Anatomy, Imaging, and Clinical Significance of the Cervicothoracic (Stellate) Ganglion
Abstract
1. Introduction
2. Methods
3. Anatomical Considerations
3.1. Anatomical Terminology
3.2. Basic Anatomy
| Variation | Prevalence | Clinical Impact | Imaging Recommendation | Key References |
|---|---|---|---|---|
| Ganglion Formation | ||||
| True fusion (C7 + T1) | 37.29–100% | Standard block approach | Ultrasound at C6-C7 | [1,3,23,42,47] |
| Separate ganglia | 14–20% | May require dual injection | MRI for planning | [4,42] |
| Includes T2–T4 | 3–10% | Extended block needed | Consider CT guidance | [42,45] |
| Associated Structures | ||||
| Nerve of Kuntz present | 33–68% | Risk of incomplete block | Thoracoscopic evaluation | [19,22,26] |
| Intermediate/vertebral ganglion | 60–94% | Additional target | High-resolution ultrasound | [20,41,42] |
| Multiple cords of the subclavian ansa | 10–15% | Complex neural pathways | MRI neurography | [48,49] |
| Vascular Relations | ||||
| Aberrant vertebral artery | 8–10% | High complication risk | Mandatory ultrasound with Doppler | [27,30,34,50] |
| Anterior vertebral artery at C6 | >90% | Injection hazard | Color Doppler essential | [34,51] |
| Variant inferior thyroid artery | 5–7% | Hematoma risk | Pre-procedural vessel mapping | [31,52,53,54] |
| Ascending and deep cervical arteries | N/A | [30,50,55,56] | ||
| Positional Variations | ||||
| Supracostal position | Variable | Standard approach effective | Lateral neck radiograph | [46,57] |
| Intrathoracic position | 25% (left > right) | Risk of pneumothorax | CT or fluoroscopy | [43,57] |
| Perforated morphology | 14.5% | Incomplete block possible | High-resolution imaging | [47] |



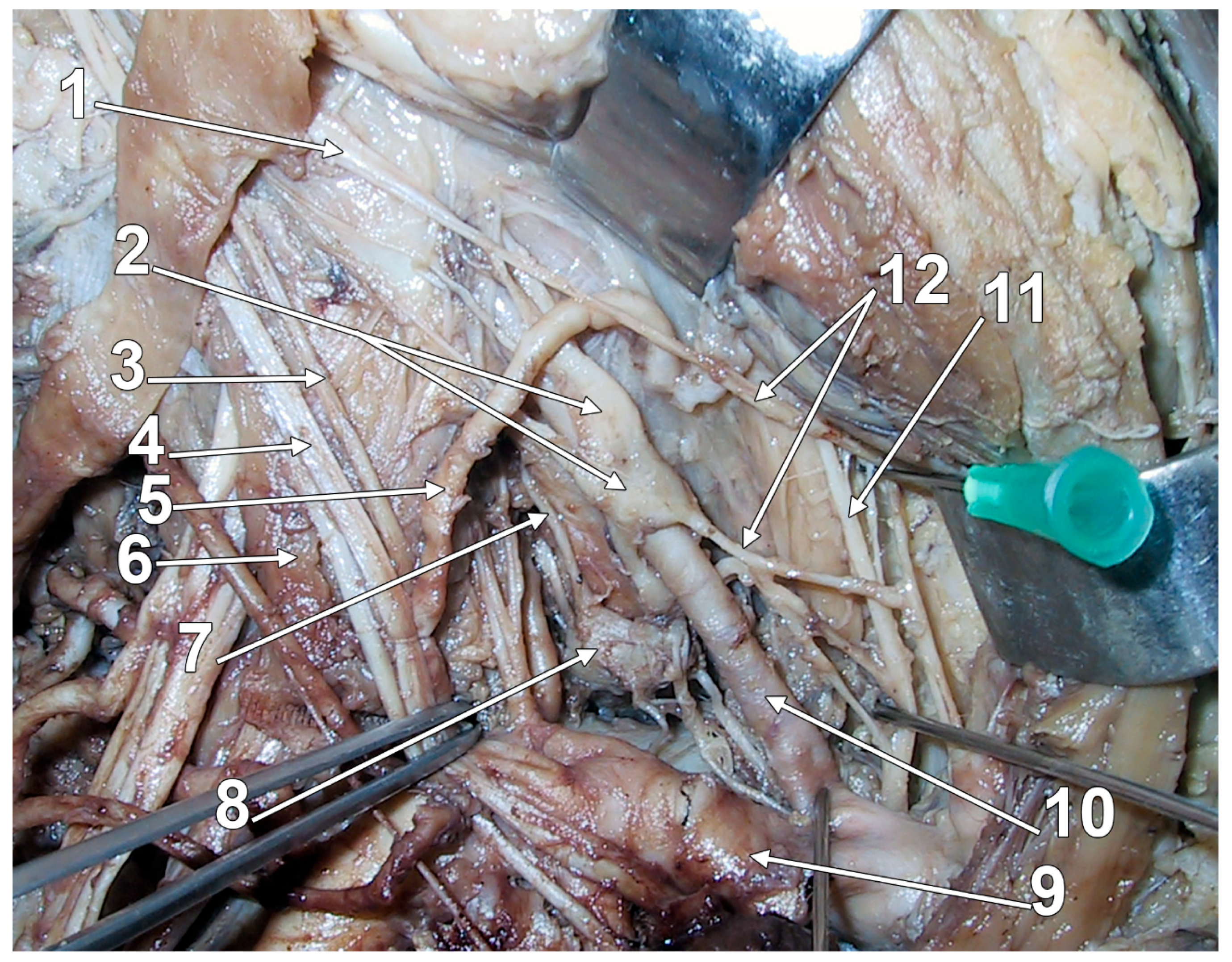
3.3. Connections of the Stellate Ganglion
| Branch | Target/Destination | Characteristics | Clinical Significance | Key References |
|---|---|---|---|---|
| gray communicating rami | C7-T2 spinal nerves (or C8-T2). | Variable number: C7 has 1–5 rami (usually 2); the 3rd ramus may ascend medial to the vertebral artery, traverse C6 foramen with vertebral vessels; an inconstant ramus may traverse C7 foramen. C8 has 3–6 rami. T1 variable. The anterior scalene muscle is an essential relation. | Supply sympathetic innervation to corresponding dermatomes and myotomes. | [40,45] |
| vertebral nerve | C4-C7 spinal nerves (indirect); vertebral artery plexus. | Cranial deep communicating ramus; most commonly connects to C6-C7; it passes through transverse foramina. | Sympathetic innervation of the vertebral artery; possible role in the vertebrobasilar circulation; may be involved in certain headaches. | [75,76,77,78] |
| direct superficial branches | C6-T1 spinal nerves; occasionally C5. | Short connections; superficial course. | Direct sympathetic supply to the upper limb via brachial plexus. | [70] |
| T1 communicating branch | First thoracic nerve. | Contains myelinated fibers from ciliospinal nucleus; short and deep; courses on the pleural dome. | Physiological mydriasis pathway; damage causes Horner’s syndrome; vulnerable in neck surgery and Pancoast tumor. | [70,79] |
| phrenic nerve connection | Phrenic nerve. | Direct connection; almost constant. | Sympathetic influence on the diaphragm; may contribute to respiratory-autonomic integration. | [44,70,80] |
| vagal connections | Vagus nerve. | Almost constant connection; sometimes a direct branch from the SG. | Parasympathetic-sympathetic interaction. | [44,49] |
| recurrent laryngeal connection | Recurrent laryngeal nerve. | Almost constant connection. | Mixed motor, sensory, and sympathetic innervation of larynx. | [44,70] |
| superior cardiac nerve (SN) | Cardiac plexus/Heart. | Originates from: superior cervical ganglion (88.5%) or sympathetic trunk between SG and MG (71.2%). | Sympathetic cardiac innervation; accompanies great vessels (brachiocephalic trunk, common carotid arteries) to reach the heart. | [42] |
| inferior cervical cardiac nerve (IN) | Cardiac plexus/Heart. | Originates from the IG or SG; observed in 86.0%. Course: Descends behind the subclavian artery, along front of trachea to the deep cardiac plexus. Connections: Connects with the recurrent laryngeal nerve and cardiac branch of middle cervical ganglion (or replaced by fine branches from IG and ansa subclavia). | Principal cardiac branch from the SG; consistently present bilaterally; major contributor to the cardiac plexus. | [42,81,82] |
| middle cardiac nerve (MN) | Cardiac plexus/Heart. | Can originate from multiple sources: MG (87.8%), vertebral ganglion (86.0%), sympathetic trunk between MG and SG (76.9%). Includes contributions from the subclavian ansa. | Major cardiac contributor; one of the main sympathetic pathways to the heart; considered a principal component of the human cardiac innervation. | [42] |
| thoracic cardiac nerve (TN) | Cardiac plexus/Heart. | Originates from thoracic ganglia or thoracic sympathetic trunk below SG; observed in 67.3%. | Complex course in the posterior mediastinum; right TN may follow a “recurrent” path along the thoracic aorta; left TN uses the aortic arch. | [42] |
| subclavian artery plexus | Subclavian artery and branches. | Direct vascular branches from the SG to nearby vessels. | Vasomotor control of subclavian territory. | [19,43] |
| brachial plexus pathway | Axillary artery (via brachial plexus). | Indirect vascular branches for the upper limb. | Sympathetic vasomotor control of the upper extremity. | [19,70] |
| internal thoracic artery branch | Internal thoracic artery. | May receive phrenic nerve contribution. | Vascular sympathetic supply. | [45] |
| inferior thyroid artery plexus | Thyroid gland; recurrent laryngeal nerve; external laryngeal nerve; common carotid plexus. | Connects with multiple neural structures. | Complex autonomic-endocrine integration. | [45] |
3.3.1. The Communicating Branches of the SG
3.3.2. The Subclavian Ansa of Vieussens

3.3.3. The Recurrent—Sympathetic Anastomosis
3.3.4. The Phrenic-Stellate Ansa
3.3.5. The Vertebral Nerve
3.3.6. The Subclavian Sympathetic Plexus

3.3.7. The Nerve of Küntz
3.3.8. The Visceral Branches of the SG
3.3.9. Functional Influences of the Stellate Ganglion
3.4. Imaging Anatomy of the SG

| Landmark/Structure | Relationship with the SG | Clinical Note | References |
|---|---|---|---|
| C6 transverse process | 1–2 cm above the SG | common needle entry, not SG level | [112,113,114] |
| C7 transverse process | directly posterior to the SG | true anatomical level of the SG | [60] |
| cricoid cartilage | variable with movement | useful in a neutral position, less in extension | [112,113,114] |
| neck of the first rib | inferior to the SG | lower boundary of the SG | [60] |
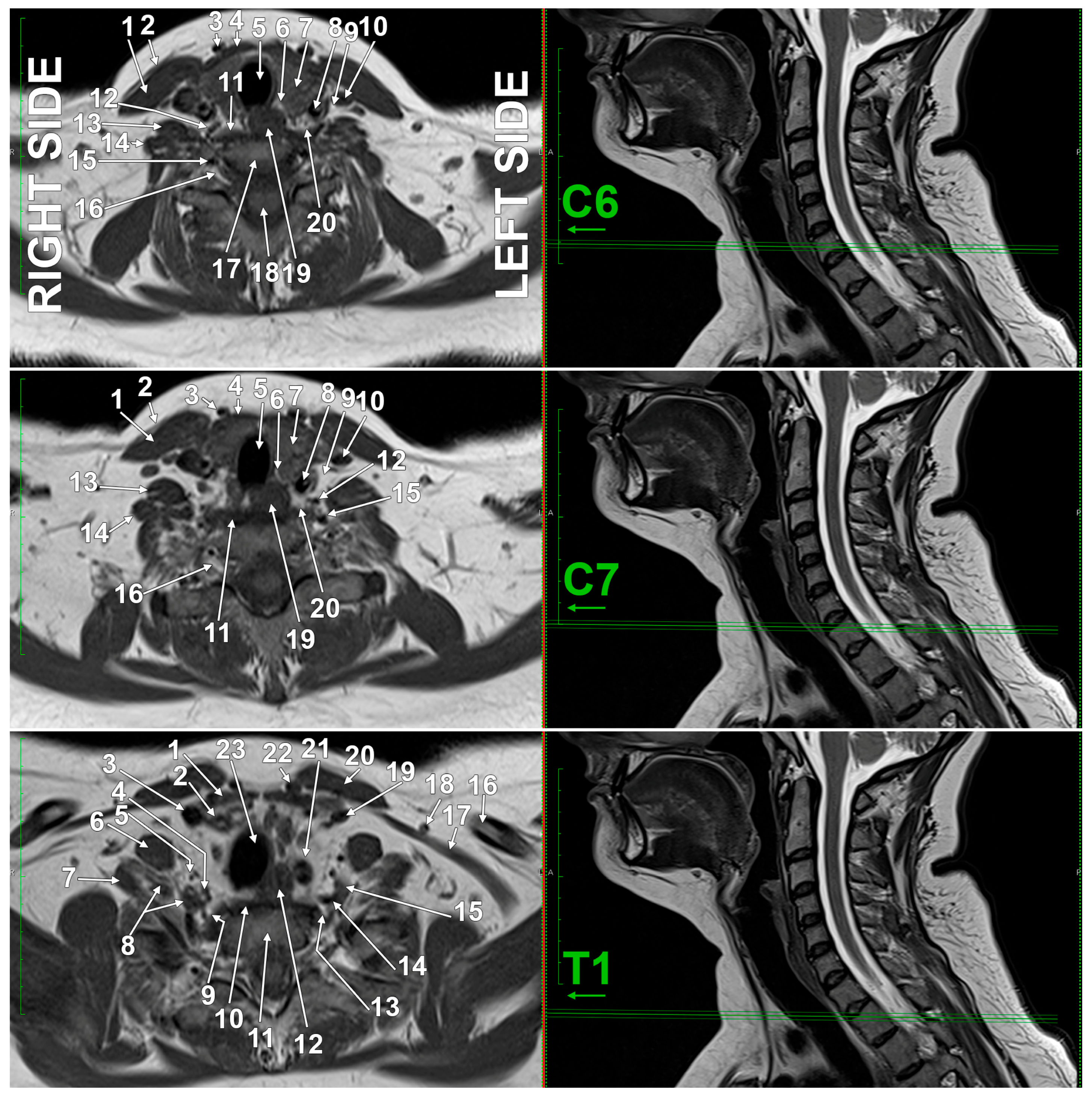
4. Histological Organization of the Stellate Ganglion
5. Clinical Applications
5.1. The Stellate Ganglion Block
| Type | Contraindication | Rationale |
|---|---|---|
| absolute | active anticoagulation therapy | the risk of hemorrhage if vascular structures are inadvertently punctured during needle placement |
| absolute | contralateral pneumothorax or pneumectomy | the procedure carries a risk of iatrogenic pneumothorax, so, if the opposite lung is already compromised, this could result in bilateral pulmonary compromise |
| absolute | recent myocardial infarction | the SGB interrupts cardiac sympathetic innervation (accelerator fibers), which may adversely affect cardiac function in the acute post-infarction period |
| relative | glaucoma | repeated SGBs have been reported to trigger or exacerbate glaucoma in susceptible patients |
| relative | significant cardiac conduction abnormalities | the blockade of upper thoracic sympathetic ganglia can worsen bradycardia by removing sympathetic compensation for impaired conduction |
