Reduced Serum sRAGE Levels Identify COPD and Reflect Disease Severity: Findings from a Cross-Sectional Study in India
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Clinical and Functional Assessments
2.4. Exposure Assessment
2.5. Laboratory and Biomarker Evaluations
2.6. Data Collection Procedure
2.7. Sample Size
2.8. Statistical Methods
2.9. Ethical Considerations
3. Results
Baseline Characteristics
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Form |
| ANOVA | Analysis of Variance |
| ATS/ERS | American Thoracic Society/European Respiratory Society |
| AUC | Area Under the Curve |
| BMEI | Biomass Exposure Index |
| BMS | Biomass Smoke |
| CAT | COPD Assessment Test |
| COPD | Chronic Obstructive Pulmonary Disease |
| FEV1 | Forced Expiratory Volume in One Second |
| FVC | Forced Vital Capacity |
| GOLD | Global Initiative for Chronic Obstructive Lung Disease |
| IQR | Interquartile Range (25th percentile, 75th percentile) |
| LMICs | Low- and Middle-Income Countries |
| mMRC | Modified Medical Research Council |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| NPV | Negative Predictive Value |
| PFT | Pulmonary Function Test |
| PLR | Platelet-to-Lymphocyte Ratio |
| PPV | Positive Predictive Value |
| SD | Standard Deviation |
| SGQRC | COPD-Specific Version of the St. George’s Respiratory Questionnaire |
| sRAGE | Soluble Receptor for Advanced Glycation End-Products |
| TS | Tobacco Smoke |
References
- Salvi, S.; Barnes, P.J. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009, 374, 733–743. [Google Scholar] [CrossRef] [PubMed]
- WHO. Chronic Obstructive Pulmonary Disease (COPD) Fact Sheet; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 13 November 2025).
- Burney, P.; Jithoo, A.; Kato, B.; Janson, C.; Mannino, D.; Niżankowska-Mogilnicka, E.; Studnicka, M.; Tan, W.; Bateman, E.; Koçabas, A.; et al. Chronic obstructive pulmonary disease mortality and prevalence: The associations with smoking and poverty—A BOLD analysis. Thorax 2014, 69, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Gordon, S.B.; Bruce, N.G.; Grigg, J.; Hibberd, P.L.; Kurmi, O.P.; Lam, K.B.H.; Mortimer, K.; Asante, K.P.; Balakrishnan, K.; Balmes, J.; et al. Respiratory risks from household air pollution in low- and middle-income countries. Lancet Respir. Med. 2014, 2, 823–860. [Google Scholar] [CrossRef]
