.O.; Adeyeye, B.K. Analysis of 2017 Risk Communication on Human Monkey Pox Outbreak in Nigeria’s News Media. Int. J. Educ. Inf. Technol. 2020, 14, 69–75. [Google Scholar] [CrossRef]
- Godinho, F.M.; Bermann, T.D.L.; De Oliveira, M.M.; Barcellos, R.B.; Ruivo, A.P.; De Melo, V.H.; Machado Dos Santos, F.; Bauermann, M.; Selayaran, T.M.; Soares, T.D.S.; et al. Endemic Transmission and International Introduction of Monkeypox Virus in Southern Brazil between 2022-2023. medRxiv 2024. [Google Scholar] [CrossRef]
- NICD: Mpox update Mpox Updates—NICD. Available online: https://www.nicd.ac.za/mpox-updates/ (accessed on 24 October 2025).
- Du, M.; Liu, M.; Liu, J. Mpox Caused by Clade Ib: Epidemiological Characteristics, Prevention, and Control. Chin. Med. J. 2025, 138, 505–508. [Google Scholar] [CrossRef]
- WHO: Mpox–African Region. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON528 (accessed on 28 October 2025).
- WHO Director: WHO Director-General Declares Mpox Outbreak a Public Health Emergency of International Concern. Available online: https://www.who.int/news/item/14-08-2024-who-director-general-declares-mpox-outbreak-a-public-health-emergency-of-international-concern (accessed on 18 August 2025).
- WHO. Global Mpox Trends. 2025. Available online: https://worldhealthorg.shinyapps.io/mpx_global/ (accessed on 28 October 2025).
- WHO: Multi-Country External Situation Report No. 56 2025. Available online: https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report--56---31-july-2025 (accessed on 19 August 2025).
- Okwor, T.; Mbala, P.K.; Evans, D.H.; Kindrachuk, J. A Contemporary Review of Clade-Specific Virological Differences in Monkeypox Viruses. Clin. Microbiol. Infect. 2023, 29, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Karagoz, A.; Tombuloglu, H.; Alsaeed, M.; Tombuloglu, G.; AlRubaish, A.A.; Mahmoud, A.; Smajlović, S.; Ćordić, S.; Rabaan, A.A.; Alsuhaimi, E. Monkeypox (Mpox) Virus: Classification, Origin, Transmission, Genome Organization, Antiviral Drugs, and Molecular Diagnosis. J. Infect. Public Health 2023, 16, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Miura, F.; Murayama, H.; Funk, S.; Wallinga, J.; Lessler, J.; Endo, A. Dynamic Landscape of Mpox Importation Risks Driven by Heavy-Tailed Sexual Contact Networks among Men Who Have Sex with Men in 2022: A Mathematical Modeling Study. medRxiv 2023. [Google Scholar] [CrossRef]
- WHO: Mpox. Available online: https://www.who.int/news-room/fact-sheets/detail/mpox (accessed on 12 August 2025).
- Swed, S.; Bohsas, H.; Alibrahim, H.; Rakab, A.; Hafez, W.; Sawaf, B.; Amir, R.M.; Motawei, A.S.; Aljabali, A.; Shoib, S.; et al. Monkeypox Post-COVID-19: Knowledge, Worrying, and Vaccine Adoption in the Arabic General Population. Vaccines 2023, 11, 759. [Google Scholar] [CrossRef]
- Kumar, S.; Guruparan, D.; Karuppanan, K.; Kumar, K.J.S. Comprehensive Insights into Monkeypox (Mpox): Recent Advances in Epidemiology, Diagnostic Approaches and Therapeutic Strategies. Pathogens 2024, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Subramaniam, G.; Senthil Kumar, K.J. The Resurgence of Monkeypox Clade Ib: A Global Health Emergency and Concern. Arch. Virol. 2025, 170, 84. [Google Scholar] [CrossRef]
- Otieno, J.R.; Ruis, C.; Onoja, A.B.; Kuppalli, K.; Hoxha, A.; Nitsche, A.; Brinkmann, A.; Michel, J.; Mbala-Kingebeni, P.; Mukadi-Bamuleka, D.; et al. Global Genomic Surveillance of Monkeypox Virus. Nat. Med. 2025, 31, 342–350. [Google Scholar] [CrossRef]
- Ogunleye, S.C.; Akinsulie, O.C.; Aborode, A.T.; Olorunshola, M.M.; Gbore, D.; Oladoye, M.; Adesola, R.O.; Gbadegoye, J.O.; Olatoye, B.J.; Lawal, M.A.; et al. The Re-Emergence and Transmission of Monkeypox Virus in Nigeria: The Role of One Health. Front. Public Health 2024, 11, 1334238. [Google Scholar] [CrossRef]
- Suraka, B.; Abubakar, Z.; Ibrahim, D. Monkeypox Virus: Transmission Pathway, Clinical Manifestation, Predisposing Factors Responsible for the Re-Emergence and Spread in Nigeria. Afro-Egypt. J. Infect. Endem. Dis. 2024, 14, 250–258. [Google Scholar] [CrossRef]
- Africa CDC: Outbreak Report, 30 July 2024: Mpox Situation in Africa. 2024. Available online: https://africacdc.org/news-item/mpox-situation-in-africa/ (accessed on 21 August 2025).
