Peripheral Nerve Ultrasound Findings in Hereditary Transthyretin Amyloidosis in Brazil
Abstract
1. Introduction
2. Materials and Methods
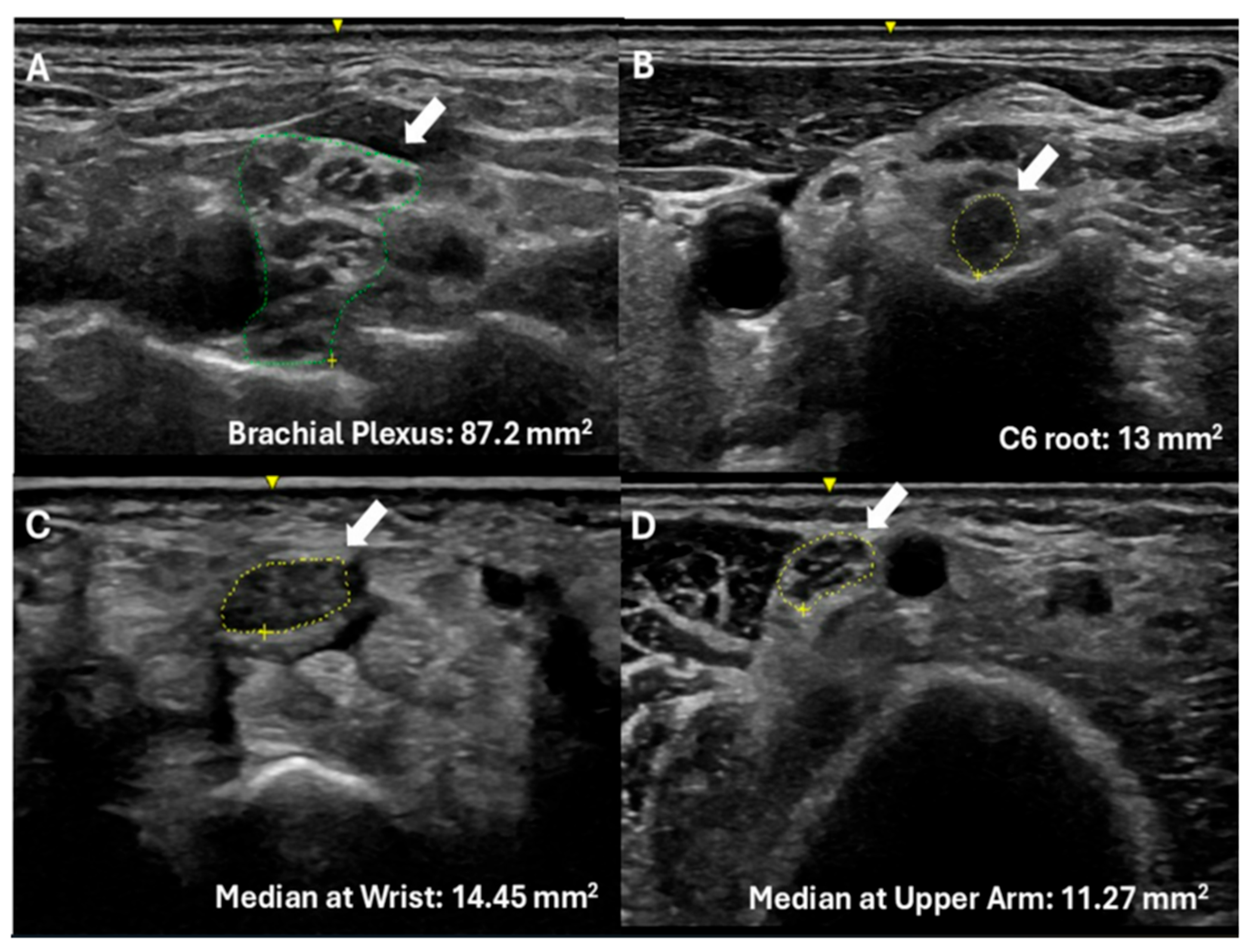
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATTRv | Hereditary transthyretin amyloidosis (variant) |
| TTR | Transthyretin |
| PN | Polyneuropathy |
| ATTRv-PN | Transthyretin amyloidosis with polyneuropathy |
| ATTRv-C | Asymptomatic transthyretin variant carrier |
| CSA | Cross-sectional area |
| CTS | Carpal tunnel syndrome |
| PND | Polyneuropathy Disability scale |
| NCS | Nerve conduction studies |
| EMG | Electromyography |
| CMT | Charcot–Marie–Tooth disease |
| CIDP | Chronic inflammatory demyelinating polyradiculoneuropathy |
| GBS | Guillain–Barré syndrome |
| IENF | Intraepidermal nerve fiber |
| NfL | Neurofilament light chain |
| MRN | Magnetic resonance neurography |
| DTI | Diffusion tensor imaging |
References
- Ando, Y.; Waddington-Cruz, M.; Sekijima, Y.; Koike, H.; Ueda, M.; Konishi, H.; Ishii, T.; Coelho, T. Optimal Practices for the Management of Hereditary Transthyretin Amyloidosis: Real-World Experience from Japan, Brazil, and Portugal. Orphanet J. Rare Dis. 2023, 18, 323. [Google Scholar] [CrossRef]
- Poli, L.; Labella, B.; Cotti Piccinelli, S.; Caria, F.; Risi, B.; Damioli, S.; Padovani, A.; Filosto, M. Hereditary Transthyretin Amyloidosis: A Comprehensive Review with a Focus on Peripheral Neuropathy. Front. Neurol. 2023, 14, 1242815. [Google Scholar] [CrossRef]
- Finsterer, J.; Iglseder, S.; Wanschitz, J.; Topakian, R.; Löscher, W.N.; Grisold, W. Hereditary Transthyretin-Related Amyloidosis. Acta Neurol. Scand. 2019, 139, 92–105. [Google Scholar] [CrossRef]
- Desai, U.; Ilieva, H.S.; Eyer, J.E.; Peltier, A.C. Peripheral Nervous System Involvement of Hereditary Transthyretin Amyloidosis in the United States: A Multi-Center Perspective. Muscle Nerve 2025, 72, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Chompoopong, P.; Mauermann, M.L.; Siddiqi, H.; Peltier, A. Amyloid Neuropathy: From Pathophysiology to Treatment in Light-Chain Amyloidosis and Hereditary Transthyretin Amyloidosis. Ann. Neurol. 2024, 96, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Berard, N.; Verschueren, A.; Fortanier, E.; Grapperon, A.M.; Kouton, L.; Rebouh, H.; Gallard, J.; Salort-Campana, E.; Attarian, S.; Delmont, E. Electrophysiological Monitoring of Asymptomatic Transthyretin Mutation Carriers. Muscle Nerve 2025, 71, 208–215. [Google Scholar] [CrossRef]