- Siddharthan, T.; Grigsby, M.R.; Goodman, D.; Chowdhury, M.; Rubinstein, A.; Irazola, V.; Gutierrez, L.; Miranda, J.J.; Bernabe-Ortiz, A.; Alam, D.; et al. Association between Household Air Pollution Exposure and Chronic Obstructive Pulmonary Disease Outcomes in 13 Low- and Middle-Income Country Settings. Am. J. Respir. Crit. Care Med. 2018, 197, 611–620. [Google Scholar] [CrossRef]
- Barnes, P.J.; Celli, B.R. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009, 33, 1165–1185. [Google Scholar] [CrossRef]
- Pratte, K.A.; Curtis, J.L.; Kechris, K.J.; Couper, D.; Cho, M.H.; Silverman, E.K.; DeMeo, D.L.; Sciurba, F.C.; Zhang, F.; Ortega, V.E.; et al. Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COPD. Respir. Res. 2021, 22, 127. [Google Scholar] [CrossRef]
- Po, J.Y.T.; FitzGerald, J.M.; Carlsten, C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: Systematic review and meta-analysis. Thorax 2011, 66, 232–239. [Google Scholar] [CrossRef]
- Nicolaou, L.; Checkley, W. Differences between cigarette smoking and biomass smoke exposure: An in silico comparative assessment of particulate deposition in the lungs. Environ. Res. 2021, 197, 111116. [Google Scholar] [CrossRef]
- Ramírez-Venegas, A.; Sansores, R.H.; Pérez-Padilla, R.; Regalado, J.; Velázquez, A.; Sánchez, C.; Mayar, M.E. Survival of patients with COPD due to biomass smoke and tobacco smoke. Chest 2006, 173, 393–397. [Google Scholar] [CrossRef]
- Sukkar, M.B.; Wood, L.G.; Tooze, M.; Simpson, J.L.; McDonald, V.; Gibson, P.G.; Wark, P.A.B. Soluble RAGE is deficient in neutrophilic asthma and COPD. Eur. Respir. J. 2012, 39, 721–729. [Google Scholar] [CrossRef]
- Keefe, J.; Yao, C.; Hwang, S.J.; Courchesne, P.; Lee, G.Y.; Dupuis, J.; Mizgerd, J.P.; O’Connor, G.; Washko, G.R.; Cho, M.H.; et al. An Integrative Genomic Strategy Identifies sRAGE as a Causal and Protective Biomarker of Lung Function. Chest 2022, 161, 76–84. [Google Scholar] [CrossRef]
- Capistrano, S.J.; van Reyk, D.; Chen, H.; Oliver, B.G. Evidence of Biomass Smoke Exposure as a Causative Factor for the Development of COPD. Toxics 2017, 5, 36. [Google Scholar] [CrossRef]