- Ndembi, N.; Mbala-Kingebeni, P.; Khalifa, B.; Mazaba, M.L.; Foláyan, M.O. Are There Ecological and Seasonal Factors Influencing the Resurgence of Mpox in Africa? J. Public Health Afr. 2024, 15, 823. [Google Scholar] [CrossRef]
- Moyo, E.; Musuka, G.; Murewanhema, G.; Moyo, P.; Dzinamarira, T. Monkeypox Outbreak: A Perspective on Africa’s Diagnostic and Containment Capacity. Int. J. Infect. Dis. 2022, 123, 127–130. [Google Scholar] [CrossRef]
- Abdul-Rahman, T.; Ghosh, S.; Lawal, L.; Bamigbade, G.B.; Olanrewaju, O.F.; Amarachi, O.R.; Aborode, A.T.; Wireko, A.A.; Faniyi, A.J.; Alao, U.H. Tackling the Resurgence of Monkeypox in Africa: Challenges and Strategies for Eradication. Int. J. Surg. Glob. Health 2024, 7, e0413. [Google Scholar] [CrossRef]
- Hassan, I.N.; Abuassa, N.; Ibrahim, M. Strengthening the Response to the Monkeypox Outbreak in Africa: Addressing Critical Gaps and Challenges. Pathog. Glob. Health 2025, 119, 147–149. [Google Scholar] [CrossRef]
- Sanicas Melvin Mpox in 2025: What We Know, What We Have and What Needs to Happen Next. Available online: https://www.idsociety.org/science-speaks-blog/2025/mpox-in-2025-what-we-know-what-we-have-and-what-needs-to-happen-next/ (accessed on 25 October 2025).
- Chen, S.; Huang, J.; Chen, J.; Liu, F.; Wang, S.; Wang, N.; Li, M.; Zhang, Z.; Huang, C.; Du, W.; et al. Mpox Virus: Virology, Molecular Epidemiology, and Global Public Health Challenges. Front. Microbiol. 2025, 16. [Google Scholar] [CrossRef]
- Yadav, R.; Chaudhary, A.A.; Srivastava, U.; Gupta, S.; Rustagi, S.; Rudayni, H.A.; Kashyap, V.K.; Kumar, S. Mpox 2022 to 2025 Update: A Comprehensive Review on Its Complications, Transmission, Diagnosis, and Treatment. Viruses 2025, 17, 753. [Google Scholar] [CrossRef] [PubMed]
- CDC: Clinical Treatment of Monkeypox. Available online: https://www.cdc.gov/monkeypox/hcp/clinical-care/index.html (accessed on 25 October 2025).
- CDC: Monkeypox in the United States and Around the World: Current Situation. Available online: https://www.cdc.gov/monkeypox/situation-summary/index.html (accessed on 25 October 2025).
- Macmillan Carrie Mpox: What You Need to Know > News > Yale Medicine. Available online: https://www.yalemedicine.org/news/monkeypox-mpox-symptoms-treatment (accessed on 25 October 2025).
- Kulkarni Prathit 2025 Update on Mpox: Epidemiology, Transmission, Diagnosis, Treatment, and Prevention. Available online: https://clinicianresources.bcm.edu/executive-summaries/2025-update-on-mpox-epidemiology-transmission-diagnosis-treatment-and-prevention/ (accessed on 25 October 2025).