- Salvalaggio, A.; Cacciavillani, M.; Tiengo, C.; Cipriani, A.; Frizziero, L.; Fedrigo, M.; Rizzo, S.; Angelini, A.; Gasparotti, R.; Briani, C. Multimodal Evaluation of Carpal Tunnel Syndrome in a Pre-Symptomatic TTR Mutation Carrier. J. Neurol. Sci. 2023, 448. [Google Scholar] [CrossRef]
- Conceição, I. Clinical Features of TTR-FAP in Portugal. In Amyloid; Taylor & Francis: Abingdon, UK, 2012; Volume 19, pp. 71–72. [Google Scholar]
- Waddington-Cruz, M.; Ando, Y.; Amass, L.; Kiszko, J.; Chapman, D.; Sekijima, Y. Feasibility of Assessing Progression of Transthyretin Amyloid Polyneuropathy Using Nerve Conduction Studies: Findings from the Transthyretin Amyloidosis Outcomes Survey (THAOS). J. Peripher. Nerv. Syst. 2021, 26, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Karam, C.; Dimitrova, D.; Christ, M.; Heitner, S.B. Carpal Tunnel Syndrome and Associated Symptoms as First Manifestation of HATTR Amyloidosis. Neurol. Clin. Pract. 2019, 9, 309–313. [Google Scholar] [CrossRef]
- Dispenzieri, A.; Coelho, T.; Conceição, I.; Waddington-Cruz, M.; Wixner, J.; Kristen, A.V.; Rapezzi, C.; Planté-Bordeneuve, V.; Gonzalez-Moreno, J.; Maurer, M.S.; et al. Clinical and Genetic Profile of Patients Enrolled in the Transthyretin Amyloidosis Outcomes Survey (THAOS): 14-Year Update. Orphanet J. Rare Dis. 2022, 17, 236. [Google Scholar] [CrossRef]
- Beauvais, D.; Labeyrie, C.; Cauquil, C.; Francou, B.; Eliahou, L.; Not, A.; Echaniz-Laguna, A.; Adam, C.; Slama, M.S.; Benmalek, A.; et al. Detailed Clinical, Physiological and Pathological Phenotyping Can Impact Access to Disease-Modifying Treatments in ATTR Carriers. J. Neurol. Neurosurg. Psychiatry 2024, 95, 489–499. [Google Scholar] [CrossRef]
- Warendorf, J.K.; van der Star, G.M.; Dooijes, D.; Notermans, N.C.; Vrancken, A.F.J.E. Red Flags and Adjusted Suspicion Index for Distinguishing Hereditary Transthyretin Amyloid Polyneuropathy from Idiopathic Axonal Polyneuropathy. Neurol. Sci. 2023, 44, 3679–3685. [Google Scholar] [CrossRef]
- Péréon, Y.; Adams, D.; Camdessanché, J.-P.; Chanson, J.-B.; Cintas, P.; Magy, L.; Signaté, A.; Solé, G.; Svahn, J.; Tard, C.; et al. Diagnosis of Hereditary Transthyretin Amyloidosis in Patients with Suspected Chronic Inflammatory Demyelinating Polyneuropathy Unresponsive to Intravenous Immunoglobulins: Results of a Retrospective Study. Orphanet J. Rare Dis. 2025, 20, 95. [Google Scholar] [CrossRef]
- Adams, D.; Beaudonnet, G.; Adam, C.; Lacroix, C.; Théaudin, M.; Cauquil, C.; Labeyrie, C. Familial Amyloid Polyneuropathy: When Does It Stop to Be Asymptomatic and Need a Treatment? Rev. Neurol. 2016, 172, 645–652. [Google Scholar] [CrossRef]
