Comparison of Echocardiography and Invasive Transseptal Catheterization for Assessing Transvalvular Gradient in Patients with Surgical Aortic Valve Prostheses: Fact or Myth?
Abstract
1. Introduction
2. Methods
2.1. Study Population
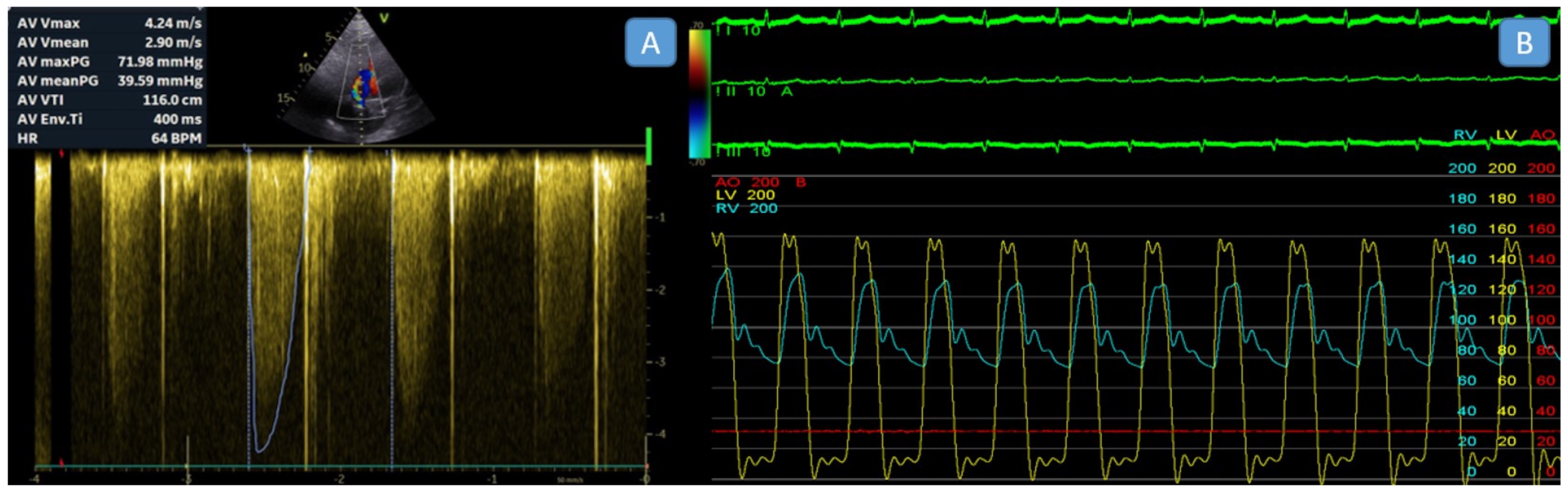
2.2. Echocardiographic Assessment
2.3. Invasive Transseptal Left Heart Catheterization
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kadri, A.N.; Hanzel, G.; Elmariah, S.; Shannon, F.; Al-Azizi, K.; Boura, J.; Mack, M.; Abbas, A.E. Invasive versus echocardiographic gradients in degenerated surgical aortic valve prostheses: A multicenter study. JTCVS Open 2021, 7, 51–60. [Google Scholar] [CrossRef]
- Abbas, A.E.; Mando, R.; Hanzel, G.; Gallagher, M.; Safian, R.; Hanson, I.; Almany, S.; Pibarot, P.; Dalal, P.; Vivacqua, A. Invasive versus echocardiographic evaluation of transvalvular gradients immediately post-transcatheter aortic valve replacement: Demonstration of significant echocardiography-catheterization discordance. Circ. Cardiovasc. Interv. 2019, 12, e007973. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.C.; Pibarot, P.; Wu, C.; Hahn, R.T.; Tang, G.H.L.; Abbas, A.E.; Playford, D.; Ruel, M.; Jilaihawi, H.; Sathananthan, J.; et al. Bioprosthetic Aortic Valve Hemodynamics: Definitions, Outcomes, and Evidence Gaps: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 527–544. [Google Scholar] [CrossRef] [PubMed]
- Bilkhu, R.; Jahangiri, M.; Otto, C.M. Patient-prosthesis mismatch following aortic valve replacement. Heart 2019, 105, s28–s33. [Google Scholar] [CrossRef]
- Hahn, R.T.; Pibarot, P. Prosthesis-patient mismatch in transcatheter and surgical aortic valve replacement. Ann. Cardiothorac. Surg. 2024, 13, 211. [Google Scholar] [CrossRef]
- Biersmith, M.; Alston, M.; Makki, N.; Hatoum, H.; Yeats, B.; Egbuche, O.; Biswas, M.; Orsinelli, D.; Boudoulas, K.D.; Dasi, L. Comparison of Catheterization Versus Echocardiographic-Based Gradients in Balloon-Expandable Versus Self-Expanding Transcatheter Aortic Valve Implantation. J. Invasive Cardiol. 2022, 34, E442–E447. [Google Scholar] [CrossRef]
- Abbas, A.E.; Mando, R.; Hanzel, G.; Goldstein, J.; Shannon, F.; Pibarot, P. Hemodynamic principles of prosthetic aortic valve evaluation in the transcatheter aortic valve replacement era. Echocardiography 2020, 37, 738–757. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Jone, P.N.; Chamsi-Pasha, M.A.; Chen, T.; Collins, K.A.; Desai, M.Y.; Grayburn, P.; Groves, D.W.; Hahn, R.T.; Little, S.H.; et al. Guidelines for the Evaluation of Prosthetic Valve Function with Cardiovascular Imaging: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance and the Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2024, 37, 2–63. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Edvardsen, T.; Delgado, V.; Dulgheru, R.; Pepi, M.; Cosyns, B.; Dweck, M.R.; Garbi, M.; et al. Recommendations for the imaging assessment of prosthetic heart valves: A report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 589–590. [Google Scholar] [CrossRef]