| Indication | Evidence Level | Study Type (n) | Success Rate | Patient Selection/Contraindications | Alternative Treatments | Key References |
|---|---|---|---|---|---|---|
| CARDIAC ARRHYTHMIAS | ||||||
| Electrical storm | Level B | Cohort studies (n = 147) | 70–80% reduction in VT/VF | Failed antiarrhythmics, hemodynamically stable | Surgical CSD, catheter ablation | [8,9,10] |
| Refractory VT | Level B | Case series (n = 58) | 60–75% ICD shock reduction | >3 ICD shocks/24 h despite medical therapy | Bilateral CSD, RFA of SG | [105,149] |
| PAIN SYNDROMES | ||||||
| CRPS Type I/II | Level A | 2 RCTs (n = 124), SR | 70–85% pain reduction | Failed conservative therapy > 3 months | IV regional blocks, spinal cord stimulation | [6,150] |
| Phantom limb pain | Level B | Case series (n = 42) | 65–70% at 2 months | Post-amputation > 6 months | Mirror therapy, gabapentin, TENS | [151,152] |
| Postherpetic neuralgia | Level B | Retrospective (n = 86) | 60–70% | Pain > 3 months post-rash | Pregabalin, lidocaine patch, RFA | [153,154] |
| Post-mastectomy pain | Level B | RCT (n = 60) | 65–75% | Chronic pain > 6 months post-surgery | Intercostal blocks, gabapentin | [16,153] |
| SLEEP DISORDERS | ||||||
| Primary insomnia | Level B | 3 RCTs (n = 186) | 70–75% PSQI improvement | PSQI > 7, failed behavioral therapy | CBT-I, benzodiazepines, melatonin | [14,155] |
| Postoperative sleep | Level B | 2 RCTs (n = 120) | 65–70% quality improvement | Major surgery, no respiratory compromise | Dexmedetomidine, melatonin | [12,15] |
| Anxiety-related insomnia | Level B | RCT (n = 80) | 68% improvement | GAD with insomnia component | SSRIs, benzodiazepines, CBT | [13] |
| CEREBROVASCULAR | ||||||
| Vasospasm post-SAH | Level C | Pilot RCT (n = 40), series | 60–65% MCA velocity reduction | Hunt-Hess grade II-IV, day 3–14 | Triple-H therapy, nimodipine, angioplasty | [156,157,158] |
| Cerebral blood flow | Level C | Case series (n = 25) | Variable improvement | Refractory vasospasm | Intra-arterial verapamil | [159] |
| AUTONOMIC DYSFUNCTION | ||||||
| Hot flashes (menopause) | Level B | SR (n = 245) | 60–65% symptom reduction | Failed hormone therapy or contraindicated | HRT, SSRIs, gabapentin | [5] |
| Hyperhidrosis | Level C | Case reports (n = 18) | 70–80% | Primary palmar, failed topicals | Surgical sympathectomy, botulinum toxin | [160] |
| EMERGING INDICATIONS | ||||||
| PTSD symptoms | Level C | Open label (n = 42) | Variable (40–60%) | Treatment-resistant PTSD | Prazosin, EMDR, prolonged exposure | [17] |
| Sudden hearing loss | Level C | Case series (n = 35) | 45–55% hearing improvement | <72 h onset, failed steroids | Intratympanic steroids, HBO | [161] |
| Vestibular migraine | Level C | Open label (n = 30) | 60% vertigo reduction | >3 attacks/month | Propranolol, topiramate, vestibular rehab | [162] |
5.2. Evidence-Based Indications
5.3. Technical Approach
| Parameter | Recommendation | Evidence Quality | Rationale | Key References |
|---|---|---|---|---|
| Approach | ||||
| Imaging guidance | Ultrasound preferred | High | Reduces complications, real-time visualization | [30,51,143] |
| Entry level | C6 > C7 | Moderate | Avoids pleura, reduces pneumothorax | [18,55,164] |
| Needle angle | In-plane visualization | High | Real-time monitoring, vessel avoidance | [30,155] |
| Lateral vs. medial | Paratracheal preferred | Moderate | Shortest route, fewer complications | [18,46] |
| Volume/concentration | ||||
| Local anesthetic volume | 4–5 ml | Moderate | Balances efficacy/spread | [14,18,165] |
| Concentration | 0.25–0.375% | Low | Reduces motor block | [14,51] |
| Test dose | 0.5–1 mL initial | Low | Detects intravascular injection | [29,168] |
| Patient positioning | ||||
| Head position | Contralateral rotation | Moderate | Increases carotid distance | [166,167] |
| Neck extension | Mild extension | Low | Improves visualization | [51,155] |
| Patient selection | ||||
| Anticoagulation | Hold if INR > 1.5 | High | Reduces hematoma risk | [52,55] |
| Bilateral blocks | Avoid | High | Risk of bilateral RLN/phrenic palsy | [18,169,170] |
| Respiratory compromise | Relative contraindication | Moderate | Risk of phrenic palsy | [161,170] |
| Monitoring | ||||
| Aspiration | Every 3–5 ml | High | Detects vascular puncture | [29,30] |
| Horner’s syndrome | Expected sign | High | Confirms successful block | [142,171] |
| Vascular Doppler | Pre-procedure scan | Moderate | Identifies aberrant vessels | [31,34] |
| Complication | Incidence | Risk Factors | Prevention Strategy | Management | Key References |
|---|---|---|---|---|---|
| Major Complications | |||||
| Vertebral artery injection | <0.5% | Blind technique, C6 approach | US guidance, aspiration | Supportive care | [30,51,168] |
| Pneumothorax | 0.5–1% | Deep needle, low approach | Limit depth, C6 level | Chest tube if >20% | [55,61,164] |
| Seizures (LA toxicity) | <0.1% | Intravascular injection | Aspiration, low volume | Benzodiazepines | [29,168] |
| Spinal/epidural block | <0.1% | Deep medial needle | Lateral approach, US guidance | Supportive care | [18,170] |
| Minor Complications | |||||
| Horner’s syndrome | 90% (expected) | Successful block | N/A (desired effect) | Reassurance | [142,171] |
| Hoarseness (RLN block) | 10–15% | Large volume, spread | Limit to 4–5 ml | Observation | [165,169,172] |
| Phrenic nerve palsy | 5–10% | Lateral spread | Medial approach | Supportive | [161,170] |
| Hematoma | 1–2% | Coagulopathy, vessel injury | Check INR/platelets | Compression | [31,52] |
| Esophageal puncture | Rare | Medial needle placement | US guidance | Conservative | [46,55] |
| Brachial plexus block | 3–5% | Lateral/posterior spread | Precise needle placement | Observation | [3,18] |
| Bilateral Block Risks | |||||
| Bilateral RLN palsy | High risk | Bilateral procedure | Avoid bilateral blocks | Airway support | [18,169] |
| Bilateral phrenic palsy | High risk | Bilateral procedure | Single-sided only | Ventilatory support | [18,170] |
5.4. Complications and Management
6. Special Considerations
7. Knowledge Gaps and Future Research Directions
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Ansa Subclavia (subclavian ansa of Vieussens) |
| BDNF | Brain-Derived Neurotrophic Factor |
| CBT-I | Cognitive Behavioral Therapy for Insomnia |
| CRPS | Complex Regional Pain Syndrome |
| CSD | Cardiac Sympathetic Denervation |
| CT | Computed Tomography |
| Cx43 | Connexin 43 |
| EAAT1/EAAT2 | Excitatory Amino Acid Transporter 1/2 |
| EMDR | Eye Movement Desensitization and Reprocessing |
| GAD | Generalized Anxiety Disorder |
| GDNF | Glial Cell Line-Derived Neurotrophic Factor |
| GFAP | Glial Fibrillary Acidic Protein |
| GLAST | Glutamate Aspartate Transporter |
| GLT1 | Glutamate Transporter 1 |
| GRP | Gastrin-Releasing Peptide |
| HBO | Hyperbaric Oxygen |
| HRT | Hormone Replacement Therapy |
| ICCN | Inferior Cervical Cardiac Nerve |
| ICD | Implantable Cardioverter Defibrillator |
| IG | Intermediate Ganglion |
| IN | Inferior Cervical Cardiac Nerve |
| INR | International Normalized Ratio |
| JAK2STAT3 | Janus Kinase 2 Signal Transducer and Activator of Transcription 3 |
| LA | Local Anesthetic |
| LC | Locus Coeruleus |
| LHA | Lateral Hypothalamic Area |
| MCA | Middle Cerebral Artery |
| MG | Middle Cervical Ganglion |
| MN | Middle Cardiac Nerve |
| MRI | Magnetic Resonance Imaging |
| NGF | Nerve Growth Factor |
| Orai1 | Calcium Release Activated Calcium Modulator 1 |
| PSQI | Pittsburgh Sleep Quality Index |
| PTSD | Post-Traumatic Stress Disorder |
| RFA | Radiofrequency Ablation |
| RLN | Recurrent Laryngeal Nerve |
| SAH | Subarachnoid Hemorrhage |
| SG | Stellate Ganglion |
| SGB | Stellate Ganglion Block |
| SGCs | Satellite Glial Cells |
| SIF | Small Intensely Fluorescent (cells) |
| SN | Superior Cardiac Nerve |
| SOCE | Store-Operated Calcium Entry |
| SR | Systematic Review |
| SSRIs | Selective Serotonin Reuptake Inhibitors |