- Mahesh, P.A.; Jayaraj, B.S.; Prabhakar, A.K.; Chaya, S.K.; Vijaysimha, R. Identification of a threshold for biomass exposure index for chronic bronchitis in rural women of Mysore district, Karnataka, India. Indian J. Med. Res. 2013, 137, 87–94. [Google Scholar] [PubMed] [PubMed Central]
- Cheng, D.T.; Kim, D.K.; Cockayne, D.; Belousov, A.; Bitter, H.; Cho, M.H.; Duvoix, A.; Edwards, L.D.; Lomas, D.A.; Miller, B.E.; et al. Systemic sRAGE is a biomarker of emphysema and associated with AGER genetic variants in COPD Patients. Am. J. Respir. Crit. Care Med. 2013, 188, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Klont, F.; Horvatovich, P.; Bowler, R.P.; van Rikxoort, E.; Charbonnier, J.P.; Kwiatkowski, M.; Lynch, D.A.; Humphries, S.; Bischoff, R.; Ten Hacken, N.H.T.; et al. Plasma sRAGE levels strongly associate with centrilobular emphysema assessed by HRCT scans. Respir. Res. 2022, 23, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iwamoto, H.; Gao, J.; Pulkkinen, V.; Toljamo, T.; Nieminen, P.; Mazur, W. Soluble receptor for advanced glycation end-products and progression of airway disease. BMC Pulm. Med. 2014, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Dutta, J.; Singh, S.; Greeshma, M.V.; Mahesh, P.A.; Mabalirajan, U. Diagnostic Challenges and Pathogenetic Differences in Biomass-Smoke-Induced versus Tobacco-Smoke-Induced COPD: A Comparative Review. Diagnostics 2024, 14, 2154. [Google Scholar] [CrossRef]
- Haider, S.H.; Oskuei, A.; Crowley, G.; Kwon, S.; Lam, R.; Riggs, J.; Mikhail, M.; Talusan, A.; Veerappan, A.; Kim, J.S.; et al. Receptor for Advanced Glycation End-Products and Environmental Exposure Related Obstructive Airways Disease: A Systematic Review. Eur. Respir. Rev. 2019, 28, 180096. [Google Scholar] [CrossRef]
- Yonchuk, J.G.; Silverman, E.K.; Bowler, R.P.; Agustí, A.; Lomas, D.A.; Miller, B.E.; Tal-Singer, R.; Mayer, R.J. Circulating sRAGE levels are associated with emphysema and chronic cor pulmonale in smokers. Am. J. Respir. Crit. Care Med. 2015, 192, 785–792. [Google Scholar] [CrossRef]
- Lindsey, J.B.; de Lemos, J.A.; Cipollone, F.; Ayers, C.R.; Rohatgi, A.; Morrow, D.A.; Khera, A.; McGuire, D.K. Association between Circulating Soluble Receptor for Advanced Glycation End Products and Atherosclerosis: Observations from the Dallas Heart Study. Diabetes Care 2009, 32, 1218–1220. [Google Scholar] [CrossRef]