- Jin, S.; Asakura, T.R.; Murayama, H.; Jung, S.; Niyukuri, D.; Nyandwi, J.; Nkengurutse, L.; Kamatari, O.; Lim, J.T.; Endo, A.; et al. Disentangling Temporal Trends of Clade Ib Monkeypox Virus Transmission in Burundi. J. Infect. Dis. 2025, jiaf475. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.L.; Isaacs, S.N. Mpox: Keep It on the Differential. Cleve. Clin. J. Med. 2023, 90, 565–575. [Google Scholar] [CrossRef]
- Sharma, A.; Prasad, H.; Kaeley, N.; Bondalapati, A.; Edara, L.; Kumar, Y.A. Monkeypox Epidemiology, Clinical Presentation, and Transmission: A Systematic Review. Int. J. Emerg. Med. 2023, 16, 20. [Google Scholar] [CrossRef]
- CDC: Clinical Features of Mpox. Available online: https://www.cdc.gov/mpox/hcp/clinical-signs/index.html (accessed on 11 August 2025).
- Silva, S.J.R.D.; Kohl, A.; Pena, L.; Pardee, K. Clinical and Laboratory Diagnosis of Monkeypox (Mpox): Current Status and Future Directions. iScience 2023, 26, 106759. [Google Scholar] [CrossRef]
- Yon, H.; Shin, H.; Shin, J.I.; Shin, J.U.; Shin, Y.H.; Lee, J.; Rhee, S.Y.; Koyanagi, A.; Jacob, L.; Smith, L.; et al. Clinical Manifestations of Human Mpox Infection: A Systematic Review and Meta-Analysis. Rev. Med. Virol. 2023, 33, e2446. [Google Scholar] [CrossRef]
- Titanji, B.K.; Hazra, A.; Zucker, J. Mpox Clinical Presentation, Diagnostic Approaches, and Treatment Strategies: A Review. JAMA 2024, 332, 1652–1662. [Google Scholar] [CrossRef]
- Chenchula, S.; Ghanta, M.K.; Amerneni, K.C.; Rajakarunakaran, P.; Chandra, M.B.; Chavan, M.; Gupta, R. A Systematic Review to Identify Novel Clinical Characteristics of Monkeypox Virus Infection and Therapeutic and Preventive Strategies to Combat the Virus. Arch. Virol. 2023, 168, 1–9. [Google Scholar] [CrossRef]
- Tomiyoshi, T. What You Need to Know about the Latest Mpox Outbreak. Available online: https://health.ucdavis.edu/news/headlines/what-you-need-to-know-about-the-latest-mpox-outbreak/2024/09 (accessed on 11 August 2025).
- Mount Sinai: Health Monkeypox Information|Mount Sinai—New York. Available online: https://www.mountsinai.org/health-library/diseases-conditions/monkeypox (accessed on 11 August 2025).
- NICD: Interim Guidelines for the Clinical Recognition, Diagnosis and Management of Mpox in South Africa. 2025. Available online: https://www.nicd.ac.za/key-reference-documents/ (accessed on 11 August 2025).
- Eisenstadt, R.; Liszewski, W.J.; Nguyen, C.V. Recognizing Minimal Cutaneous Involvement or Systemic Symptoms in Monkeypox. JAMA Dermatol. 2022, 158, 1457–1458. [Google Scholar] [CrossRef]
- Tagka, A.; Geronikolou, S.; Evaggelopoulos, A.; Grigoropoulou, S.; Kavatha, D.; Botsi, C.; Papadopoulou, A.; Tryfinopoulou, K.; Katsoulidou, A.; Pappa, S.; et al. Simultaneous Multiple-Stages Mpox Genital Lesions on the Same Site in a Traveler to Greece: A Case Report. Vaccines 2023, 11, 901. [Google Scholar] [CrossRef]
- Hammad, M.A.M.; Miller, J.A.; Magill, R.G.; Fattash, A.T.; Yazar, R.O.; Abou Chawareb, E.; Sanford, D.; Amini, E.; Jenkins, L.; Barham, D.W.; et al. Awareness of Monkeypox Virus among Sexual Medicine Experts Is Low: A Multi-Institutional Survey. Int. J. Impot. Res. 2025, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cleveland Clinic: Mpox (Monkeypox) Causes, Symptoms, Treatment & Prevention. Available online: https://my.clevelandclinic.org/health/diseases/22371-monkeypox (accessed on 25 October 2025).