- Di Stefano, V.; Lupica, A.; Alonge, P.; Pignolo, A.; Augello, S.M.; Gentile, F.; Gagliardo, A.; Giglia, F.; Brinch, D.; Cappello, M.; et al. Genetic Screening for Hereditary Transthyretin Amyloidosis with Polyneuropathy in Western Sicily: Two Years of Experience in a Neurological Clinic. Eur. J. Neurol. 2024, 31, e16065. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.; Foguel, D.; Berensztejn, A.; Pedrosa, R.; da Silva, P. The Phenotypical Expression of an European Inherited TTR Amyloidosis in Brazil. Orphanet J. Rare Dis. 2015, 10, O7. [Google Scholar] [CrossRef]
- Cruz, M.W.; Pinto, M.V.; Pinto, L.F.; Gervais, R.; Dias, M.; Perez, C.; Mundayat, R.; Ong, M.L.; Pedrosa, R.C.; Foguel, D. Baseline Disease Characteristics in Brazilian Patients Enrolled in Transthyretin Amyloidosis Outcome Survey (THAOS). Arq. Neuropsiquiatr. 2019, 77, 96–100. [Google Scholar] [CrossRef]
- Lavigne-Moreira, C.; Marques, V.D.; Gonçalves, M.V.M.; de Oliveira, M.F.; Tomaselli, P.J.; Nunez, J.C.; do Nascimento, O.J.M.; Barreira, A.A.; Marques, W. The Genetic Heterogeneity of Hereditary Transthyretin Amyloidosis in a Sample of the Brazilian Population. J. Peripher. Nerv. Syst. 2018, 23, 134–137. [Google Scholar] [CrossRef]
- Fernandes, F.; Luzuriaga, G.d.C.J.; da Fonseca, G.W.P.; Correia, E.B.; Carvalho, A.A.S.; Macedo, A.V.S.; Coelho-Filho, O.R.; Scheinberg, P.; Antunes, M.O.; Schwartzmann, P.V.; et al. Clinical and Genetic Profiles of Patients with Hereditary and Wild-Type Transthyretin Amyloidosis: The Transthyretin Cardiac Amyloidosis Registry in the State of São Paulo, Brazil (REACT-SP). Orphanet J. Rare Dis. 2024, 19, 273. [Google Scholar] [CrossRef] [PubMed]
- Telleman, J.A.; Grimm, A.; Goedee, S.; Visser, L.H.; Zaidman, C.M. Nerve Ultrasound in Polyneuropathies. Muscle Nerve 2018, 57, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Camelo-Filho, A.E.; Lima, P.L.G.S.B.; Cavalcante, F.L.H.B.; Miyajima, O.R.; Santos, C.F.; da Rosa, R.F.; Pessoa, A.L.S.; Braga-Neto, P.; Nóbrega, P.R. Polyneuropathy in Cerebrotendinous Xanthomatosis: Diagnostic Challenges and Potential for Therapeutic Intervention. Brain Sci. 2024, 14, 1159. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, L.; van Alfen, N. Neuromuscular Ultrasound as a Marker for Inherited Sensory Neuronopathy. Muscle Nerve 2023, 68, 718–721. [Google Scholar] [CrossRef]
- Pelosi, L.; Ghosh, A.; Leadbetter, R.; Lance, S.; Rodrigues, M.; Roxburgh, R. Nerve Ultrasound Detects Abnormally Small Nerves in Patients with Spinal and Bulbar Muscular Atrophy. Muscle Nerve 2022, 65, 599–602. [Google Scholar] [CrossRef]
