Diagnostic Stratification of Pancreatic Ductal Adenocarcinoma via Metallomics and Blood-Based Biomarkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Patient Selection
2.2. Analysis of Serum and Urinary Metals
2.3. Statistical Analysis
3. Results
3.1. Univariate Analysis
3.2. Multivariate Analysis (PCA–LDA)
3.3. Correlations with Inflammatory Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| ABC | Adaptive Box–Cox |
| AISI | Aggregate Index of Systemic Inflammation |
| BLM | Bloom syndrome protein (DNA helicase) |
| CA19-9 | Carbohydrate Antigen 19-9 |
| Cd | Cadmium |
| Cr | Chromium |
| Cu | Copper |
| Fe | Iron |
| HGB/RDW | Hemoglobin/Red Cell Distribution Width ratio |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| LDA | Linear Discriminant Analysis |
| MLR | Monocyte-to-Lymphocyte Ratio |
| Mn | Manganese |
| Na | Not available |
| Ni | Nickel |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| PCA | Principal Component Analysis |
| PDAC | Pancreatic Ductal Adenocarcinoma |
| PLR | Platelet-to-Lymphocyte Ratio |
| QC | Quality Control |
| ROS | Reactive Oxygen Species |
| Sb | Antimony |
| Se | Selenium |
| SII | Systemic Immune-Inflammation Index |
| SIRI | Systemic Inflammation Response Index |
| Sn | Tin |
| V | Vanadium |
| Zn | Zinc |
References
- Mack, S.; Koessler, T.; Bichard, P.; Frossard, J.-L. Pancreatic cancer: Epidemiology, risk factors, and prevention. Onco 2025, 5, 37. [Google Scholar] [CrossRef]
- Pu, N.; He, T.; Wu, W.; Yin, H.; Habib, J.R.; Chen, Q.; Xu, Z.; Jiang, Z.; Jin, Y.; Lou, W. Adjuvant chemotherapy improves survival in resected early-onset pancreatic cancer after neoadjuvant therapy: A retrospective cohort study based on the seer database. Oncol. Adv. 2025, 3, 61–72. [Google Scholar] [CrossRef]
- Qadir, R.M.A.B.; Umair, M.B.; Tariq, U.B.; Ahmad, A.; Kiran, W.; Shahid, M.H. Unraveling pancreatic cancer: Epidemiology, risk factors, and global trends. Cureus 2024, 16, e72816. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Peshin, S.; Takrori, E.; Kodali, N.A.; Bashir, F.; Singal, S. Advances in the management of pancreatic cancer: Current strategies and emerging therapies. Int. J. Mol. Sci. 2025, 26, 7055. [Google Scholar] [CrossRef] [PubMed]
- Sciano, F.; Terrana, F.; Pecoraro, C.; Parrino, B.; Cascioferro, S.; Diana, P.; Giovannetti, E.; Carbone, D. Exploring the therapeutic potential of focal adhesion kinase inhibition in overcoming chemoresistance in pancreatic ductal adenocarcinoma. Future Med. Chem. 2024, 16, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Shih, W.-H.; Huang, H.-L.; HuangFu, W.-C.; Lin, T.E.; Sung, T.-Y.; Li, M.-C.; Huang, G.-L.; Chang, Y.-W.; Yen, S.-C.; Hsieh, H.-P. Discovery of novel tank-binding kinase 1 (tbk1) inhibitor against pancreatic ductal adenocarcinoma. Int. J. Biol. Macromol. 2024, 283, 137296. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Liu, Y.; Tang, C.; Zhu, H. Pancreatic cancer: Pathogenesis and clinical studies. MedComm 2025, 6, e70162. [Google Scholar] [CrossRef]
- Sarantis, P.; Koustas, E.; Papadimitropoulou, A.; Papavassiliou, A.G.; Karamouzis, M.V. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J. Gastrointest. Oncol. 2020, 12, 173. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, F. Diagnostic value of serum carbohydrate antigen 19-9 in pancreatic cancer: A meta-analysis. Tumor Biol. 2014, 35, 7459–7465. [Google Scholar] [CrossRef]