- Bhandari, G.; Acharya, A.; Chettri, A.K.; Sharma, S. Neurological Manifestations of Mpox Virus during the Recent Global Outbreak: A Systematic Review. BMC Infect. Dis. 2025, 25, 1188. [Google Scholar] [CrossRef] [PubMed]
- Satheshkumar, P.S.; Gigante, C.M.; Mbala-Kingebeni, P.; Nakazawa, Y.; Anderson, M.; Balinandi, S.; Mulei, S.; Fuller, J.; McQuiston, J.H.; McCollum, A.M.; et al. Emergence of Clade Ib Monkeypox Virus—Current State of Evidence. Emerg. Infect. Dis. 2025, 31, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Nzoyikorera, N.; Nduwimana, C.; Schuele, L.; Nieuwenhuijse, D.F.; Koopmans, M.; Otani, S.; Aarestrup, F.M.; Ihorimbere, T.; Niyomwungere, D.; Ndihokubwayo, A.; et al. Monkeypox Clade Ib Virus Introduction into Burundi: First Findings, July to Mid-August 2024. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2024, 29, 2400666. [Google Scholar] [CrossRef]
- Saied, A.A.; Dhawan, M.; Metwally, A.A.; Fahrni, M.L.; Choudhary, P.; Choudhary, O.P. Disease History, Pathogenesis, Diagnostics, and Therapeutics for Human Monkeypox Disease: A Comprehensive Review. Vaccines 2022, 10, 2091. [Google Scholar] [CrossRef]
- Africa CDC Mpox Testing Strategy, 2024. Available online: https://africacdc.org/download/mpox-testing-strategy-november-2024/ (accessed on 12 August 2025).
- Atceken, N.; Bayaki, I.; Can, B.; Yigci, D.; Tasoglu, S. Mpox Disease, Diagnosis, and Point of Care Platforms. Bioeng. Transl. Med. 2025, 10, e10733. [Google Scholar] [CrossRef]
- Grossegesse, M.; Stern, D.; Hofmann, N.; Surtees, R.; Kohl, C.; Michel, J.; Nitsche, A. Serological Methods for the Detection of Antibodies against Monkeypox Virus Applicable for Laboratories with Different Biosafety Levels. J. Med. Virol. 2023, 95, e29261. [Google Scholar] [CrossRef]
- Fan, G.; Kuang, J.; Zhang, S.; Yang, Y.; Liu, Y.; Lu, H. Diagnostic Approaches for Monkeypox Virus. iLABMED 2024, 2, 6–13. [Google Scholar] [CrossRef]
- Liang, M.; Gao, F.; Liu, M.; Zhang, L.; Zheng, Y.; Miao, J.; He, C.; Chen, Z. IC-ELISAs for the Quantitative Detection of Monkeypox Virus Cross-Immunogenic Modified Vaccinia Virus Ankara Antigens L1 and A33. J. Virol. Methods 2025, 338, 115204. [Google Scholar] [CrossRef]
- Laidlaw, S.M.; Ulaeto, D.; Lonsdale, S.; Clark, G.; Sumner, R.; Edwards, T.; Adams, E.; Logist, A.-S.; Van Holm, B.; Maluquer de Motes, C.; et al. Detection of Mpox and Other Orthopoxviruses Using a Lateral Flow Device as a Point-of-Care Diagnostic. Microbiol. Spectr. 2025, 13, e02456-24. [Google Scholar] [CrossRef]
- Madihi, S.; Benani, A. A Comprehensive Review of Current Diagnostic Techniques for Monkeypox Virus Detection. Biologicals 2025, 91, 101841. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xiao, F.; Jia, N.; Sun, C.; Fu, J.; Xu, Z.; Cui, X.; Huang, H.; Qu, D.; Zhou, J.; et al. Loop-Mediated Isothermal Amplification Combined with Lateral Flow Biosensor for Rapid and Sensitive Detection of Monkeypox Virus. Front. Public Health 2023, 11, 1132896. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.D.; Caldas, G.C.; Ferreira, V.N.; Barth, O.M.; da Silva, A.d.P.D.; Silva, M.S.T.; Grinsztejn, B.; Veloso, V.G.; Souza, T.M.; da Silva, E.E.; et al. Monkeypox (Mpox) Virus Isolation and Ultrastructural Characterisation from a Brazilian Human Sample Case. Mem. Inst. Oswaldo Cruz 2023, 118, e230090. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-H.; No, J.S.; Kim, J.-W.; Lee, M.; Shin, H.; Choi, M.-M.; Yi, H.; Rhie, G. Complete Genome Sequence of Monkeypox Virus Strain MPXV-ROK-P1-2022 Isolated from the First Monkeypox Patient in the Republic of Korea. Microbiol. Resour. Announc. 2022, 11, e00853-22. [Google Scholar] [CrossRef]
- Roshdy, W.H.; El-Shesheny, R.; Moatasim, Y.; Kamel, M.N.; Showky, S.; Gomaa, M.; Naguib, A.; El Guindy, N.; Fahim, M.; Khalifa, M.; et al. Whole-Genome Sequence of a Human Monkeypox Virus Strain Detected in Egypt. Microbiol. Resour. Announc. 2023, 12, e00006-23. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, C.; A, R.; Zhao, L.; Zhang, Z.; Tan, W. Perspective on the Application of Genome Sequencing for Monkeypox Virus Surveillance. Virol. Sin. 2023, 38, 327–333. [Google Scholar] [CrossRef]
- Cordeiro, R.; Francisco, R.; Pelerito, A.; Lopes de Carvalho, I.; Núncio, M.S. Mpox Surveillance and Laboratory Response in Portugal: Lessons Learned from Three Outbreak Waves (2022–2025). Infect. Dis. Rep. 2025, 17, 86. [Google Scholar] [CrossRef]
- CDC: Guidelines for Collecting and Handling Specimens for Monkeypox Testing. Available online: https://www.cdc.gov/monkeypox/hcp/diagnosis-testing/collecting-specimens.html (accessed on 27 October 2025).