- Camelo-Filho, A.E.; Lima, P.L.G.S.B.; da Rosa, R.F.; Soares, T.B.S.; Pessoa, A.L.S.; Nóbrega, P.R.; Braga-Neto, P. Nerve Ultrasound Detects Nerve Atrophy in Patients With Ataxia-Telangiectasia: A Pilot Study. Muscle Nerve 2025, 71, 1091–1095. [Google Scholar] [CrossRef]
- Granata, G.; Luigetti, M.; Coraci, D.; Del Grande, A.; Romano, A.; Bisogni, G.; Bramanti, P.; Rossini, P.M.; Sabatelli, M.; Padua, L. Ultrasound Evaluation in Transthyretin-Related Amyloid Neuropathy. Muscle Nerve 2014, 50, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Podnar, S.; Sarafov, S.; Tournev, I.; Omejec, G.; Zidar, J. Peripheral Nerve Ultrasonography in Patients with Transthyretin Amyloidosis. Clin. Neurophysiol. 2017, 128, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Salvalaggio, A.; Coraci, D.; Cacciavillani, M.; Obici, L.; Mazzeo, A.; Luigetti, M.; Pastorelli, F.; Grandis, M.; Cavallaro, T.; Bisogni, G.; et al. Nerve Ultrasound in Hereditary Transthyretin Amyloidosis: Red Flags and Possible Progression Biomarkers. J. Neurol. 2021, 268, 189–198. [Google Scholar] [CrossRef]
- Du, K.; Xu, K.; Chu, X.; Tang, Y.; Lv, H.; Zhang, W.; Wang, Z.; Yuan, Y.; Meng, L. Vagus Nerve Ultrasound in Transthyretin Familial Amyloid Polyneuropathy: A Pilot Study. J. Neuroimaging 2022, 32, 285–291. [Google Scholar] [CrossRef]
- Salvalaggio, A.; Coraci, D.; Obici, L.; Cacciavillani, M.; Luigetti, M.; Mazzeo, A.; Pastorelli, F.; Grandis, M.; Cavallaro, T.; Bisogni, G.; et al. Progressive Brachial Plexus Enlargement in Hereditary Transthyretin Amyloidosis. J. Neurol. 2022, 269, 1905–1912. [Google Scholar] [CrossRef]
- Arvidsson, S.; Eriksson, R.; Anan, I.; Heldestad, V. Enlarged Cross-Sectional Area in Peripheral Nerves in Swedish Patients with Hereditary V30M Transthyretin Amyloidosis. Ann. Med. 2023, 55, 2239269. [Google Scholar] [CrossRef]
- Asteggiano, C.; Paoletti, M.; Vegezzi, E.; Deligianni, X.; Santini, F.; Bergsland, N.; Papinutto, N.; Todisco, M.; Cosentino, G.; Cortese, A.; et al. Quantitative MRI assessment Using Variable Echo Time Imaging of Peripheral Nerve Injury in ATTRv Amyloidosis Patients. Eur. J. Neurol. 2025, 32, e70172. [Google Scholar] [CrossRef]
- Romano, A.; Guglielmino, V.; Bisogni, G.; Di Paolantonio, A.; Truini, A.; Minnella, A.M.; Sciarrone, M.A.; Vitali, F.; Maceroni, M.; Galosi, E.; et al. Early Detection of Nerve Involvement in Presymptomatic TTR Mutation Carriers: Exploring Potential Markers of Disease Onset. Neurol. Sci. 2024, 45, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, H.; Chao, C.; Lin, Y.; Tseng, P.; Su, M.; Hsieh, S. Neck Triangle Nerve Enlargement in Hereditary Transthyretin Amyloidosis Correlates with Changes in the Autonomic, Cardiac, and Gastrointestinal Systems. J. Intern. Med. 2024, 296, 495–509. [Google Scholar] [CrossRef]