- Abbas, A.E.; Mando, R.; Kadri, A.; Khalili, H.; Hanzel, G.; Shannon, F.; Al-Azizi, K.; Waggoner, T.; Kassas, S.; Pilgrim, T. Comparison of transvalvular aortic mean gradients obtained by intraprocedural echocardiography and invasive measurement in balloon and self-expanding transcatheter valves. J. Am. Heart Assoc. 2021, 10, e021014. [Google Scholar] [CrossRef]
- Pfenniger, A.; Stolte, T.; Reichl, J.J.; Leibundgut, G.; Wagener, M.; Kaiser, C.; Boeddinghaus, J.; Mahfoud, F.; Nestelberger, T. Comparison of invasive and non-invasive gradients before and after TAVI and their implications on clinical outcomes. Cardiovasc. Interv. Ther. 2024, 40, 362–377. [Google Scholar] [CrossRef]
- Lim, M.J.; Kern, M.J. Aortic Stenosis. Hemodynamic Rounds: Interpretation of Cardiac Pathophysiology from Pressure Waveform Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 79–100. [Google Scholar]
- Abbas, A.E.; Khalili, H.; Madanat, L.; Elmariah, S.; Shannon, F.; Al-Azizi, K.; Waggoner, T.; Pilgrim, T.; Okuno, T.; Bavry, A.; et al. Echocardiographic Versus Invasive Aortic Valve Gradients in Different Clinical Scenarios. J. Am. Soc. Echocardiogr. 2023, 36, 1302–1314. [Google Scholar] [CrossRef]
- DeSa, T.B.; Tecson, K.M.; Lander, S.R.; Stoler, R.C.; Vallabhan, R.C.; Hebeler, R.F.; Henry, A.C.; Grayburn, P.A. Comparison of Echocardiographic and Catheter Mean Gradient to Assess Stenosis After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2023, 191, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D. Raised Prosthetic Valve Gradients: What Should be the Approach? J. Indian Acad. Echocardiogr. Cardiovasc. Imaging 2019, 3, 156–162. [Google Scholar] [CrossRef]
- Dietrich, M.; Mankad, R. Elevated Prosthetic Valve Gradients: What to Consider When Determining an Etiology. J. Cardiothorac. Vasc. Anesth. 2021, 35, 2223–2227. [Google Scholar] [CrossRef]
- Joury, A.; Duran, A.; Stewart, M.; Gilliland, Y.E.; Spindel, S.M.; Qamruddin, S. Prosthesis-patient mismatch following aortic and mitral valves replacement—A comprehensive review. Prog. Cardiovasc. Dis. 2022, 72, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Marshall, E.S.; Fanari, Z.; Kostal, M.J.; West, J.T.; Kolm, P.; Weintraub, W.S.; Doorey, A.J. Discrepancies between direct catheter and echocardiography-based values in aortic stenosis. Catheter. Cardiovasc. Interv. 2016, 87, 488–497. [Google Scholar] [CrossRef]
- Schwartzenberg, S.; Sagie, A.; Shapira, Y.; Monakier, D.; Yedidya, I.; Ofek, H.; Kazum, S.; Kornowski, R.; Vaturi, M. Echocardiographic assessment of aortic stenosis under sedation underestimates stenosis severity. J. Am. Soc. Echocardiogr. 2019, 32, 1051–1057. [Google Scholar] [CrossRef]