- PAHO: Laboratory Guidelines for the Detection and Diagnosis of Monkeypox Virus Infection—27 August 2024—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/documents/laboratory-guidelines-detection-and-diagnosis-monkeypox-virus-infection-27-august-2024 (accessed on 12 August 2025).
- WHO Interim: Diagnostic Testing for the Monkeypox Virus (MPXV): Interim Guidance, 10 May 2024. Available online: https://www.who.int/publications/i/item/WHO-MPX-Laboratory-2024.1 (accessed on 12 August 2025).
- Hatmal, M.M.; Al-Hatamleh, M.A.I.; Olaimat, A.N.; Ahmad, S.; Hasan, H.; Ahmad Suhaimi, N.A.; Albakri, K.A.; Abedalbaset Alzyoud, A.; Kadir, R.; Mohamud, R. Comprehensive Literature Review of Monkeypox. Emerg. Microbes Infect. 2022, 11, 2600–2631. [Google Scholar] [CrossRef]
- Mostafa, H.H.; Wall, G.; Su, S.-C.; Hysa, G.; Gong, L.; Dadjeu, U.C.; Cheung, H.; Pekosz, A.; De Smet, D.; Sklenovská, N.; et al. Multi-Center Evaluation of the Research Use Only NeuMoDx Monkeypox Virus (MPXV) Fully Automated Real-Time PCR Assay. J. Clin. Microbiol. 2024, 62, e00028-24. [Google Scholar] [CrossRef]
- Schuele, L.; Masirika, L.M.; Udahemuka, J.C.; Siangoli, F.B.; Mbiribindi, J.B.; Ndishimye, P.; Aarestrup, F.M.; Koopmans, M.; Oude Munnink, B.B.; Molenkamp, R.; et al. Real-Time PCR Assay to Detect the Novel Clade Ib Monkeypox Virus, September 2023 to May 2024. Eurosurveillance 2024, 29, 2400486. [Google Scholar] [CrossRef]
- Hershan, A.A. Virology, Epidemiology, Transmissions, Diagnostic Tests, Prophylaxis and Treatments of Human Mpox: Saudi Arabia Perspective. Front. Cell. Infect. Microbiol. 2025, 15, 1530900. [Google Scholar] [CrossRef]
- Henry, S.; Champagne, M.; Ayouba, A.; Peeters, M.; Rio, L.; Bistoquet, M.; Alcocer Cordellat, C.; Godreuil, S.; Tuaillon, E.; Foulongne, V. Serological Response to Mpox and Direct Virus Detection in Asymptomatic Patient Prior to the First Diagnosed Case: A Retrospective Study of the 2022 Montpellier Epidemic. J. Med. Virol. 2025, 97, e70365. [Google Scholar] [CrossRef]
- Kupritz, J.; Pahwa, S.; Pallikkuth, S. Serosurvey of Immunity to Monkeypox (Mpox) Virus Antigens in People Living with HIV in South Florida. Pathogens 2023, 12, 1355. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-D.; Chao, T.-L.; Chen, K.-H.; Sun, H.-Y.; Lin, K.-Y.; Chuang, Y.-C.; Huang, Y.-S.; Lin, C.-Y.; Hsu, W.-T.; Huang, C.-F.; et al. Short-Term Evolution of Mpox-Specific IgG and Neutralizing Antibodies among Individuals Undergoing MVA-BN Vaccination. Int. J. Infect. Dis. 2025, 153, 107830. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.E.; Hammarlund, E.; Slifka, M.K. Optimization of Peptide-Based ELISA for Serological Diagnostics: A Retrospective Study of Human Monkeypox Infection. Vector Borne Zoonotic Dis. 2012, 12, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.; Saini, G.; Garcia-Leon, A.; Alalwan, D.; Doran, P.; Landay, A.; Nguyen, L.B.L.; O’Broin, C.; Savinelli, S.; O’Halloran, J.A.; et al. Development and Validation of a Quantitative Orthopoxvirus Immunoassay to Evaluate and Differentiate Serological Responses to Mpox Infection and Vaccination. eBioMedicine 2025, 113, 105622. [Google Scholar] [CrossRef]