- Fukami, K.; Yamagishi, S.; Okuda, S. Role of AGEs-RAGE System in Cardiovascular Disease. Curr. Pharm. Des. 2014, 20, 2395–2402. [Google Scholar] [CrossRef]
- Grauen Larsen, H.; Yndigegn, T.; Marinkovic, G.; Grufman, H.; Mares, R.; Nilsson, J.; Goncalves, I.; Schiopu, A. The Soluble Receptor for Advanced Glycation End-Products (sRAGE) Has a Dual Phase-Dependent Association with Residual Cardiovascular Risk after an Acute Coronary Event. Atherosclerosis 2019, 287, 16–23. [Google Scholar] [CrossRef]
- Hirano, T.; Doi, K.; Matsunaga, K.; Takahashi, S.; Donishi, T.; Suga, K.; Oishi, K.; Yasuda, K.; Mimura, Y.; Harada, M.; et al. A Novel Role of Growth Differentiation Factor (GDF)-15 in Overlap with Sedentary Lifestyle and Cognitive Risk in COPD. J. Clin. Med. 2020, 9, 2737. [Google Scholar] [CrossRef] [PubMed]
- Jaitovich, A.; Barreiro, E. Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease. What We Know and Can Do for Our Patients. Am. J. Respir. Crit. Care Med. 2018, 198, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Curran, C.S.; Kopp, J.B. RAGE Pathway Activation and Function in Chronic Kidney Disease and COVID-19. Front. Med. 2022, 9, 970423. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.; Kumar, G.A.; Dhaliwal, R.S.; Paulson, K.; Agrawal, A.; Koul, P.A.; Mahesh, P.A.; Nair, S.; Singh, V.; Aggarwal, A.N.; et al. The burden of chronic respiratory diseases and their heterogeneity across India: The Global Burden of Disease Study 1990–2016. Lancet Glob. Health 2018, 6, e1363–e1374. [Google Scholar] [CrossRef]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Kline Leidy, N. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–656. [Google Scholar] [CrossRef]
- Smith, D.J.; Yerkovich, S.T.; Towers, M.A.; Carroll, M.L.; Thomas, R.; Upham, J.W. Reduced soluble receptor for advanced glycation end-products in COPD. Eur. Respir. J. 2011, 37, 516–522. [Google Scholar] [CrossRef]
- MacNee, W. ABC of chronic obstructive pulmonary disease: Pathology, pathogenesis, and pathophysiology. BMJ 2006, 332, 1202–1204. [Google Scholar] [CrossRef]

| Variable | TS-COPD (n = 25) | TS-CONTROL (n = 25) | BMS-COPD (n = 25) | BMS-Control (n = 25) | Healthy Controls (n = 50) |
|---|---|---|---|---|---|