| STIM1 | Stromal Interaction Molecule 1 |
| TENS | Transcutaneous Electrical Nerve Stimulation |
| TN | Thoracic Cardiac Nerve |
| TOS | Thoracic Outlet Syndrome |
| US | Ultrasound |
| VN | Vertebral Nerve |
| VT/VF | Ventricular Tachycardia/Fibrillation |
References
- Feneis, H.; Dauber, W. Pocket Atlas of Human Anatomy: Based on the International Nomenclature; Thieme: Stuttgart, Germany; New York, NY, USA, 2000. [Google Scholar]
- Pather, N.; Partab, P.; Singh, B.; Satyapal, K.S. Cervico-thoracic ganglion: Its clinical implications. Clin. Anat. 2006, 19, 323–326. [Google Scholar] [CrossRef]
- Samrid, R.; King, M.; Pujol, J.; Reina, M.A.; Iwanaga, J.; Tubbs, R.S. Anatomical and histological classification of the stellate ganglion: Implications for clinical nerve blocks. Surg. Radiol. Anat. 2024, 47, 26. [Google Scholar] [CrossRef] [PubMed]
- Wrete, M. The anatomy of the sympathetic trunks in man. J. Anat. 1959, 93, 448–459. [Google Scholar]
- Feigin, G.; Figueroa, S.V.; Englesakis, M.F.; D’Souza, R.; Hoydonckx, Y.; Bhatia, A. Stellate ganglion block for non-pain indications: A scoping review. Pain. Med. 2023, 24, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cha, J.; Choi, S.N.; Heo, G.; Yoo, Y.; Moon, J.Y. Multicenter Prospective Randomized Comparison of Ultrasound-Guided Stellate Ganglion Versus Thoracic Paravertebral Block for Sympathetic Blockade in Chronic Upper Extremity Pain. Anesth. Analg. 2025, 140, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wen, S.; Tan, X.; Yi, X.; Cao, S. Stellate ganglion intervention for chronic pain: A review. Ibrain 2022, 8, 210–218. [Google Scholar] [CrossRef]
- Nademanee, K.; Taylor, R.; Bailey, W.E.; Rieders, D.E.; Kosar, E.M. Treating electrical storm: Sympathetic blockade versus advanced cardiac life support-guided therapy. Circulation 2000, 102, 742–747. [Google Scholar] [CrossRef]
- Reinertsen, E.; Sabayon, M.; Riso, M.; Lloyd, M.; Spektor, B. Stellate ganglion blockade for treating refractory electrical storm: A historical cohort study. Can. J. Anaesth. 2021, 68, 1683–1689. [Google Scholar] [CrossRef]
- Tian, Y.; Wittwer, E.D.; Kapa, S.; McLeod, C.J.; Xiao, P.; Noseworthy, P.A.; Mulpuru, S.K.; Deshmukh, A.J.; Lee, H.C.; Ackerman, M.J.; et al. Effective Use of Percutaneous Stellate Ganglion Blockade in Patients with Electrical Storm. Circ. Arrhythm. Electrophysiol. 2019, 12, e007118. [Google Scholar] [CrossRef]
- Ding, X.-D.; Ding, Z.-G.; Wang, W.; Liu, Y.-P.; Zhong, J.; Chen, H.-X. Ultrasound guided injections of botulinum toxin type A into stellate ganglion to treat insomnia. Exp. Ther. Med. 2017, 14, 1136–1140. [Google Scholar] [CrossRef]
- Gu, C.; Zhai, M.; Lu, A.; Liu, L.; Hu, H.; Liu, X.; Li, X.; Cheng, X. Ultrasound-guided stellate ganglion block improves sleep quality in elderly patients early after thoracoscopic surgery for lung cancer: A randomized controlled study. J. South. Med. Univ. 2022, 42, 1807–1814. [Google Scholar] [CrossRef]
- Liu, N.; Ma, Q.; Zhou, M.; Yang, L.; Wang, W.; Wang, Y. Efficacy and exploratory analysis of potential mechanisms of stellate ganglion block in alleviating sleep disturbance in generalized anxiety disorder: A randomized controlled trial excluding comorbid depression. Front. Neurol. 2025, 16, 1554841. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Sun, Y.; Zhao, J.; Shen, Y.; Wang, C.H.; Luo, S.Z.; Li, Y.W. Efficacy and safety of stellate ganglion block with different volumes of ropivacaine to improve sleep quality in patients with insomnia: A comparative study. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 10233–10239. [Google Scholar] [CrossRef]
- Yan, S.; Wang, Y.; Yu, L.; Xia, W.; Xue, F.; Yu, Y.; Yuan, B.; Li, N.; Li, H.; Liang, H.; et al. Stellate ganglion block alleviates postoperative sleep disturbance in patients undergoing radical surgery for gastrointestinal malignancies. J. Clin. Sleep. Med. 2023, 19, 1633–1642. [Google Scholar] [CrossRef]
- Yang, R.Z.; Li, Y.Z.; Liang, M.; Yu, J.J.; Chen, M.L.; Qiu, J.J.; Lin, S.Z.; Wu, X.D.; Zeng, K. Stellate Ganglion Block Improves Postoperative Sleep Quality and Analgesia in Patients with Breast Cancer: A Randomized Controlled Trial. Pain. Ther. 2023, 12, 491–503. [Google Scholar] [CrossRef]
- Lipov, E.; Gluncic, V.; Lukic, I.K.; Candido, K. How does stellate ganglion block alleviate immunologically-linked disorders? Med. Hypotheses 2020, 144, 110000. [Google Scholar] [CrossRef] [PubMed]
- Elias, M. Cervical sympathetic and stellate ganglion blocks. Pain. Physician 2000, 3, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Vanlommel, J.; Van Look, L.; Peetermans, M.; Uyttebroek, L.; van Nassauw, L.; Van Schil, P. Anatomical variations of the upper thoracic sympathetic chain: A review. Eur. J. Cardiothorac. Surg. 2022, 61, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Manuel, N.; Lekha, K.; Varghese, G.; Ashalatha, P.; Das, A. Incidence of vertebral ganglion in cervical sympathetic trunk. Int. J. Life Sci. Biotechnol. Pharma Res. 2025, 14, 1123–1128. [Google Scholar]
- Ren, T.; Laurikainen, E.; Quirk, W.S.; Miller, J.M.; Nuttall, A.L. Effects of stellate ganglion stimulation on bilateral cochlear blood flow. Ann. Otol. Rhinol. Laryngol. 1993, 102, 378–384. [Google Scholar] [CrossRef]
- Chung, I.H.; Oh, C.S.; Koh, K.S.; Kim, H.J.; Paik, H.C.; Lee, D.Y. Anatomic variations of the T2 nerve root (including the nerve of Kuntz) and their implications for sympathectomy. J. Thorac. Cardiovasc. Surg. 2002, 123, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Filion, W.; Lamb, C. Anatomical variation of the sympathetic trunk and aberrant rami communicantes and their clinical implications. Ann. Anat. 2023, 245, 151999. [Google Scholar] [CrossRef] [PubMed]
- Marhold, F.; Izay, B.; Zacherl, J.; Tschabitscher, M.; Neumayer, C. Thoracoscopic and anatomic landmarks of Kuntz’s nerve: Implications for sympathetic surgery. Ann. Thorac. Surg. 2008, 86, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.C.; Jarral, O.A.; Shipolini, A.R.; McCormack, D.J. Does the nerve of Kuntz exist? Interact. Cardiovasc. Thorac. Surg. 2011, 13, 175–178. [Google Scholar] [CrossRef]
- Zaidi, Z.F.; Ashraf, A. The nerve of Kuntz: Incidence, location and variations. J. Appl. Sci. Res. 2010, 6, 659–664. [Google Scholar]
- Chaudhary, B.; Tripathy, P.R.; Gaikwad, M.R. Vertebral arteries bilaterally passing through stellate (cervicothoracic) ganglion. Folia Morphol. 2020, 79, 621–626. [Google Scholar] [CrossRef]
- Kim, E.D.; Yoo, W.J.; Kim, Y.N.; Park, H.J. Ultrasound-guided pulsed radiofrequency treatment of the cervical sympathetic chain for complex regional pain syndrome: A retrospective observational study. Medicine 2017, 96, e5856. [Google Scholar] [CrossRef]
- Korevaar, W.C.; Burney, R.G.; Moore, P.A. Convulsions during stellate ganglion block: A case report. Anesth. Analg. 1979, 58, 329–330. [Google Scholar] [CrossRef]
- Narouze, S. Ultrasound-guided stellate ganglion block: Safety and efficacy. Curr. Pain. Headache Rep. 2014, 18, 424. [Google Scholar] [CrossRef]
- Oh, D.; Lee, H.S. Atypical Course of Vertebral Artery Identified by Ultrasound Prescan before Performing a Stellate Ganglion Block. J. Med. Ultrasound 2022, 30, 143–145. [Google Scholar] [CrossRef]
- Singh, P.; Bhoi, D. Aberrant Branch of Vertebral Artery: A Needling Challenge for Ultrasound-Guided Stellate Ganglion Block. Ind. J. Pain. 2023, 37, 136–137. [Google Scholar] [CrossRef]
- Torres, N.; Lara, A.; Slaktoski, D.; Rodríguez, P.; Ramos, F. Stellate Ganglion Encircling Atypical Vertebral Artery. J. Anat. Var. Clin. Case Rep. 2024, 2, 113. [Google Scholar] [CrossRef]
- Tudose, R.C.; Rusu, M.C.; Hostiuc, S. The Vertebral Artery: A Systematic Review and a Meta-Analysis of the Current Literature. Diagnostics 2023, 13, 2036. [Google Scholar] [CrossRef] [PubMed]