- Hunt, J.H.; Jones, J.L.; Gebo, K.A.; Hansoti, B.; Traut, C.C.; Hamill, M.M.; Keller, S.C.; Gilliams, E.A.; Manabe, Y.C.; Mostafa, H.H.; et al. Discordant Performance of Mpox Serological Assays. J. Virol. Methods 2024, 329, 115004. [Google Scholar] [CrossRef]
- Sagdat, K.; Batyrkhan, A.; Kanayeva, D. Exploring Monkeypox Virus Proteins and Rapid Detection Techniques. Front. Cell. Infect. Microbiol. 2024, 14. [Google Scholar] [CrossRef]
- Feng, J.; Xue, G.; Cui, X.; Du, B.; Feng, Y.; Cui, J.; Zhao, H.; Gan, L.; Fan, Z.; Fu, T.; et al. Development of a Loop-Mediated Isothermal Amplification Method for Rapid and Visual Detection of Monkeypox Virus. Microbiol. Spectr. 2022, 10, e0271422. [Google Scholar] [CrossRef]
- Yu, C.; Zuo, L.; Miao, J.; Mao, L.; Selekon, B.; Gonofio, E.; Nakoune, E.; Berthet, N.; Wong, G. Development of a Novel Loop-Mediated Isothermal Amplification Method for the Rapid Detection of Monkeypox Virus Infections. Viruses 2022, 15, 84. [Google Scholar] [CrossRef]
- Jang, W.S.; Lee, J.M.; Lee, E.; Park, S.; Lim, C.S. Loop-Mediated Isothermal Amplification and Lateral Flow Immunochromatography Technology for Rapid Diagnosis of Influenza A/B. Diagnostics 2024, 14, 967. [Google Scholar] [CrossRef] [PubMed]
- Millenia-biotech: Loop Mediated Isothermal Amplification (LAMP)—Lateral Flow. Available online: https://www.milenia-biotec.com/en/isothermal-amplification-lateral-flow/ (accessed on 13 September 2025).
- Davi, S.D.; Kissenkötter, J.; Faye, M.; Böhlken-Fascher, S.; Stahl-Hennig, C.; Faye, O.; Faye, O.; Sall, A.A.; Weidmann, M.; Ademowo, O.G.; et al. Recombinase Polymerase Amplification Assay for Rapid Detection of Monkeypox Virus. Diagn. Microbiol. Infect. Dis. 2019, 95, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Chen, X.; Wang, Y.; Liang, J.; Liu, X.; Wang, Y. Rapid, Sensitive, and Highly Specific Detection of Monkeypox Virus by CRISPR-Based Diagnostic Platform. Front. Public Health 2023, 11, 1137768. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Rong, Z.; Gu, Z.; Wei, H.; Wang, Y.; Song, R.; Wang, S.; Wang, S. Detection of Monkeypox Virus Based on a Convenient and Sensitive Single-Step RPA-CRISPR/Cas12a Strategy. RSC Adv. 2024, 14, 14775–14783. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Lin, K.; Han, Y.; Gu, Z.; Wei, H.; Mu, K.; Wang, D.; Liu, L.; Jin, R.; et al. Ultrasensitive Single-Step CRISPR Detection of Monkeypox Virus in Minutes with a Vest-Pocket Diagnostic Device. Nat. Commun. 2024, 15, 3279. [Google Scholar] [CrossRef]
- Low, S.J.; O’Neill, M.T.; Kerry, W.J.; Krysiak, M.; Papadakis, G.; Whitehead, L.W.; Savic, I.; Prestedge, J.; Williams, L.; Cooney, J.P.; et al. Rapid Detection of Monkeypox Virus Using a CRISPR-Cas12a Mediated Assay: A Laboratory Validation and Evaluation Study. Lancet Microbe 2023, 4, e800–e810. [Google Scholar] [CrossRef]