| Age (years) (Mean ± SD) | 62.00 ± 6.50 | 56.64 ± 9.13 | 68.00 ± 7.74 | 61.16 ± 10.26 | 50.52 ± 9.92 |
| BMI (Mean ± SD) | 21.60 ± 4.13 | 22.39 ± 3.36 | 22.31 ± 2.98 | 23.56 ± 2.62 | 23.62 ± 2.33 |
| Gender | |||||
| Male (n) | 25 | 25 | - | - | 25 |
| Female (n) | - | - | 25 | 25 | 25 |
| FEV1/FVC Ratio (Mean ± SD) | 0.59 ± 0.11 | 0.79 ± 0.05 | 0.60 ± 0.08 | 0.80 ± 0.04 | 0.81 ± 0.05 |
| FVC % Predicted (Mean ± SD) | 83.56 ± 20.33 | 100.16 ± 10.98 | 78.64 ± 21.77 | 102.50 ± 12.61 | 100.90 ± 16.27 |
| FEV1% Predicted (Mean ± SD) | 62.32 ± 21.42 | 97.12 ± 12.53 | 62.40 ± 20.15 | 101.80 ± 13.37 | 99.56 ± 14.99 |
| Biomass Exposure Years (Median, IQR) | – | – | 23 (17–28) | 15 (10–16) | – |
| BMEI (Median, IQR) | – | – | 90 (75–102) | 48 (40–60) | – |
| Pack-Years (Median, IQR) | 41 (26–55) | 23 (13–41) | – | – | – |
| Smoking Index (Median, IQR) | 810 (525–1100) | 450 (256–816) | – | – | – |
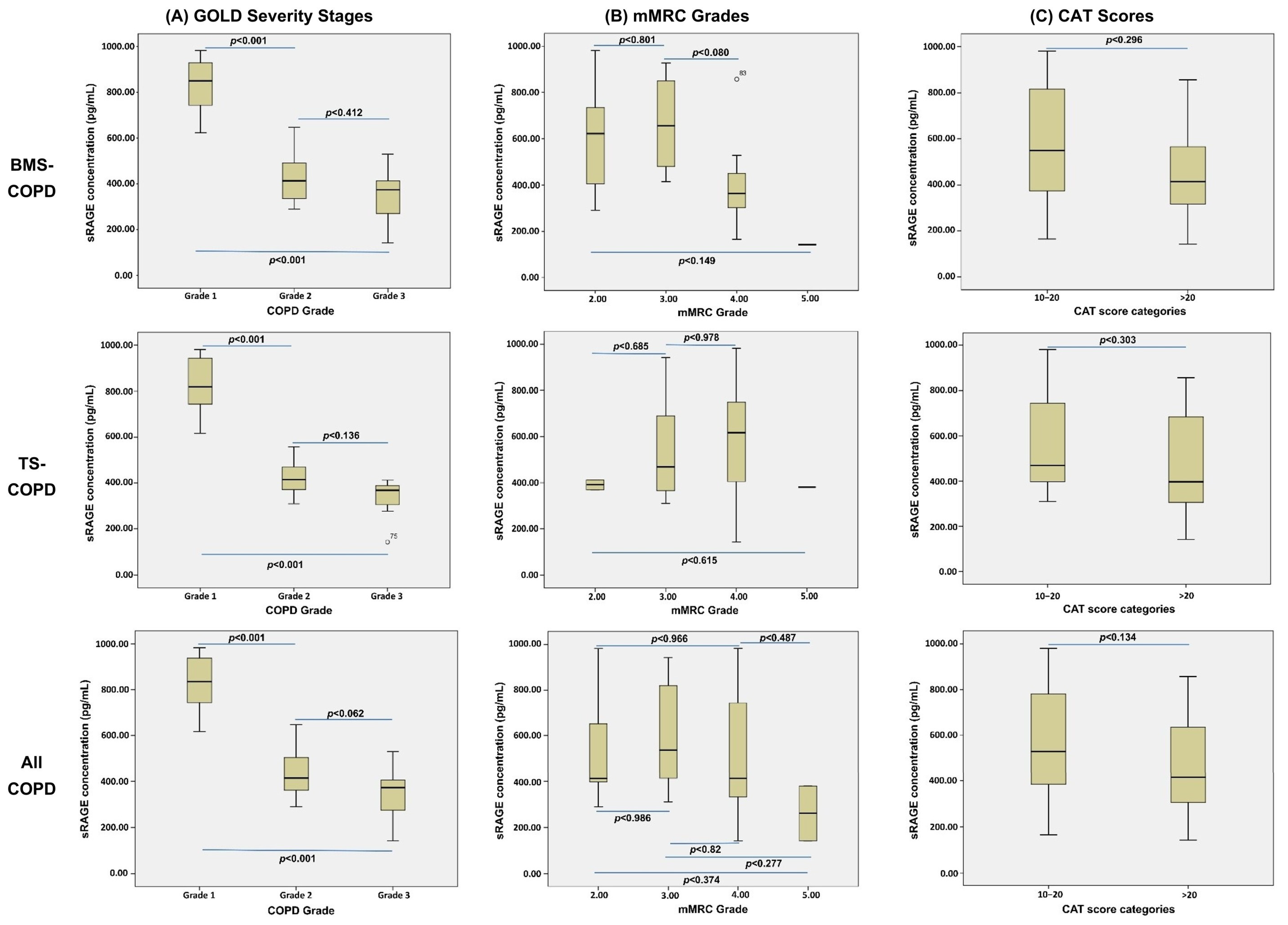
| GOLD Severity Grading (n) | |||||
| I | 9 | 9 | |||
| II | 9 | 7 | |||
| III | 7 | 9 | |||
| CAT Score (Median, IQR) | 20 (16–23) | 7 (4–10) | 17 (16–21) | 8 (4–9) | 0 (0–0) |
| mMRC Grade (Median, IQR) | 3 (3–4) | 1 (1–1) | 3 (2–4) | 1 (1–1) | 0 (0–0) |
| SGQRC Symptoms (Median, IQR) | 56.80 (46.68–71.72) | 0 (0–25.36) | 53.57 (44.93–72.04) | 0 (0–10.95) | 0 (0–0) |
| SGQRC Activity (Median, IQR) | 67.87 (44.90–77.00) | 0 (0–7.74) | 67.87 (54.01–84.21) | 0 (0–0) | 0 (0–0) |
| SGQRC Impact (Median, IQR) | 19.30 (14.33–31.96) | 2.54 (2.54–5.09) | 29.99 (19.30–48.00) | 2.54 (2.54–5.09) | 0 (0–0) |
| SGQRC Total (Median, IQR) | 44.58 (31.02–51.09) | 3.25 (1.31–10.69) | 45.78 (41.41–57.14) | 3.25 (1.31–6.98) | 0 (0–0) |
| NLR (Mean ± SD) | 2.56 ± 2.28 | 1.85 ± 1.06 | 7.92 ± 11.33 | 1.76 ± 1.10 | 1.87 ± 0.94 |
| PLR (Mean ± SD) | 115.88 ± 44.45 | 129.99 ± 66.40 | 192.50 ± 145.09 | 115.22 ± 54.57 | 128.06 ± 43.13 |
| sRAGE (Mean ± SD) | 545 ± 238 | 1207 ± 228 | 540 ± 251 | 1237 ± 266 | 1462 ± 347 |
| Parameter | sRAGE Concentration | |
|---|---|---|
| Pearson Correlation | p-Value | |
| FEV1/FVC Ratio | 0.748 | <0.001 |
| FVC Percent Predicted | 0.543 | <0.001 |
| FEV1 Percent Predicted | 0.694 | <0.001 |
| CAT Score | −0.768 | <0.001 |
| mMRC Grade | −0.791 | <0.001 |
| SGQRC Total | −0.803 | <0.001 |