- England, J.D.; Gronseth, G.S.; Franklin, G.; Miller, R.G.; Asbury, A.K.; Carter, G.T.; Cohen, J.A.; Fisher, M.A.; Howard, J.F.; Kinsella, L.J.; et al. Distal symmetric polyneuropathy: A definition for clinical research: Report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005, 64, 199–207. [Google Scholar] [CrossRef]
- Yamamoto, S.; Wilczek, H.E.; Nowak, G.; Larsson, M.; Oksanen, A.; Iwata, T.; Gjertsen, H.; Söderdahl, G.; Wikström, L.; Ando, Y.; et al. Liver Transplantation for Familial Amyloidotic Polyneuropathy (FAP): A Single-Center Experience Over 16 Years. Am. J. Transplant. 2007, 7, 2597–2604. [Google Scholar] [CrossRef] [PubMed]
- Dyck, P.J.B.; González-Duarte, A.; Obici, L.; Polydefkis, M.; Wiesman, J.F.; Antonino, I.; Litchy, W.J.; Dyck, P.J. Development of Measures of Polyneuropathy Impairment in HATTR Amyloidosis: From NIS to MNIS + 7. J. Neurol. Sci. 2019, 405, 116424. [Google Scholar] [CrossRef] [PubMed]
- Bedewi, M.A.; Kotb, M.A. Ultrasound Reference Values of C5, C6, and C7 Brachial Plexus Roots at the Interscalene Groove. Neurol. Sci. 2021, 42, 2425–2429. [Google Scholar] [CrossRef]
- Fisse, A.L.; Katsanos, A.H.; Gold, R.; Pitarokoili, K.; Krogias, C. Cross-sectional Area Reference Values for Peripheral Nerve Ultrasound in Adults: A Systematic Review and Meta-analysis—Part I: Upper Extremity Nerves. Eur. J. Neurol. 2021, 28, 1684–1691. [Google Scholar] [CrossRef]
- Wong, S.M.; Griffith, J.F.; Hui, A.C.; Lo, S.K.; Fu, M.; Wong, K.S. Carpal tunnel syndrome: Diagnostic usefulness of sonography. Radiology 2004, 232, 93–99. [Google Scholar] [CrossRef]
- Hobson-Webb, L.D.; Massey, J.M.; Juel, V.C.; Sanders, D.B. The ultrasonographic wrist-to-forearm median nerve area ratio in carpal tunnel syndrome. Clin. Neurophysiol. 2008, 119, 1353–1357. [Google Scholar] [CrossRef]
- Rimes-Dias, K.A.; Costa, J.C.; Canella, D.S. Obesity and health service utilization in Brazil: Data from the National Health Survey. BMC Public Health 2022, 22, 1474. [Google Scholar] [CrossRef]
- Russell, A.; Khayambashi, S.; Fine, N.M.; Chhibber, S.; Hahn, C. Characteristics of Carpal Tunnel Syndrome in Wild-Type Transthyretin Amyloidosis. Can. J. Neurol. Sci. 2024, 51, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Milandri, A.; Farioli, A.; Gagliardi, C.; Longhi, S.; Salvi, F.; Curti, S.; Foffi, S.; Caponetti, A.G.; Lorenzini, M.; Ferlini, A.; et al. Carpal Tunnel Syndrome in Cardiac Amyloidosis: Implications for Early Diagnosis and Prognostic Role across the Spectrum of Aetiologies. Eur. J. Heart Fail. 2020, 22, 507–515. [Google Scholar] [CrossRef]