- Ahamed, M.A.; Politza, A.J.; Liu, T.; Khalid, M.A.U.; Zhang, H.; Guan, W. CRISPR-Based Strategies for Sample-to-Answer Monkeypox Detection: Current Status and Emerging Opportunities. Nanotechnology 2025, 36, 042001. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, R.; Hu, X.; Wang, X. The Current Status and Future Prospects of CRISPR-Based Detection of Monkeypox Virus: A Review. Anal. Chim. Acta 2025, 1336, 343295. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, X.; Chen, X.; Huang, J.; Wei, X.; Ying, X.; Tan, Q.; Wang, Y.; Li, S. Development of a CRISPR/Cas12a-Recombinase Polymerase Amplification Assay for Visual and Highly Specific Identification of the Congo Basin and West African Strains of Mpox Virus. J. Med. Virol. 2023, 95, e28757. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, Y.; Yin, B.; Xu, L.; Chen, H.; Qiao, R.; Chen, A.; Zhu, N.; Wu, X. An Ultrasensitive and Specific CRISPR-Cas13a-Mediated Point-of-Care Assay for Monkeypox Detection and PCR-Based Clade Detection. Infect. Dis. Poverty 2025, 14, 56. [Google Scholar] [CrossRef]
- Hirano, R.; Yoshimi, K.; Asano, K.; Takeshita, K.; Ishii, K.J.; Sato, K.; Mashimo, T. Sustainable and Portable CRISPR-Based Diagnostics for High-Sensitivity Mpox Detection. medRxiv 2024. [Google Scholar] [CrossRef]
- Zhao, F.; Hu, Y.; Fan, Z.; Huang, B.; Wei, L.; Xie, Y.; Huang, Y.; Mei, S.; Wang, L.; Wang, L.; et al. Rapid and Sensitive One-Tube Detection of Mpox Virus Using RPA-Coupled CRISPR-Cas12 Assay. Cell Rep. Methods 2023, 3, 100620. [Google Scholar] [CrossRef] [PubMed]
- Venturi, C.; Guadagno, A.; Varesano, S.; Ricucci, V.; Nigro, N.; Di Biagio, A.; Bassetti, M.; Nozza, P.; Drago, F.; Parodi, A.; et al. Histopathologic and Transmission Electron Microscopic Findings in Monkeypox Cutaneous Lesions. Infez. Med. 2024, 32, 76–82. [Google Scholar] [CrossRef]
- Arunagiri, T.; Ganesan, A.; Ravi Kumaran, V.; Mani, S.; Chanduluru, H.K.; Vellapandian, C.; Kannaiah, K.P. Diagnostic Strategies in the Era of Monkeypox Resurgence: A Comprehensive Analysis. Cureus 2024. [Google Scholar] [CrossRef]
- Langat, S.K.; Limbaso, K.; Khamadi, S.; Nyunja, A.; Pilarowski, G.; Okunga, E.; Ofula, V.; Oluniyi, P.; Koka, H.; Koskei, E.; et al. Genomic Sequence Analysis of the First Mpox Virus Detected in Kenya. 2024. Available online: https://www.biorxiv.org/content/10.1101/2024.08.20.608891v1 (accessed on 13 August 2025).
- Isabel, S.; Eshaghi, A.; Duvvuri, V.R.; Gubbay, J.B.; Cronin, K.; Li, A.; Hasso, M.; Clark, S.T.; Hopkins, J.P.; Patel, S.N.; et al. Targeted Amplification-Based Whole Genome Sequencing of Monkeypox Virus in Clinical Specimens. Microbiol. Spectr. 2023, 12, e02979-23. [Google Scholar] [CrossRef]
- WHO: Mpox—Democratic Republic of the Congo. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON522 (accessed on 21 August 2025).
- Ofori, B.; Twum, S.; Nkansah Yeboah, S.; Ansah, F.; Amofa Nketia Sarpong, K. Towards the Development of Cost-Effective Point-of-Care Diagnostic Tools for Poverty-Related Infectious Diseases in Sub-Saharan Africa. PeerJ 2024, 12, e17198. [Google Scholar] [CrossRef]
- Africa CDC. New Test Added to Recommended List of Molecular Diagnostic Tests for Mpox. 2025. Available online: https://africacdc.org/news-item/new-test-added-to-recommended-list-of-molecular-diagnostic-tests-for-mpox/ (accessed on 21 August 2025).
- Impact Global Health: Strengthening Africa’s Mpox Diagnostic Capacity for Enhanced Epidemic Preparedness and Response. Available online: https://www.impactglobalhealth.org//insights/report-library/strengthening-africas-mpox-diagnostic-capacity-for-enhanced-epidemic-preparedness-and-response (accessed on 13 August 2025).