- Annabi, E.H.; Arefieg, J.; Shiller, S. Stellate Ganglion Blockade. In Treatment of Chronic Pain Conditions: A Comprehensive Handbook; Springer: New York, NY, USA, 2017; pp. 145–146. [Google Scholar]
- Hogan, Q.H.; Erickson, S.J. MR imaging of the stellate ganglion: Normal appearance. AJR Am. J. Roentgenol. 1992, 158, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Nakajo, M.; Mori, S.; Nakayama, H.; Yoshiura, T.; Xu, S.; Rimmer, M.; Sato, T.; Sato, M.; Malik, V.; Salamon, N.; et al. Three-Dimensional Magnetic Resonance Imaging of the Human Stellate Ganglion. Clin. Anat. 2025, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Slappendel, R.; Thijssen, H.O.; Crul, B.J.; Merx, J.L. The stellate ganglion in magnetic resonance imaging: A quantification of the anatomic variability. Anesthesiology 1995, 83, 424–426. [Google Scholar] [CrossRef]
- FIPAT Terminology. FIPAT. Terminologia Anatomica. FIPAT.library.dal.ca. 2019. Available online: https://neuron.mefst.hr/docs/katedre/anatomija/medicina/Terminologia%20anatomica/Terminologia-Anatomica-2nd-Ed-2019.pdf (accessed on 1 February 2025).
- Potts, T.K. The Main Peripheral Connections of the Human Sympathetic Nervous System. J. Anat. 1925, 59, 129–135. [Google Scholar]
- Kiray, A.; Arman, C.; Naderi, S.; Guvencer, M.; Korman, E. Surgical anatomy of the cervical sympathetic trunk. Clin. Anat. 2005, 18, 179–185. [Google Scholar] [CrossRef]
- Kawashima, T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat. Embryol. 2005, 209, 425–438. [Google Scholar] [CrossRef]
- Rouviere, H.; Delmas, A. Anatomie Humaine. Tête et Cou; Masson: Paris, France, 1985. [Google Scholar]
- Ellison, J.P.; Williams, T.H. Sympathetic nerve pathways to the human heart, and their variations. Am. J. Anat. 1969, 124, 149–162. [Google Scholar] [CrossRef]
- Standring, S.; Anand, N.; Birch, R.; Collins, P.; Crossman, A.; Gleeson, M.; Jawaheer, G.; Smith, A.L.; Spratt, J.D.; Stringer, M.D.; et al. Gray’s Anatomy: The Anatomical Basis of Clinical Practice; Elsevier: London, UK, 2016. [Google Scholar]
- Walls, W.K. The anatomical approach in stellate ganglion injection. Br. J. Anaesth. 1955, 27, 616–621. [Google Scholar] [CrossRef]
- Marcer, N.; Bergmann, M.; Klie, A.; Moor, B.; Djonov, V. An anatomical investigation of the cervicothoracic ganglion. Clin. Anat. 2012, 25, 444–451. [Google Scholar] [CrossRef]
- Caliot, P.; Bousquet, V.; Cabanie, P.; Midy, D. The nerve loops crossing below the subclavian artery and their anatomical variations. Anat. Clin. 1984, 6, 209–213. [Google Scholar] [CrossRef]
- Loukas, M.; Zhan, X.L.; Tubbs, R.S.; Mirchandani, D.; Shoja, M.M. The ansa subclavia: A review of the literature. Folia Morphol. 2008, 67, 166–170. [Google Scholar]
- Huntoon, M.A. The vertebral artery is unlikely to be the sole source of vascular complications occurring during stellate ganglion block. Pain Pract. 2010, 10, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Song, S.O.; Jung, G. A lateral paracarotid approach for ultrasound-guided stellate ganglion block with a linear probe. J. Anesth. 2017, 31, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Hirata, K.; Shibata, S.; Shigematsu, K.; Higa, K.; Yamaura, K. Risk Vessels of Retropharyngeal Hematoma During Stellate Ganglion Block. Reg. Anesth. Pain Med. 2017, 42, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Mattei, A.; Massimiliano, C.; Cataldo, R.; Agro, F.E. The Clarus Video System as a useful diagnostic tool. Anaesthesia 2011, 66, 135–136. [Google Scholar] [CrossRef]
- Narouze, S. Beware of the “serpentine” inferior thyroid artery while performing stellate ganglion block. Anesth. Analg. 2009, 109, 289–290. [Google Scholar] [CrossRef]
- Siegenthaler, A.; Mlekusch, S.; Schliessbach, J.; Curatolo, M.; Eichenberger, U. Ultrasound imaging to estimate risk of esophageal and vascular puncture after conventional stellate ganglion block. Reg. Anesth. Pain Med. 2012, 37, 224–227. [Google Scholar] [CrossRef]
- Goel, V.; Patwardhan, A.M.; Ibrahim, M.; Howe, C.L.; Schultz, D.M.; Shankar, H. Complications associated with stellate ganglion nerve block: A systematic review. Reg. Anesth. Pain Med. 2019, 44, 669–678. [Google Scholar] [CrossRef]
- Paturet, G. Traite d’Anatomie Humaine; Masson: Paris, France, 1964. [Google Scholar]
- Sebileau, P. L’Appareil Suspenseur de la Plèvre; G. Steinheil: Paris, France, 1891. [Google Scholar]
- Ionescu, T. Le Sympathique Cervico-Thoracique; Masson: Paris, France, 1923. [Google Scholar]
- Beheshti, M.; Rezaee, A.; Langsteger, W. 68Ga-PSMA-HBED Uptake on Cervicothoracic (Stellate) Ganglia, a Common Pitfall on PET/CT. Clin. Nucl. Med. 2017, 42, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Bryce-Smith, R. Stellate ganglion block. Anaesthesia 1952, 7, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Hogan, Q.H.; Erickson, S.J.; Haddox, J.D.; Abram, S.E. The spread of solutions during stellate ganglion block. Reg. Anesth. 1992, 17, 78–83. [Google Scholar] [CrossRef]
- Johnson, O.W.; Chick, J.F.; Chauhan, N.R.; Fairchild, A.H.; Fan, C.M.; Stecker, M.S.; Killoran, T.P.; Suzuki-Han, A. The thoracic duct: Clinical importance, anatomic variation, imaging, and embolization. Eur. Radiol. 2016, 26, 2482–2493. [Google Scholar] [CrossRef]
- Chaudhry, A.; Kamali, A.; Herzka, D.A.; Wang, K.C.; Carrino, J.A.; Blitz, A.M. Detection of the Stellate and Thoracic Sympathetic Chain Ganglia with High-Resolution 3D-CISS MR Imaging. Am. J. Neuroradiol. 2018, 39, 1550–1554. [Google Scholar] [CrossRef]
- Gunduz, O.H.; Kenis-Coskun, O. Ganglion blocks as a treatment of pain: Current perspectives. J. Pain. Res. 2017, 10, 2815–2826. [Google Scholar] [CrossRef]
- Millhouse, P.W.; Bloom, R.W.; Beckstrand, J.N.; McClure, M.L.; Eckmann, M.S.; Feeko, K.J.; Mojica, J.J. The Ganglia of the Head and Neck: Clinical Relevance for the Interventional Pain Physician. Curr. Pain Headache Rep. 2025, 29, 80. [Google Scholar] [CrossRef]
- Wink, J.; van Delft, R.; Notenboom, R.G.E.; Wouters, P.F.; DeRuiter, M.C.; Plevier, J.W.M.; Jongbloed, M.R.M. Human adult cardiac autonomic innervation: Controversies in anatomical knowledge and relevance for cardiac neuromodulation. Auton. Neurosci. 2020, 227, 102674. [Google Scholar] [CrossRef]
- Axford, M. Some Observations on the Cervical Sympathetic in Man. J. Anat. 1928, 62, 301–318. [Google Scholar]
- Xiuqing, C.; Bo, S.; Shizhen, Z. Nerves accompanying the vertebral artery and their clinical relevance. Spine 1988, 13, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Delmas, J.; Laux, G. Anatomie Médico-Chirurgicale du Système Nerveux Végétatif (Sympathique & Parasympathique); Masson et Cie.: Paris, France, 1933. [Google Scholar]
- Mannu, D.A. Ricerche anatomo-comparative sul Simpatico cervicale. Int. Mschr. Anat. Physiol. 1914, 30, 49. [Google Scholar]
- Siwe, S.A. The cervical part of the gangliated cord, with special reference to its connections with the spinal nerves and certain cerebral nerves. Am. J. Anat. 1931, 48, 479–497. [Google Scholar] [CrossRef]
- Erdine, S. Sympathetic Blocks of the Head and Neck. In Interventional Pain Management: Image-Guided Procedures; Raj, P.P., Lou, L., Erdine, S., Staats, P.S., Waldman, S.D., Racz, G., Hammer, M., Niv, D., Ruiz-Lopez, R., et al., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2008; pp. 108–126. [Google Scholar]
- Nozdrachev, A.D.; Fateev, M.M.; Jimenez, B.; Morales, M.A. Circuits and projections of cat stellate ganglion. Arch. Med. Res. 2003, 34, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N.; Lambert, G.A.; Duckworth, J.W. The anatomy and physiology of the vertebral nerve in relation to cervical migraine. Cephalalgia 1981, 1, 11–24. [Google Scholar] [CrossRef]
- Johal, J.; BelElary, S.S.; Lax, E.A.; Maharaja, G.K.; Oskouian, R.J.; Loukas, M.; Tubbs, R.S. The vertebral nerve: A comprehensive review of its form and function. J. Clin. Neurosci. 2017, 41, 1–5. [Google Scholar] [CrossRef]