- Pibarot, P.; Magne, J.; Leipsic, J.; Côté, N.; Blanke, P.; Thourani, V.H.; Hahn, R. Imaging for predicting and assessing prosthesis-patient mismatch after aortic valve replacement. JACC Cardiovasc. Imaging 2019, 12, 149–162. [Google Scholar] [CrossRef]
- Vriesendorp, M.D.; De Lind Van Wijngaarden, R.A.; Head, S.J.; Kappetein, A.-P.; Hickey, G.L.; Rao, V.; Weissman, N.J.; Reardon, M.J.; Moront, M.G.; Sabik, J.F., III. The fallacy of indexed effective orifice area charts to predict prosthesis–patient mismatch after prosthesis implantation. Eur. Heart J.-Cardiovasc. Imaging 2020, 21, 1116–1122. [Google Scholar] [CrossRef]
- Di Biase, L.; Burkhardt, J.D.; Santangeli, P.; Mohanty, P.; Sanchez, J.E.; Horton, R.; Gallinghouse, G.J.; Themistoclakis, S.; Rossillo, A.; Lakkireddy, D.; et al. Periprocedural Stroke and Bleeding Complications in Patients Undergoing Catheter Ablation of Atrial Fibrillation with Different Anticoagulation Management. Circulation 2014, 129, 2638–2644. [Google Scholar] [CrossRef]
- Tzeis, S.; Gerstenfeld, E.P.; Kalman, J.; Saad, E.B.; Sepehri Shamloo, A.; Andrade, J.G.; Barbhaiya, C.R.; Baykaner, T.; Boveda, S.; Calkins, H.; et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. EP Eur. 2024, 26, euae043. [Google Scholar] [CrossRef]
| Variables | (n = 14) |
|---|---|
| Age, years | 49.2 ± 16 |
| Gender, female | 10 (71.4%) |
| Body weight, kg | 80 ± 9.0 |
| Height, m | 165.92 ± 7.9 |
| BMI, kg/m2 | 29.3 ± 5.3 |
| BSA, m2 | 1.91 ± 0.10 |
| Coronary artery disease | 2 (14.3%) |
| Stroke | 1 (7.1%) |
| Hypertension | 4 (28.6%) |
| Index AVR indication | 1 (7.1%) 13 (92.9%) |
| Aortic stenosis + regurgitation | |
| Aortic stenosis | |
| Prosthetic valve type | |
| Mechanic prosthesis | 12 (85.7%) |
| Bioprothesis | 2 (14.3%) |
| Prosthetic valve size | |
| 19 mm | 3 (21.4%) |
| 21 mm | 11 (78.6%) |
| Previous cardiovascular surgery | 1.3 ± 0.6 |
| Echocardiographic parameters | |
| Ascending aorta diameter, mm | 33.4 ± 3.43 |
| LVEDD, mm | 46.14 ± 3.39 |
| LVEF, % | 61.57 ± 4.53% |
| Aortic valvular gradient, peak, mmHg | 77 ± 13.1 |
| Aortic valvular gradient, mean, mmHg | 44.2 ± 8.9 |
| AVA | 0.87 (0.62–1.23) |
| EOA index | 0.47 (0.34–0.62) |
| Time interval between AVR and cardiac catheterization, years | 6.7 (2.5–11.5) |
| Time interval between echocardiography and cardiac catheterization, days | 7.2 (2–19) |
| NYHA class | |
| I | 2 (14.3%) |
| II | 12 (85.7%) |
| BNP level | 89.80 ± 67.5 |
| Invasive transaortic peak-to-peak gradient, mmHg | 47.5 ± 21.74 |
| Patient | Age/Gender (Years) | Echo Mean Gradient (mmHg) | Echo Peak Gradient (mmHg) | Invasive Peak to Peak Gradient (mmHg) | Doppler Velocity Index | Valve Type | Company Name/Size | Fluoroscopic Leaflet Motion |