| NLR | −0.265 | <0.001 |
| PLR | −0.145 | 0.072 |
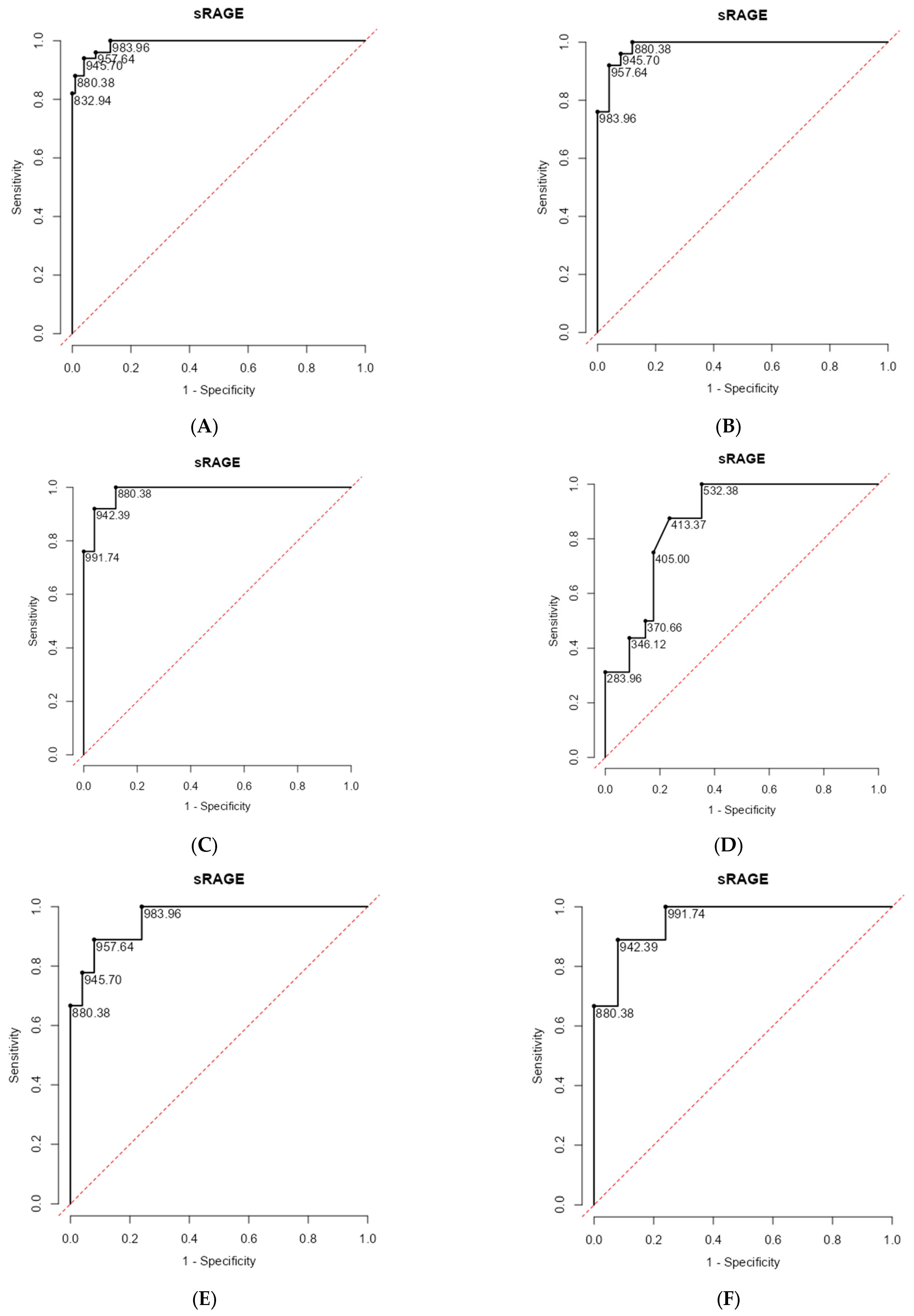
| sRAGE Cutoff Point | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Youden’s Index | AUC | |
|---|---|---|---|---|---|---|---|
| Patients with COPD and without COPD | 946 | 0.940 | 0.960 | 0.922 | 0.970 | 1.90 | 0.990 |
| Patients with TS COPD and TS without COPD | 958 | 0.920 | 0.960 | 0.958 | 0.923 | 1.88 | 0.986 |
| Patients with BMS COPD and BMS without COPD | 880 | 1.000 | 0.880 | 0.893 | 1.000 | 1.88 | 0.984 |
| Patients with non-severe (mild and moderate) and severe COPD | 413 | 0.875 | 0.7647 | 0.636 | 0.929 | 1.64 | 0.866 |
| Patients with TS COPD (mild) and TS without COPD | 957.6 | 0.920 | 0.889 | 0.479 | 0.990 | 0.809 | 0.960 |
| Patients with BMS COPD (mild) and BMS without COPD | 942.4 | 0.920 | 0.889 | 0.479 | 0.990 | 0.809 | 0.956 |
| 95% CI | |||||||
|---|---|---|---|---|---|---|---|
| COPD Status | Predictor | Estimate | Lower | Upper | SE | Z | p |
| Patients with TS COPD and without COPD | Age in yrs | 0.084 | −0.421 | 0.589 | 0.258 | 0.325 | 0.745 |
| BMI (kg/m2) | −0.376 | −2.193 | 1.442 | 0.927 | −0.405 | 0.685 | |
| sRAGE (pg/mL) | −0.023 | −0.046 | 0.000 | 0.012 | −1.958 | 0.050 | |
| Smoking pack-years | −1.996 | −2.407 | −1.585 | 0.210 | −9.521 | <0.001 | |
| Patients with TS without COPD-NORMAL | Age in yrs | 0.078 | −0.433 | 0.588 | 0.260 | 0.298 | 0.766 |
| BMI (kg/m2) | −0.314 | −2.179 | 1.552 | 0.952 | −0.329 | 0.742 | |
| sRAGE (pg/mL) | −0.004 | −0.027 | 0.020 | 0.012 | −0.298 | 0.765 | |
| Smoking pack-years | −2.032 | −2.458 | −1.606 | 0.217 | −9.358 | <0.001 | |
| Patients with BMS COPD and without COPD | Age in yrs | 0.244 | 0.033 | 0.456 | 0.108 | 2.265 | 0.024 |
| BMI (kg/m2) | 0.151 | −0.352 | 0.655 | 0.257 | 0.589 | 0.556 | |
| sRAGE (pg/mL) | −0.029 | −0.051 | −0.008 | 0.011 | −2.647 | 0.008 | |
| Smoking pack-years | 1.009 | 0.797 | 1.221 | 0.108 | 9.313 | <0.001 | |
| Patients with BMS without COPD-NORMAL | Age in yrs | 0.107 | 0.045 | 0.169 | 0.032 | 3.361 | <0.001 |
| BMI (kg/m2) | 0.009 | −0.219 | 0.238 | 0.116 | 0.082 | 0.935 | |
| sRAGE (pg/mL) | −0.002 | −0.004 | −0.000 | 0.001 | −2.314 | 0.021 | |
| Smoking pack-years | 0.424 | 0.360 | 0.489 | 0.033 | 12.916 | <0.001 | |