- Kawashima, T. Anatomy of the cardiac nervous system with clinical and comparative morphological implications. Anat. Sci. Int. 2011, 86, 30–49. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Loukas, M.; Remy, A.C.; Shoja, M.M.; Salter, E.G.; Oakes, W.J. The vertebral nerve revisited. Clin. Anat. 2007, 20, 644–647. [Google Scholar] [CrossRef]
- Tripathy, K.; Simakurthy, S.; Jan, A. Ciliospinal Reflex; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Verlinden, T.J.; van Dijk, P.; Herrler, A.; de Gier-de Vries, C.; Lamers, W.H.; Köhler, S.E. The human phrenic nerve serves as a morphological conduit for autonomic nerves and innervates the caval body of the diaphragm. Sci. Rep. 2018, 8, 11697. [Google Scholar] [CrossRef]
- Janes, R.D.; Brandys, J.C.; Hopkins, D.A.; Johnstone, D.E.; Murphy, D.A.; Armour, J.A. Anatomy of human extrinsic cardiac nerves and ganglia. Am. J. Cardiol. 1986, 57, 299–309. [Google Scholar] [CrossRef]
- Li, J.; Zheng, L. The Mechanism of Cardiac Sympathetic Activity Assessment Methods: Current Knowledge. Front. Cardiovasc. Med. 2022, 9, 931219. [Google Scholar] [CrossRef]
- Sear, J.W. Role of cardiac reflexes in the control of heart rate: What does the anesthesiologist need to know? Anesth. Analg. 2012, 114, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Galosy, R.A.; Clarke, L.K. The effect of left dorsal and ventral ansa subclavian transection on cardiac changes during behavioral stress in dogs. Physiol. Behav. 1980, 25, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Raffaelli, M.; Iacobone, M.; Henry, J.F. The “false” nonrecurrent inferior laryngeal nerve. Surgery 2000, 128, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Yetisir, F.; Salman, A.E.; Ozkardes, A.B.; Aydin, S.M.; Kilic, M. Fusion of a cervical sympathetic ganglion with the recurrent inferior laryngeal nerve: A case of false positive non-recurrent inferior laryngeal nerve. Turk. J. Surg. 2013, 29, 150–152. [Google Scholar] [CrossRef]
- Forman, S.M.; Kuhnert, S.; Glueck, E.; Brauer, P.; Lemmons, C.; Kilmer, M.; Barry, A. An Exploration of the Sympathetic Component of the Recurrent Laryngeal Nerve. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Steinberg, J.L.; Khane, G.J.; Fernandes, C.M.; Nel, J.P. Anatomy of the recurrent laryngeal nerve: A redescription. J. Laryngol. Otol. 1986, 100, 919–927. [Google Scholar] [CrossRef]
- Loukas, M.; Du Plessis, M.; Louis, R.G., Jr.; Tubbs, R.S.; Wartmann, C.T.; Apaydin, N. The subdiaphragmatic part of the phrenic nerve—Morphometry and connections to autonomic ganglia. Clin. Anat. 2016, 29, 120–128. [Google Scholar] [CrossRef]
- François-Franck, C.A. Cours du Collège de France de 1880 à 1904; Doin: Paris, France, 1904. [Google Scholar]
- François-Franck, C.A. Recherches Sur l’Anatomie et la Physiologie des Nerfs Vasculaires de la Tête. Ph.D. Thesis, University of Paris, Paris, France, 1875. [Google Scholar]
- Hollinshead, W. The Neck. In Anatomy for Surgeons: The Head and Neck, 3rd ed.; J. B. Lippincott Company: Philadelphia, PA, USA, 1982; pp. 443–531. [Google Scholar]
- Yan, J.; Ogino, K.; Hitomi, J. The terminal insertional segments and communications of the vertebral nerve in the human cervical region. Surg. Radiol. Anat. 2009, 31, 165–171. [Google Scholar] [CrossRef]
- Hoffman, H.H.; Kuntz, A. Vertebral nerve and plexus; components, anatomical relationships, and surgical implications. AMA Arch. Surg. 1957, 74, 430–437. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Salter, E.G.; Wellons, J.C., 3rd; Blount, J.P.; Oakes, W.J. The triangle of the vertebral artery. Neurosurgery 2005, 56, 252–255. [Google Scholar] [CrossRef]
- Van den Broek, A. Untersuchungen über den Bau des sympathischen Nervensystems der Säugetiere. Morphol. Jahrb. 1908, 37, 202–288. [Google Scholar]
- Benes, M.; Kachlik, D.; Belbl, M.; Whitley, A.; Havlikova, S.; Kaiser, R.; Kunc, V.; Kunc, V. A meta-analysis on the anatomical variability of the brachial plexus: Part II—Branching of the supraclavicular part. Ann. Anat. 2021, 238, 151788. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, A. Distribution of the sympathetic rami to the brachial plexus: Its relation to sympathectomy affecting the upper extremity. Arch. Surg. 1927, 15, 871–877. [Google Scholar] [CrossRef]
- Ramsaroop, L.; Singh, B.; Moodley, J.; Partab, P.; Pather, N.; Satyapal, K.S. A thoracoscopic view of the nerve of Kuntz. Surg. Endosc. 2003, 17, 1498. [Google Scholar] [CrossRef]
- Esler, M. The sympathetic regulation of the heart. Eur. Heart J. 2016, 37, 2808–2809. [Google Scholar] [CrossRef]
- Margus, C.; Correa, A.; Cheung, W.; Blaikie, E.; Kuo, K.; Hockensmith, A.; Kinas, D.; She, T. Stellate Ganglion Nerve Block by Point-of-Care Ultrasonography for Treatment of Refractory Infarction-Induced Ventricular Fibrillation. Ann. Emerg. Med. 2020, 75, 257–260. [Google Scholar] [CrossRef]
- Van Weperen, V.Y.H.; Ripplinger, C.M.; Vaseghi, M. Autonomic control of ventricular function in health and disease: Current state of the art. Clin. Auton. Res. 2023, 33, 491–517. [Google Scholar] [CrossRef]
- Rogers, M.C.; Abildskov, J.A.; Preston, J.B. Cardiac effects of stimulation and block of the stellate ganglion. Anesthesiology 1973, 39, 525–533. [Google Scholar] [CrossRef]
- Kuder, T.; Nowak, E. Autonomic cardiac nerves: Literature review. Folia Morphol. 2015, 74, 1–8. [Google Scholar] [CrossRef]
- Vaseghi, M.; Zhou, W.; Shi, J.; Ajijola, O.A.; Hadaya, J.; Shivkumar, K.; Mahajan, A. Sympathetic innervation of the anterior left ventricular wall by the right and left stellate ganglia. Heart Rhythm 2012, 9, 1303–1309. [Google Scholar] [CrossRef]
- Yanowitz, F.; Preston, J.B.; Abildskov, J.A. Functional distribution of right and left stellate innervation to the ventricles. Production of neurogenic electrocardiographic changes by unilateral alteration of sympathetic tone. Circ. Res. 1966, 18, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Strack, A.M.; Sawyer, W.B.; Hughes, J.H.; Platt, K.B.; Loewy, A.D. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res. 1989, 491, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Laurikainen, E.A.; Kim, D.; Didier, A.; Ren, T.; Miller, J.M.; Quirk, W.S.; Nuttall, A.L. Stellate ganglion drives sympathetic regulation of cochlear blood flow. Hear. Res. 1993, 64, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, C.; Iwama, K. Electrical stimulation of the stellate ganglion and the vertebral nerve. J. Neurosurg. 1972, 36, 756–762. [Google Scholar] [CrossRef]
- Erickson, S.J.; Hogan, Q.H. CT-guided injection of the stellate ganglion: Description of technique and efficacy of sympathetic blockade. Radiology 1993, 188, 707–709. [Google Scholar] [CrossRef]
- Rathmell, J.P. Atlas of Image-Guided Intervention in Regional Anesthesia and Pain Medicine; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Park, J.S.; Kim, K.J.; Lee, Y.W.; Yoon, D.M.; Yoon, K.B.; Han, M.Y.; Choi, J.B. Estimation of Stellate Ganglion Block Injection Point Using the Cricoid Cartilage as Landmark Through X-ray Review. Korean J. Pain. 2011, 24, 141–145. [Google Scholar] [CrossRef]
- Rauck, R.L. Stellate ganglion block. Tech. Reg. Anesth. Pain. Manag. 2001, 5, 88–93. [Google Scholar] [CrossRef]
- Janik, J.E.; Hoeft, M.A.; Ajar, A.H.; Alsofrom, G.F.; Borrello, M.T.; Rathmell, J.P. Variable osteology of the sixth cervical vertebra in relation to stellate ganglion block. Reg. Anesth. Pain. Med. 2008, 33, 102–108. [Google Scholar] [CrossRef]
- Kim, S.; Kang, S.J.; Nguyen, H.S.; Jeong, S.W. Store-operated calcium entry in the satellite glial cells of rat sympathetic ganglia. Korean J. Physiol. Pharmacol. 2024, 28, 93–103. [Google Scholar] [CrossRef]
- Hanani, M. Satellite glial cells in sympathetic and parasympathetic ganglia: In search of function. Brain Res. Rev. 2010, 64, 304–327. [Google Scholar] [CrossRef]