- Caputo, C.; Falco, M.; Grimaldi, A.; Lombardi, A.; Miceli, C.C.; Cocule, M.; Montella, M.; Pompella, L.; Tirino, G.; Campione, S. Identification of tissue mirna signatures for pancreatic ductal adenocarcinoma. Cancers 2024, 16, 824. [Google Scholar] [CrossRef]
- Coradduzza, D.; Garroni, G.; Congiargiu, A.; Balzano, F.; Cruciani, S.; Sedda, S.; Nivoli, A.; Maioli, M. Micrornas, stem cells in bipolar disorder, and lithium therapeutic approach. Int. J. Mol. Sci. 2022, 23, 10489. [Google Scholar] [CrossRef] [PubMed]
- Coradduzza, D.; Ghironi, A.; Azara, E.; Culeddu, N.; Cruciani, S.; Zinellu, A.; Maioli, M.; De Miglio, M.R.; Medici, S.; Fozza, C. Role of polyamines as biomarkers in lymphoma patients: A pilot study. Diagnostics 2022, 12, 2151. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Pisano, A.; Bocca, B.; Fenu, G.; Farace, C.; Etzi, F.; Perra, T.; Sabalic, A.; Porcu, A.; Madeddu, R. Toxic metal and essential element concentrations in the blood and tissues of pancreatic ductal adenocarcinoma patients. Toxics 2024, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Coradduzza, D.; Congiargiu, A.; Azara, E.; Mammani, I.M.A.; De Miglio, M.R.; Zinellu, A.; Carru, C.; Medici, S. Heavy metals in biological samples of cancer patients: A systematic literature review. Biometals 2024, 37, 803–817. [Google Scholar] [CrossRef]
- Coradduzza, D.; Sanna, A.; Di Lorenzo, B.; Congiargiu, A.; Marra, S.; Cossu, M.; Tedde, A.; De Miglio, M.R.; Zinellu, A.; Mangoni, A.A. Associations between plasma and urinary heavy metal concentrations and the risk of prostate cancer. Sci. Rep. 2025, 15, 14274. [Google Scholar] [CrossRef]
- Frydrych, A.; Krośniak, M.; Jurowski, K. The role of chosen essential elements (zn, cu, se, fe, mn) in food for special medical purposes (fsmps) dedicated to oncology patients—Critical review: State-of-the-art. Nutrients 2023, 15, 1012. [Google Scholar] [CrossRef]
- Wróblewski, M.; Wróblewska, W.; Sobiesiak, M. The role of selected elements in oxidative stress protection: Key to healthy fertility and reproduction. Int. J. Mol. Sci. 2024, 25, 9409. [Google Scholar] [CrossRef]
- Shadfar, S.; Parakh, S.; Jamali, M.S.; Atkin, J.D. Redox dysregulation as a driver for DNA damage and its relationship to neurodegenerative diseases. Transl. Neurodegener. 2023, 12, 18. [Google Scholar] [CrossRef]
- Angelé-Martínez, C.; Goodman, C.; Brumaghim, J. Metal-mediated DNA damage and cell death: Mechanisms, detection methods, and cellular consequences. Metallomics 2014, 6, 1358–1381. [Google Scholar] [CrossRef]
- Coradduzza, D.; Congiargiu, A.; Chen, Z.; Zinellu, A.; Carru, C.; Medici, S. Ferroptosis and senescence: A systematic review. Int. J. Mol. Sci. 2023, 24, 3658. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive oxygen species signaling and oxidative stress: Transcriptional regulation and evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ros) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wu, H.; Yang, Y.; Huang, J.; He, X.; Gong, Y. Harnessing the interaction between redox signaling and senescence to restrain tumor drug resistance. Front. Cell Dev. Biol. 2025, 13, 1639772. [Google Scholar] [CrossRef]