|---|---|---|---|---|---|---|---|---|
| 1 | 66/F | 42 | 75 | 70 | 0.24 | SAVR/Bileaflet | St. Jude/21 | Normal |
| 2 | 52/F | 52 | 97 | 70 | 0.26 | SAVR/Bileaflet | St. Jude/19 | Reduced |
| 3 | 25/F | 41 | 70 | 65 | 0.23 | Bioprosthetic valve | St. Jude/21 | Normal |
| 4 | 42/F | 52 | 95 | 15 | 0.33 | SAVR/Bileaflet | St. Jude/19 | Normal |
| 5 | 57/F | 61 | 87 | 30 | 0.31 | Bioprosthetic valve | St. Jude/21 | Normal |
| 6 | 67/F | 47 | 84 | 32 | 0.35 | SAVR/Bileaflet | St. Jude/21 | Normal |
| 7 | 40/M | 42 | 77 | 35 | 0.29 | SAVR/Bileaflet | Medtronic-Hall/21 | Normal |
| 8 | 57/F | 42 | 66 | 40 | 0.30 | SAVR/Bileaflet | St. Jude/21 | Normal |
| 9 | 26/M | 32 | 56 | 60 | 0.35 | SAVR/Bileaflet | St. Jude/21 | Normal |
| 10 | 24/M | 35 | 68 | 54 | 0.37 | SAVR/Bileaflet | St. Jude/21 | Normal |
| 11 | 75/F | 60 | 95 | 80 | 0.27 | SAVR/Bileaflet | St. Jude/19 | Reduced |
| 12 | 58/F | 38 | 59 | 19 | 0.33 | SAVR/Bileaflet | St. Jude/21 | Normal |
| 13 | 57/F | 39 | 72 | 19 | 0.20 | SAVR/Bileaflet | St. Jude/21 | Normal |
| 14 | 43/M | 37 | 77 | 65 | 0.20 | SAVR/Bileaflet | Medtronic-Hall/21 | Normal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ates, A.H.; Kivrak, A.; Canpolat, U.; Dogan, M.; Kılıc, G.S.; Menemencioglu, C.; Karakulak, U.N.; Kaya, E.B.; Sahiner, M.L.; Aytemir, K. Comparison of Echocardiography and Invasive Transseptal Catheterization for Assessing Transvalvular Gradient in Patients with Surgical Aortic Valve Prostheses: Fact or Myth? Diagnostics 2025, 15, 2980. https://doi.org/10.3390/diagnostics15232980
Ates AH, Kivrak A, Canpolat U, Dogan M, Kılıc GS, Menemencioglu C, Karakulak UN, Kaya EB, Sahiner ML, Aytemir K. Comparison of Echocardiography and Invasive Transseptal Catheterization for Assessing Transvalvular Gradient in Patients with Surgical Aortic Valve Prostheses: Fact or Myth? Diagnostics. 2025; 15(23):2980. https://doi.org/10.3390/diagnostics15232980
Chicago/Turabian StyleAtes, Ahmet Hakan, Ahmet Kivrak, Ugur Canpolat, Mert Dogan, Gul Sinem Kılıc, Can Menemencioglu, Ugur Nadir Karakulak, Ergun Barıs Kaya, Mehmet Levent Sahiner, and Kudret Aytemir. 2025. "Comparison of Echocardiography and Invasive Transseptal Catheterization for Assessing Transvalvular Gradient in Patients with Surgical Aortic Valve Prostheses: Fact or Myth?" Diagnostics 15, no. 23: 2980. https://doi.org/10.3390/diagnostics15232980
APA StyleAtes, A. H., Kivrak, A., Canpolat, U., Dogan, M., Kılıc, G. S., Menemencioglu, C., Karakulak, U. N., Kaya, E. B., Sahiner, M. L., & Aytemir, K. (2025). Comparison of Echocardiography and Invasive Transseptal Catheterization for Assessing Transvalvular Gradient in Patients with Surgical Aortic Valve Prostheses: Fact or Myth? Diagnostics, 15(23), 2980. https://doi.org/10.3390/diagnostics15232980