- Hanani, M.; Spray, D.C. Emerging importance of satellite glia in nervous system function and dysfunction. Nat. Rev. Neurosci. 2020, 21, 485–498. [Google Scholar] [CrossRef]
- Van Weperen, V.Y.H.; Littman, R.J.; Arneson, D.V.; Contreras, J.; Yang, X.; Ajijola, O.A. Single-cell transcriptomic profiling of satellite glial cells in stellate ganglia reveals developmental and functional axial dynamics. Glia 2021, 69, 1281–1291. [Google Scholar] [CrossRef]
- Van Weperen, V.Y.-S.H.; Contreras, J.; Littman, R.; Ajijola, O.A. Single-cell RNA Sequencing reveals molecular heterogeneity of glia within mouse sympathetic ganglia. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Blum, E.; Procacci, P.; Conte, V.; Hanani, M. Systemic inflammation alters satellite glial cell function and structure. A possible contribution to pain. Neuroscience 2014, 274, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Ledda, M.; Blum, E.; De Palo, S.; Hanani, M. Augmentation in gap junction-mediated cell coupling in dorsal root ganglia following sciatic nerve neuritis in the mouse. Neuroscience 2009, 164, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Glueckert, R.; Linthicum, F.H.; Rieger, G.; Blumer, M.; Bitsche, M.; Pechriggl, E.; Rask-Andersen, H.; Schrott-Fischer, A. Possible role of gap junction intercellular channels and connexin 43 in satellite glial cells (SGCs) for preservation of human spiral ganglion neurons: A comparative study with clinical implications. Cell Tissue Res. 2014, 355, 267–278. [Google Scholar] [CrossRef]
- Pannese, E. The structure of the perineuronal sheath of satellite glial cells (SGCs) in sensory ganglia. Neuron Glia Biol. 2010, 6, 3–10. [Google Scholar] [CrossRef]
- Dias, C.; Fernandes, E.; Barbosa, R.M.; Laranjinha, J.; Ledo, A. Astrocytic aerobic glycolysis provides lactate to support neuronal oxidative metabolism in the hippocampus. Biofactors 2023, 49, 875–886. [Google Scholar] [CrossRef]
- Wu, A.; Lee, D.; Xiong, W.C. Lactate Metabolism, Signaling, and Function in Brain Development, Synaptic Plasticity, Angiogenesis, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 13398. [Google Scholar] [CrossRef]
- Barnes, J.R.; Mukherjee, B.; Rogers, B.C.; Nafar, F.; Gosse, M.; Parsons, M.P. The Relationship Between Glutamate Dynamics and Activity-Dependent Synaptic Plasticity. J. Neurosci. 2020, 40, 2793–2807. [Google Scholar] [CrossRef] [PubMed]
- Pajarillo, E.; Rizor, A.; Lee, J.; Aschner, M.; Lee, E. The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics. Neuropharmacology 2019, 161, 107559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chu, J.M.; Wong, G.T. Cerebral Glutamate Regulation and Receptor Changes in Perioperative Neuroinflammation and Cognitive Dysfunction. Biomolecules 2022, 12, 597. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.; Paterson, D.J.; Herring, N. Post-Ganglionic Sympathetic Neurons can Directly Sense Raised Extracellular Na+ via SCN7a/Nax. Front. Physiol. 2022, 13, 931094. [Google Scholar] [CrossRef]
- Selak, I.; Skaper, S.D.; Varon, S. Ionic behaviors and neuronal survival in developing ganglia. III. Studies with embryonic chick sympathetic neurons. J. Cell Physiol. 1983, 114, 229–234. [Google Scholar] [CrossRef]
- Hadjiconstantinou, M.; Potter, P.E.; Neff, N.H. Trans-synaptic modulation via muscarinic receptors of serotonin-containing small intensely fluorescent cells of superior cervical ganglion. J. Neurosci. 1982, 2, 1836–1839. [Google Scholar] [CrossRef]
- Helen, P.; Panula, P.; Yang, H.Y.; Rapoport, S.I. Bombesin/gastrin-releasing peptide (GRP)- and Met5-enkephalin-Arg6-Gly7-Leu8-like immunoreactivities in small intensely fluorescent (SIF) cells and nerve fibers of rat sympathetic ganglia. J. Histochem. Cytochem. 1984, 32, 1131–1138. [Google Scholar] [CrossRef]
- Chau, Y.-P.; Lu, K.-S. Differential permeability of blood microvasculatures in various sympathetic ganglia of rodents. Anat. Embryol. 1996, 194, 259–269. [Google Scholar] [CrossRef]
- Mekhail, N.A.; Estafanous, F.G.; Vitullo, J.C.; Khairallah, P.A. Portal-like microcirculation in rat sympathetic ganglia. Acta Anat. 1990, 138, 200–207. [Google Scholar] [CrossRef]
- Tanaka, K.; Chiba, T. Microvascular organization of sympathetic ganglia, with special reference to small intensely-fluorescent cells. Microsc. Res. Tech. 1996, 35, 137–145. [Google Scholar] [CrossRef]
- Jager, S.E.; Pallesen, L.T.; Richner, M.; Harley, P.; Hore, Z.; McMahon, S.; Denk, F.; Vaegter, C.B. Changes in the transcriptional fingerprint of satellite glial cells following peripheral nerve injury. Glia 2020, 68, 1375–1395. [Google Scholar] [CrossRef]
- Konnova, E.A.; Deftu, A.F.; Chung, P.C.S.; Pertin, M.; Kirschmann, G.; Decosterd, I.; Suter, M.R. Characterisation of GFAP-Expressing Glial Cells in the Dorsal Root Ganglion after Spared Nerve Injury. Int. J. Mol. Sci. 2023, 24, 15559. [Google Scholar] [CrossRef]
- Enes, J.; Haburcak, M.; Sona, S.; Gerard, N.; Mitchell, A.C.; Fu, W.; Birren, S.J. Satellite glial cells modulate cholinergic transmission between sympathetic neurons. PLoS ONE 2020, 15, e0218643. [Google Scholar] [CrossRef]
- Lingam, R.K.; Santer, R.M. An investigation into the extrinsic blood supply and the microvasculature of the rat stellate ganglion. Ann. Anat. 1993, 175, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.L. The arterial supply to the stellate ganglion. J. Anat. 1953, 87, 219–227. [Google Scholar] [PubMed]
- Noma, N.; Kamo, H.; Nakaya, Y.; Dezawa, K.; Young, A.; Khan, J.; Imamura, Y. Stellate ganglion block as an early intervention in sympathetically maintained headache and orofacial pain caused by temporal arteritis. Pain Med. 2013, 14, 392–397. [Google Scholar] [CrossRef]
- Kakuyama, M.; Toda, H.; Osawa, M.; Fukuda, K. The bilateral effect of stellate ganglion block on the facial skin blood flow. Reg. Anesth. Pain Med. 2000, 25, 389–392. [Google Scholar] [CrossRef]
- Raut, M.S.; Maheshwari, A. Stellate ganglion block: Important weapon in the anesthesiologists’ armamentarium. J. Cardiothor. Vasc. Anesth. 2018, 32, e36–e37. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Zhang, J.; Chen, W.R.; Liu, H.Y.; Ji, F.H. Ultrasound-guided Stellate Ganglion Block Improves Gastrointestinal Function After Thoracolumbar Spinal Surgery. Clin. Ther. 2017, 39, 2322–2330. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, H.; Mao, D.; Shao, J.; Deng, L.; Wang, T.; Liao, Z.; Li, X.; Chen, Y.; Yao, J.; et al. Effects of stellate ganglion block on inflammation and autophagy of spinal cord neurons in rats with neuropathic pain after spinal cord injury. Am. J. Transl. Res. 2025, 17, 3063–3073. [Google Scholar] [CrossRef]
- Deng, X.; Sun, T.; Zhao, D.; Sana, S.; Li, W. Stellate ganglion block potentially ameliorates postoperative cognitive decline in aged rats by regulating the neuroendocrine response to stress. Heliyon 2023, 9, e14337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Li, H.; Hu, Y.; Yu, S.; Liu, Q.; Chen, Y. Stellate Ganglion Block Improves Postoperative Cognitive Dysfunction in aged rats by SIRT1-mediated White Matter Lesion Repair. Neurochem. Res. 2022, 47, 3838–3853. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, C.M.; Meyer, T.M.; Stoevesandt, D.; Kielstein, H.; Kielstein, J.T. Evaluation of the ideal length of the Seldinger needle for internal jugular vein catheter placement. Sci. Rep. 2022, 12, 2745. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Yamakawa, K.; Hamon, D.; Nakamura, K.; Shivkumar, K.; Vaseghi, M. Cardiac sympathetic innervation via middle cervical and stellate ganglia and antiarrhythmic mechanism of bilateral stellectomy. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H392–H405. [Google Scholar] [CrossRef]
- Kastler, A.; Aubry, S.; Sailley, N.; Michalakis, D.; Siliman, G.; Gory, G.; Lajoie, J.L.; Kastler, B. CT-guided stellate ganglion blockade vs. radiofrequency neurolysis in the management of refractory type I complex regional pain syndrome of the upper limb. Eur. Radiol. 2013, 23, 1316–1322. [Google Scholar] [CrossRef]