| Patients with TS COPD and TS without COPD | Age in yrs | 0.010 | −0.117 | 0.137 | 0.065 | 0.152 | 0.879 |
| BMI (kg/m2) | −0.041 | −0.599 | 0.517 | 0.285 | −0.143 | 0.886 | |
| sRAGE (pg/mL) | −0.021 | −0.033 | −0.008 | 0.006 | −3.212 | 0.001 | |
| Smoking pack-years | 0.035 | −0.044 | 0.115 | 0.041 | 0.868 | 0.385 | |
| Patients with BMS COPD and BMS without COPD | Age in yrs | 0.119 | −0.069 | 0.307 | 0.096 | 1.239 | 0.215 |
| BMI (kg/m2) | 0.078 | −0.386 | 0.541 | 0.237 | 0.329 | 0.742 | |
| sRAGE (pg/mL) | −0.025 | −0.044 | −0.006 | 0.010 | −2.529 | 0.011 | |
| Smoking pack-years | 1.424 | 1.228 | 1.621 | 0.100 | 14.194 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandanna Seri, V.; Kaleem Ullah, M.; Siddaiah, J.B.; Chaya, S.K.; Lokesh, K.S.; Khan, S.A.; Aladakatti, A.R.; Shahul, S.; Vasanthan, V.; Karnik, M.; et al. Reduced Serum sRAGE Levels Identify COPD and Reflect Disease Severity: Findings from a Cross-Sectional Study in India. Diagnostics 2025, 15, 2910. https://doi.org/10.3390/diagnostics15222910
Chandanna Seri V, Kaleem Ullah M, Siddaiah JB, Chaya SK, Lokesh KS, Khan SA, Aladakatti AR, Shahul S, Vasanthan V, Karnik M, et al. Reduced Serum sRAGE Levels Identify COPD and Reflect Disease Severity: Findings from a Cross-Sectional Study in India. Diagnostics. 2025; 15(22):2910. https://doi.org/10.3390/diagnostics15222910
Chicago/Turabian StyleChandanna Seri, Venkateshkumar, Mohammed Kaleem Ullah, Jayaraj Biligere Siddaiah, Sindaghatta Krishnarao Chaya, Komarla Sundararaja Lokesh, Suhail Azam Khan, Aishwarya R. Aladakatti, Shamnaz Shahul, Vivek Vasanthan, Medha Karnik, and et al. 2025. "Reduced Serum sRAGE Levels Identify COPD and Reflect Disease Severity: Findings from a Cross-Sectional Study in India" Diagnostics 15, no. 22: 2910. https://doi.org/10.3390/diagnostics15222910
APA StyleChandanna Seri, V., Kaleem Ullah, M., Siddaiah, J. B., Chaya, S. K., Lokesh, K. S., Khan, S. A., Aladakatti, A. R., Shahul, S., Vasanthan, V., Karnik, M., Madhunapantula, S. V., Ramaiah, S., Srinivas, S., Padmakaran, V., Shankar, M., Parthasarathi, A., & Mahesh, P. A. (2025). Reduced Serum sRAGE Levels Identify COPD and Reflect Disease Severity: Findings from a Cross-Sectional Study in India. Diagnostics, 15(22), 2910. https://doi.org/10.3390/diagnostics15222910