- Padua, L.; LoMonaco, M.; Gregori, B.; Valente, E.M.; Padua, R.; Tonali, P. Neurophysiological classification and sensitivity in 500 carpal tunnel syndrome hands. Acta Neurol. Scand. 1997, 96, 211–217. [Google Scholar] [CrossRef]
- Castro, J.; Miranda, B.; de Castro, I.; Conceição, I. Changes in Nerve Conduction Studies Predate Clinical Symptoms Onset in Early Onset Val30Met Hereditary ATTR Amyloidosis. Eur. J. Neurol. 2022, 29, 826–832. [Google Scholar] [CrossRef]
- Gasparotti, R.; Salvalaggio, A.; Corbo, D.; Agazzi, G.; Cacciavillani, M.; Lozza, A.; Fenu, S.; De Vigili, G.; Tagliapietra, M.; Fabrizi, G.M.; et al. Magnetic Resonance Neurography and Diffusion Tensor Imaging of the Sciatic Nerve in Hereditary Transthyretin Amyloidosis Polyneuropathy. J. Neurol. 2023, 270, 4827–4840. [Google Scholar] [CrossRef]
- Ebenezer, G.J.; Liu, Y.; Judge, D.P.; Cunningham, K.; Truelove, S.; Carter, N.D.; Sebastian, B.; Byrnes, K.; Polydefkis, M. Cutaneous Nerve Biomarkers in Transthyretin Familial Amyloid Polyneuropathy. Ann. Neurol. 2017, 82, 44–56. [Google Scholar] [CrossRef]
- Ticau, S.; Sridharan, G.V.; Tsour, S.; Cantley, W.L.; Chan, A.; Gilbert, J.A.; Erbe, D.; Aldinc, E.; Reilly, M.M.; Adams, D.; et al. Neurofilament Light Chain as a Biomarker of Hereditary Transthyretin-Mediated Amyloidosis. Neurology 2021, 96, e412–e422. [Google Scholar] [CrossRef] [PubMed]
- Thimm, A.; Carpinteiro, A.; Oubari, S.; Papathanasiou, M.; Kessler, L.; Rischpler, C.; Malik, R.A.; Herrmann, K.; Reinhardt, H.C.; Rassaf, T.; et al. Corneal Confocal Microscopy Identifies Corneal Nerve Loss and Increased Langerhans Cells in Presymptomatic Carriers and Patients with Hereditary Transthyretin Amyloidosis. J. Neurol. 2023, 270, 3483–3491. [Google Scholar] [CrossRef] [PubMed]
- Schaan, A.P.; Gusmão, L.; Jannuzzi, J.; Modesto, A.; Amador, M.; Marques, D.; Rabenhorst, S.H.; Montenegro, R.; Lopes, T.; Yoshioka, F.K.; et al. New Insights on Intercontinental Origins of Paternal Lineages in Northeast Brazil. BMC Evol. Biol. 2020, 20, 15. [Google Scholar] [CrossRef]
- Barker, N.; Judge, D.P. Counseling Family Members and Monitoring for Evidence of Disease in Asymptomatic Carriers of Amyloid Transthyretin Cardiac Amyloidosis. Am. J. Cardiol. 2022, 185, S43–S50. [Google Scholar] [CrossRef] [PubMed]
| ATTRv-PN | ATTRv-C | |
|---|---|---|
| Number of patients | 31 | 41 |
| Gender | 17 males (55%)/14 females (45%) | 26 females (63%)/15 males (37%) |