- Goyal, S.; Kumar, A.; Kantha, M.; Sharma, R.S.; Agrawal, S.; Singh, G.K. Ultrasound-guided Stellate Ganglion Block for Upper Extremity Phantom Limb Pain-A Case Series. Ind. J. Pain. 2024, 38, 65–68. [Google Scholar] [CrossRef]
- Mendoza, W.A.S.; Gónima-Valero, E.; Sarmiento, D.A.; Amaya, S.; López, M.J.A. Stellate ganglion block as an analgesic rescue alternative in phantom limb syndrome following an amputation: A case report. Rev. Chil. Anesth. 2022, 51, 598–601. [Google Scholar]
- Abbas, D.N.; Reyad, R.M. Thermal Versus Super Voltage Pulsed Radiofrequency of Stellate Ganglion in Post-Mastectomy Neuropathic Pain Syndrome: A Prospective Randomized Trial. Pain Physician 2018, 21, 351–362. [Google Scholar] [CrossRef]
- Forouzanfar, T.; van Kleef, M.; Weber, W.E. Radiofrequency lesions of the stellate ganglion in chronic pain syndromes: Retrospective analysis of clinical efficacy in 86 patients. Clin. J. Pain. 2000, 16, 164–168. [Google Scholar] [CrossRef]
- Mo, K.; Qian, L.; Tian, J.; Liao, J.; Tan, F.; Kong, W.; Yu, X.; Chi, X. Ultrasound-guided stellate ganglion blockade—Patient positioning is everything: A case report demonstrating the efficacy of a modified out-of-plane approach. Front. Neurosci. 2023, 17, 1288484. [Google Scholar] [CrossRef]
- Jain, V.; Rath, G.P.; Dash, H.H.; Bithal, P.K.; Chouhan, R.S.; Suri, A. Stellate ganglion block for treatment of cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage—A preliminary study. J. Anaesthesiol. Clin. Pharmacol. 2011, 27, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Wendel, C.; Scheibe, R.; Wagner, S.; Tangemann, W.; Henkes, H.; Ganslandt, O.; Schiff, J.H. Decrease of blood flow velocity in the middle cerebral artery after stellate ganglion block following aneurysmal subarachnoid hemorrhage: A potential vasospasm treatment? J. Neurosurg. 2020, 133, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lin, F.; Bai, Y.; Liang, F.; Wang, X.; Wang, B.; Jian, M.; Wang, Y.; Liu, H.; Wang, A.; et al. Early stellate ganglion block for improvement of postoperative cerebral blood flow velocity after aneurysmal subarachnoid hemorrhage: Results of a pilot randomized controlled trial. J. Neurosurg. 2023, 139, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Pileggi, M.; Mosimann, P.J.; Isalberti, M.; Piechowiak, E.I.; Merlani, P.; Reinert, M.; Cianfoni, A. Stellate ganglion block combined with intra-arterial treatment: A “one-stop shop” for cerebral vasospasm after aneurysmal subarachnoid hemorrhage—A pilot study. Neuroradiology 2021, 63, 1701–1708. [Google Scholar] [CrossRef]
- Sheen, S.; Kalia, H. A Case of Intractable Hyperhidrosis in Spinal Cord Injury: Role of Stellate Ganglion Block. Adv. Clin. Med. Res. Healthc. Deliv. 2022, 2, 18. [Google Scholar] [CrossRef]
- Tuz, M.; Erodlu, F.; Dodru, H.; Uygur, K.; Yavuz, L. Transient locked-in syndrome resulting from stellate ganglion block in the treatment of patients with sudden hearing loss. Acta Anaesthesiol. Scand. 2003, 47, 485–487. [Google Scholar] [CrossRef]
- Dajing, J.; Mei, Z. Clinical efficacy of stellate ganglion block combined with betahistine hydrochloride in the treatment of vestibular migraine. Front. Med. Sci. Res. 2025, 7. [Google Scholar] [CrossRef]
- Wendel, C.; Oberhauser, C.; Schiff, J.; Henkes, H.; Ganslandt, O. Stellate Ganglion Block and Intraarterial Spasmolysis in Patients with Cerebral Vasospasm: A Retrospective Cohort Study. Neurocritical Care 2024, 40, 603–611. [Google Scholar] [CrossRef]
- Okuda, Y.; Kitajima, T.; Asai, T. Stellate ganglion block, cervical sympathetic block and cervicothoracic sympathetic block. Eur. J. Anaesthesiol. 1999, 16, 272–273. [Google Scholar] [CrossRef]
- Hardy, P.A.J.; Wells, J.C.D. Extent of sympathetic blockade after stellate ganglion block with bupivacaine. Pain 1989, 36, 193–196. [Google Scholar] [CrossRef]
- Park, D.Y.; Kang, S.; Kang, H.J.; Choi, J.K.; Do Kim, J.; Yoon, J.S. Impact of Neck Position on the Probability of Common Carotid Artery Puncture During Ultrasound-Guided Stellate Ganglion Block. PM&R 2019, 11, 463–469. [Google Scholar] [CrossRef]
- Reddy, R.; Lerman, I.; Chen, J. A Letter to the Editor: Impact of Neck Position on the Probability of Common Carotid Artery Puncture during Ultrasound-Guided Stellate Ganglion Block. PM&R 2019, 11, 1250–1251. [Google Scholar] [CrossRef]
- Rastogi, S.; Tripathi, S. Cardiac arrest following stellate ganglion block performed under ultrasound guidance. Anaesthesia 2010, 65, 1042. [Google Scholar] [CrossRef]
- Lim, K.J.; Choi, Y.J. Bilateral Horner’s Syndrome after a Stellate Ganglion Block. Korean J. Anesth. 2002, 43, 241–244. [Google Scholar] [CrossRef]
- Nelson, A. Stroke: A Complication of Stellate Ganglion Block. In Challenging Cases and Complication Management in Pain Medicine; Anitescu, M., Benzon, H., Wallace, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 111–118. [Google Scholar]
- Gan, Y.; Chen, J.; Xian, L.; Shi, Y. Objective Evaluation of Stellate Ganglion Block Effects Using Ultrasound Wave Intensity Technology: A Study on Hemodynamics. J. Pain. Res. 2024, 17, 2063–2070. [Google Scholar] [CrossRef]
- Wallace, M.S.; Milholland, A.V. Contralateral spread of local anesthetic with stellate ganglion block. Reg. Anesth. 1993, 18, 55–59. [Google Scholar] [CrossRef]
- Boukens, B.J.D.; Dacey, M.; Meijborg, V.M.F.; Janse, M.J.; Hadaya, J.; Hanna, P.; Swid, M.A.; Opthof, T.; Ardell, J.L.; Shivkumar, K.; et al. Mechanism of ventricular premature beats elicited by left stellate ganglion stimulation during acute ischaemia of the anterior left ventricle. Cardiovasc. Res. 2021, 117, 2083–2091. [Google Scholar] [CrossRef]
- Meijborg, V.M.F.; Boukens, B.J.D.; Janse, M.J.; Salavatian, S.; Dacey, M.J.; Yoshie, K.; Opthof, T.; Swid, M.A.; Hoang, J.D.; Hanna, P.; et al. Stellate ganglion stimulation causes spatiotemporal changes in ventricular repolarization in pig. Heart Rhythm. 2020, 17, 795–803. [Google Scholar] [CrossRef]
- Ter Bekke, R.M.A.; Moers, A.M.E.; de Jong, M.M.J.; Johnson, D.M.; Schwartz, P.J.; Vanoli, E.; Volders, P.G.A. Proarrhythmic proclivity of left-stellate ganglion stimulation in a canine model of drug-induced long-QT syndrome type 1. Int. J. Cardiol. 2019, 286, 66–72. [Google Scholar] [CrossRef]
- Lador, A.; Wang, S.; Schurmann, P.A.; Chihara, R.; Dave, A.S.; Valderrabano, M. Stellate ganglion instrumentation for pharmacological blockade, nerve recording, and stimulation in patients with ventricular arrhythmias: Preliminary experience. Heart Rhythm. 2023, 20, 797–805. [Google Scholar] [CrossRef]
- Wong, C.W. Stimulation of left stellate ganglion prolongs Q-T interval in patients with palmar hyperhidrosis. Am. J. Physiol. 1997, 273, H1696–H1698. [Google Scholar] [CrossRef]
- Ajijola, O.A.; Howard-Quijano, K.; Scovotti, J.; Vaseghi, M.; Lee, C.; Mahajan, A.; Shivkumar, K. Augmentation of cardiac sympathetic tone by percutaneous low-level stellate ganglion stimulation in humans: A feasibility study. Physiol. Rep. 2015, 3, e12328. [Google Scholar] [CrossRef]
- Pace, J.B. Sympathetic control of pulmonary vascular impedance in anesthetized dogs. Circ. Res. 1971, 29, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, X.; Huang, B.; Wang, Z.; Zhou, L.; Wang, M.; Yu, L.; Jiang, H. Noninvasive low-frequency electromagnetic stimulation of the left stellate ganglion reduces myocardial infarction-induced ventricular arrhythmia. Sci. Rep. 2016, 6, 30783. [Google Scholar] [CrossRef] [PubMed]