- WHO Ingrim Rapid Response: Clinical Management and Infection Prevention and Control for Monkeypox Interim Rapid Response Guidance 10 June 2022. Available online: https://www.who.int/publications/i/item/WHO-MPX-Clinical-and-IPC-2022.1 (accessed on 15 August 2025).
- Gamo Guerrero, M.; Simón Gozalbo, A.; Martín Díaz, M.; Díez Madueño, K.; Del Río Pena, E.; De la Cueva, P.; Talaván, T.; Jiménez, E.; Torres, J.; Valencia, J.; et al. Interdisciplinary Management of Mpox-Related Local Complications: Report on a Series of Cases. Front. Med. 2023, 10, 1184924. [Google Scholar] [CrossRef]
- Shamim, M.A.; Satapathy, P.; Padhi, B.K.; Veeramachaneni, S.D.; Akhtar, N.; Pradhan, A.; Agrawal, A.; Dwivedi, P.; Mohanty, A.; Pradhan, K.B.; et al. Pharmacological Treatment and Vaccines in Monkeypox Virus: A Narrative Review and Bibliometric Analysis. Front. Pharmacol. 2023, 14, 1149909. [Google Scholar] [CrossRef]
- Shang, W.; Cao, G.; Wu, Y.; Kang, L.; Wang, Y.; Gao, P.; Liu, J.; Liu, M. Spatiotemporal Cluster of Mpox in Men Who Have Sex with Men: A Modeling Study in 83 Countries. J. Med. Virol. 2023, 95, e29166. [Google Scholar] [CrossRef]


| Diagnosis Approaches | Sample Required | Setting | Time | Feature |
|---|---|---|---|---|
| PCR | Skin lesion swabs, Oropharyngeal (throat) swabs | Laboratory setting/POC | 45–60 min | Gold standard [53] |
| LAMP | Skin lesion swabs | POC | 30–60 min | Isothermal, high sensitivity |
| RPA | Skin lesion swabs | POC | 3–15 min | Isothermal, high sensitivity, comparability with multiplex [38] |
| CRISPR/CAS | Skin lesion swabs | POC | 45 min | Atomic sensitivity level, integrated with POC platforms, single-based specificity for the target genome [54] |
| ELISA | Serum, plasma | POC | 2–4 h | Processing large number of samples at once, high sensitivity and specificity [55,56,57] |
| LFA | Serum, plasma, saliva | POC | 10–15 min | RDT, fast, user-friendly and simple, minimal equipment requirement, portable [58,59] |
| MPX-LAMP-LFA | Lesion swabs, blood | POC | 60 min | No cross-reactivity, high specificity, simple and portable [60] |
| Viral isolation | Skin lesion swabs | Laboratory setting | 2–6 days | Viral identification, confirmatory [61] |
| Whole genome sequencing | Skin lesion swabs | Laboratory setting/POC | 12–48 h | Reducing cross-reactivity, high quality, and accuracy [62,63,64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sambo, C.N.; Skepu, A.; Nxumalo, N.P.; Polori, K.L. Diagnostic Advances and Public Health Challenges for Monkeypox Virus: Clade-Specific Insight and the Urgent Need for Rapid Testing in Africa. Diagnostics 2025, 15, 2991. https://doi.org/10.3390/diagnostics15232991
Sambo CN, Skepu A, Nxumalo NP, Polori KL. Diagnostic Advances and Public Health Challenges for Monkeypox Virus: Clade-Specific Insight and the Urgent Need for Rapid Testing in Africa. Diagnostics. 2025; 15(23):2991. https://doi.org/10.3390/diagnostics15232991
Chicago/Turabian StyleSambo, Caroline N., Amanda Skepu, Nolwandle P. Nxumalo, and Ketlareng L. Polori. 2025. "Diagnostic Advances and Public Health Challenges for Monkeypox Virus: Clade-Specific Insight and the Urgent Need for Rapid Testing in Africa" Diagnostics 15, no. 23: 2991. https://doi.org/10.3390/diagnostics15232991
APA StyleSambo, C. N., Skepu, A., Nxumalo, N. P., & Polori, K. L. (2025). Diagnostic Advances and Public Health Challenges for Monkeypox Virus: Clade-Specific Insight and the Urgent Need for Rapid Testing in Africa. Diagnostics, 15(23), 2991. https://doi.org/10.3390/diagnostics15232991