| Age (y) | 56.25 (range: 34 to 81) | 42.82 (range: 20 to 69) |
| BMI | 26.1 kg/m2 (5.6) | 25.8 kg/m2 (5.2) |
| TTR variants | Ile107Val (10), Val30Met (9), Val122Ile (9), Ala97Ser (1) Gli67Glu (1), Val30Met and Val122Ile (1) | Val122Ile (30), Ile107Val (7), Val30Met (2), Ala97Ser (1), Thr80Ala (1) |
| PND stage | 1 (24)/2 (4)/3a (2)/3b (1) | 0 (41) |
| Treatment | Tafamidis (22); Patisiran (3); Inotersen (2); Liver transplantation (2); Vutrisiran (1); No treatment (1) | No treatment (41) |
| Site | n | ATTRv-PN CSA | Ref CSA | p Value |
|---|---|---|---|---|
| Median Nerve at the wrist | 31 | 10.17 (2.95) | 8.3 | 0.0075 |
| Median Nerve at the forearm | 31 | 6.85 (2.84) | 6.4 | 0.9064 |
| Median Nerve at the arm | 12 | 9.8 (1.8) | 8.3 | 0.0239 |
| C6 root | 12 | 8.55 (1.38) | 5.8 | 0.0001 |
| Brachial plexus | 12 | 70.82 (18.8) | 46.13 | 0.0001 |
| Site | n | ATTRv-Carriers CSA | Ref CSA | p Value |
|---|---|---|---|---|
| Median Nerve at the wrist | 41 | 10.44 (2.76) | 8.3 | 0.0001 |
| Median Nerve at the forearm | 41 | 6.12 (1.19) | 6.4 | 0.139 |
| Median Nerve at the arm | 24 | 8.9 (2.04) | 8.3 | 0.163 |
| C6 root | 24 | 8.42 (2.10) | 5.8 | 0.0001 |
| Brachial plexus | 24 | 61.97 (14.1) | 46.13 | 0.0001 |
| ATTRv-PN | ATTRv-C | |
|---|---|---|
| Number of patients | 31 | 41 |
| Clinical Complaints | 64.5% | 14.6% |
| Median Nerve at Wrist (>10 mm2) | 47.16% | 52.4% |
| Wrist/Forearm ratio (>1.4) | 61.3% | 73.8% |
| Median Nerve CSA Range (mm2) | 5.4–14.1 | 6.47–19.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camelo-Filho, A.E.; Covaleski, A.P.P.M.; Brito, L.A.; Rodrigues, C.L.; Moreira, A.L. Peripheral Nerve Ultrasound Findings in Hereditary Transthyretin Amyloidosis in Brazil. Diagnostics 2025, 15, 2556. https://doi.org/10.3390/diagnostics15202556
Camelo-Filho AE, Covaleski APPM, Brito LA, Rodrigues CL, Moreira AL. Peripheral Nerve Ultrasound Findings in Hereditary Transthyretin Amyloidosis in Brazil. Diagnostics. 2025; 15(20):2556. https://doi.org/10.3390/diagnostics15202556
Chicago/Turabian StyleCamelo-Filho, Antonio Edvan, Anna Paula Paranhos Miranda Covaleski, Lara Albuquerque Brito, Cleonisio Leite Rodrigues, and Ana Lucila Moreira. 2025. "Peripheral Nerve Ultrasound Findings in Hereditary Transthyretin Amyloidosis in Brazil" Diagnostics 15, no. 20: 2556. https://doi.org/10.3390/diagnostics15202556
APA StyleCamelo-Filho, A. E., Covaleski, A. P. P. M., Brito, L. A., Rodrigues, C. L., & Moreira, A. L. (2025). Peripheral Nerve Ultrasound Findings in Hereditary Transthyretin Amyloidosis in Brazil. Diagnostics, 15(20), 2556. https://doi.org/10.3390/diagnostics15202556