- Swissa, M.; Zhou, S.; Gonzalez-Gomez, I.; Chang, C.M.; Lai, A.C.; Cates, A.W.; Fishbein, M.C.; Karagueuzian, H.S.; Chen, P.S.; Chen, L.S. Long-term subthreshold electrical stimulation of the left stellate ganglion and a canine model of sudden cardiac death. J. Am. Coll. Cardiol. 2004, 43, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.J.; Shinohara, T.; Park, H.W.; Frick, K.; Ice, D.S.; Choi, E.K.; Han, S.; Maruyama, M.; Sharma, R.; Shen, C.; et al. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation 2011, 123, 2204–2212. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, X.; Wan, J.; Wong, J.; Bedwell, A.A.; Persohn, S.A.; Shen, C.; Fishbein, M.C.; Chen, L.S.; Chen, Z.; et al. Subcutaneous nerve stimulation for rate control in ambulatory dogs with persistent atrial fibrillation. Heart Rhythm 2019, 16, 1383–1391. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, Y.; Wong, J.; Tsai, W.C.; Jiang, Z.; Kabir, R.A.; Han, S.; Shen, C.; Fishbein, M.C.; Chen, L.S.; et al. Subcutaneous nerve stimulation reduces sympathetic nerve activity in ambulatory dogs with myocardial infarction. Heart Rhythm 2020, 17, 1167–1175. [Google Scholar] [CrossRef]
- Rao, B.H.; Lokre, A.; Patnala, N.; Padmanabhan, T.N.C. Stellate ganglion ablation by conventional radiofrequency in patients with electrical storm. Europace 2023, 25, euad290. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Iida, H.; Sumi, K.; Takenaka, M.; Yoshimura, N.; Dohi, S. Preliminary study of the efficacy of radiofrequency lesions of stellate ganglion in chronic pain patients. Pain Med. 2010, 11, 142–144. [Google Scholar] [CrossRef]
- Benarroch, E.E. Locus coeruleus. Cell Tissue Res. 2018, 373, 221–232. [Google Scholar] [CrossRef]
- Urschel, H.C.; Patel, A. Thoracic Outlet Syndromes. Curr. Treat. Options Cardiovasc. Med. 2003, 5, 163–168. [Google Scholar] [CrossRef]
- Kuhn, J.E.; Lebus, V.G.; Bible, J.E. Thoracic outlet syndrome. J. Am. Acad. Orthop. Surg. 2015, 23, 222–232. [Google Scholar] [CrossRef]
- Li, N.; Dierks, G.; Vervaeke, H.E.; Jumonville, A.; Kaye, A.D.; Myrcik, D.; Paladini, A.; Varrassi, G.; Viswanath, O.; Urits, I. Thoracic Outlet Syndrome: A Narrative Review. J. Clin. Med. 2021, 10, 962. [Google Scholar] [CrossRef] [PubMed]
- Panther, E.J.; Reintgen, C.D.; Cueto, R.J.; Hao, K.A.; Chim, H.; King, J.J. Thoracic outlet syndrome: A review. J. Shoulder Elb. Surg. 2022, 31, e545–e561. [Google Scholar] [CrossRef] [PubMed]
- Urschel, H.C., Jr. The transaxillary approach for treatment of thoracic outlet syndromes. Semin. Thorac. Cardiovasc. Surg. 1996, 8, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Stone, H.L. Effects of unilateral stellectomy upon cardiac performance during exercise in dogs. Circ. Res. 1979, 44, 637–645. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Verrier, R.L.; Lown, B. Effect of stellectomy and vagotomy on ventricular refractoriness in dogs. Circ. Res. 1977, 40, 536–540. [Google Scholar] [CrossRef]
- Coyer, B.H.; Pryor, R.; Kirsch, W.M.; Blount, S.G., Jr. Left stellectomy in the long QT syndrome. Chest 1978, 74, 584–586. [Google Scholar] [CrossRef]
- Nelson, S.D.; Lynch, J.J.; Sanders, D.; Montgomery, D.G.; Lucchesi, B.R. Electrophysiologic actions and antifibrillatory efficacy of subacute left stellectomy in a conscious, post-infarction canine model of ischemic ventricular fibrillation. Int. J. Cardiol. 1989, 22, 365–376. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Snebold, N.G.; Brown, A.M. Effects of unilateral cardiac sympathetic denervation on the ventricular fibrillation threshold. Am. J. Cardiol. 1976, 37, 1034–1040. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Stone, H.L. Left stellectomy in the prevention of ventricular fibrillation caused by acute myocardial ischemia in conscious dogs with anterior myocardial infarction. Circulation 1980, 62, 1256–1265. [Google Scholar] [CrossRef]
- Park, H.; Park, H.; Mun, D.; Kim, M.; Pak, H.N.; Lee, M.H.; Joung, B. Sympathetic nerve blocks promote anti-inflammatory response by activating the JAK2-STAT3-mediated signaling cascade in rat myocarditis models: A novel mechanism with clinical implications. Heart Rhythm 2018, 15, 770–779. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Stone, H.L. Left stellectomy and denervation supersensitivity in conscious dogs. Am. J. Cardiol. 1982, 49, 1185–1190. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Stone, H.L. Tonic influence of the sympathetic nervous system on myocardial reactive hyperemia and on coronary blood flow distribution in dogs. Circ. Res. 1977, 41, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Damiani, D.; Agosta, V.T.; D’Andria Ursoleo, J.; Bottussi, A.; Licheri, M.; Muriana, P.; Monaco, F. Perioperative and long-term outcomes of bilateral cardiac sympathetic denervation via video-assisted thoracoscopic surgery in patients with refractory ventricular arrhythmias. Int. J. Cardiol. 2025, 421, 132890. [Google Scholar] [CrossRef] [PubMed]
- Juhos, P.; Janik, M.; Hatala, R.; Benacka, O.; Balaz, R.; Lucenic, M.; Tarabova, K.; Laucek, P. Bilateral thoracoscopic cardiac sympathetic denervation in patients with refractory ventricular tachyarrhythmias non-responsive to other treatment. Bratisl. Med. J. 2022, 123, 528–532. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.; Cullen, P.; Rackauskas, M.; Kelly, R.; O’Sullivan, K.E.; Galvin, J.; Eaton, D. Left cardiac sympathetic denervation: Case series and technical report. Ir. J. Med. Sci. 2017, 186, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Assis, F.R.; Yu, D.H.; Zhou, X.; Sidhu, S.; Bapna, A.; Engelman, Z.J.; Misra, S.; Okada, D.R.; Chrispin, J.; Berger, R.; et al. Minimally invasive transtracheal cardiac plexus block for sympathetic neuromodulation. Heart Rhythm 2019, 16, 117–124. [Google Scholar] [CrossRef]
- Shenkin, H.A.; Cabieses, F.; Van Den Noordt, G. The effect of bilateral stellectomy upon the cerebral circulation of man. J. Clin. Investig. 1951, 30, 90–93. [Google Scholar] [CrossRef]
- Ajijola, O.A.; Hoover, D.B.; Simerly, T.M.; Brown, T.C.; Yanagawa, J.; Biniwale, R.M.; Lee, J.M.; Sadeghi, A.; Khanlou, N.; Ardell, J.L.; et al. Inflammation, oxidative stress, and glial cell activation characterize stellate ganglia from humans with electrical storm. JCI Insight 2017, 2, e94715. [Google Scholar] [CrossRef]
- Hobbs, L.; Fuller, R.; Cameron-Smith, E. Stellate Ganglion Blockade for Corticobasal Syndrome Pain: A Case Report and Potential New Discovery. Mov. Disord. Clin. Pract. 2023, 10, 848–849. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Zhou, Y.; Zhao, T.; Wang, N.; Sun, L.; Han, J.; Ren, Z.; Wang, B.; Han, X. Fatal familial insomnia: A new case description with response to thoracic sympathetic nerve thermocoagulation and stellate ganglion block. Sleep Med. 2025, 127, 24–27. [Google Scholar] [CrossRef]
| Knowledge Gaps | Future Research Directions |
|---|---|
| Anatomical research needs |
|
| Clinical evidence |
|
| Mechanistic understanding |
|
| Technological advances |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, M.C.; Munteanu, I.M.; Vrapciu, A.D.; Jianu, A.M.; Hostiuc, S.; Tudose, R.C.; Motoc, A.G.M. Anatomy, Imaging, and Clinical Significance of the Cervicothoracic (Stellate) Ganglion. Diagnostics 2025, 15, 2911. https://doi.org/10.3390/diagnostics15222911
Rusu MC, Munteanu IM, Vrapciu AD, Jianu AM, Hostiuc S, Tudose RC, Motoc AGM. Anatomy, Imaging, and Clinical Significance of the Cervicothoracic (Stellate) Ganglion. Diagnostics. 2025; 15(22):2911. https://doi.org/10.3390/diagnostics15222911
Chicago/Turabian StyleRusu, Mugurel Constantin, Ionuţ Mădălin Munteanu, Alexandra Diana Vrapciu, Adelina Maria Jianu, Sorin Hostiuc, Răzvan Costin Tudose, and Andrei Gheorghe Marius Motoc. 2025. "Anatomy, Imaging, and Clinical Significance of the Cervicothoracic (Stellate) Ganglion" Diagnostics 15, no. 22: 2911. https://doi.org/10.3390/diagnostics15222911
APA StyleRusu, M. C., Munteanu, I. M., Vrapciu, A. D., Jianu, A. M., Hostiuc, S., Tudose, R. C., & Motoc, A. G. M. (2025). Anatomy, Imaging, and Clinical Significance of the Cervicothoracic (Stellate) Ganglion. Diagnostics, 15(22), 2911. https://doi.org/10.3390/diagnostics15222911