- Ohara, Y.; Valenzuela, P.; Hussain, S.P. The interactive role of inflammatory mediators and metabolic reprogramming in pancreatic cancer. Trends Cancer 2022, 8, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Pan, J.; Bao, Q. Ferroptosis in senescence and age-related diseases: Pathogenic mechanisms and potential intervention targets. Mol. Biol. Rep. 2025, 52, 238. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Khan, N.; Fatima, K.; Farooq, S.; Ramzan, M.; El-Seedi, H.R.; Uddin, J.; Muhsinah, A.B.; Musharraf, S.G. Serum metallomics reveals insights into the associations of elements with the progression of preleukemic diseases toward acute leukemia. Biol. Methods Protoc. 2024, 9, bpad027. [Google Scholar] [CrossRef]
- Manaprasertsak, A. Tracing Chemical Alterations in Cancer Cells and Tissues. Ph.D. Thesis, Lund University, Lund, Sweden, 2025. [Google Scholar]
- Byeon, S.; Toit-Thompson, T.D.; Hipperson, L.; Maloney, S.; Wenzel, R.; Gill, A.J.; Samra, J.S.; Mittal, A.; Sahni, S. Serum and tissue metallome of pancreatic ductal adenocarcinoma. Cancer Sci. 2024, 115, 1446–1458. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, S.; Yu, N.; Li, H. Zip4: A promising early diagnostic and therapeutic targets for pancreatic cancer. Am. J. Cancer Res. 2024, 14, 4652. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Luo, J.; Qi, Z.; Li, S.; Shen, L.; Li, J.; Fang, X.; Huang, J.; Liu, B. Sox4-zip14-zinc metabolism mediates oncogenesis and suppresses t cell immunity in nasopharyngeal carcinoma. Cell Rep. Med. 2025, 6, 102300. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Yang, J.; Zhan, H.; Zhou, Z.; Jiang, Y.; Shi, X.; Fan, X.; Zhang, J.; Luo, W. Zinc dependent regulation of zeb1 and yap1 co-activation promotes emt plasticity and metastasis in pancreatic cancer. Gastroenterology 2021, 160, 1771. [Google Scholar] [CrossRef] [PubMed]
- Sibono, L.; Grosso, M.; Tejedor-Calvo, E.; Casula, M.; Marco-Montori, P.; Garcia-Barreda, S.; Manis, C.; Caboni, P. A critical analysis of adaptive box-cox transformation for skewed distributed data management: Metabolomics of spanish and argentinian truffles as a case study. Anal. Chim. Acta 2025, 1345, 343704. [Google Scholar] [CrossRef]
- Yu, H.; Sang, P.; Huan, T. Adaptive box–cox transformation: A highly flexible feature-specific data transformation to improve metabolomic data normality for better statistical analysis. Anal. Chem. 2022, 94, 8267–8276. [Google Scholar] [CrossRef]
- Ballabio, D.; Consonni, V. Classification tools in chemistry. Part 1: Linear models. Pls-da. Anal. Methods 2013, 5, 3790–3798. [Google Scholar] [CrossRef]
- Hollander, M.; Wolfe, D.A.; Chicken, E. Nonparametric Statistical Methods; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Zhang, K.; Hua, Y.-Q.; Wang, D.; Chen, L.-Y.; Wu, C.-J.; Chen, Z.; Liu, L.-M.; Chen, H. Systemic immune-inflammation index predicts prognosis of patients with advanced pancreatic cancer. J. Transl. Med. 2019, 17, 30. [Google Scholar] [CrossRef]
- Neumann, C.C.; Schneider, F.; Hilfenhaus, G.; Vecchione, L.; Felsenstein, M.; Ihlow, J.; Geisel, D.; Sander, S.; Pratschke, J.; Stintzing, S. Inflammation-based prognostic scores in pancreatic cancer patients—A single-center analysis of 1294 patients within the last decade. Cancers 2023, 15, 2367. [Google Scholar] [CrossRef]
- Niu, Z.-h.; Lin, L.; Peng, H.-Y.; Zheng, X.-Z.; Wang, M.-Y.; Sun, F.-X.; Xu, C.-J. The prognostic value of systemic inflammation response index in digestive system carcinomas: A systematic review and meta-analysis. BMC Gastroenterol. 2025, 25, 34. [Google Scholar] [CrossRef]
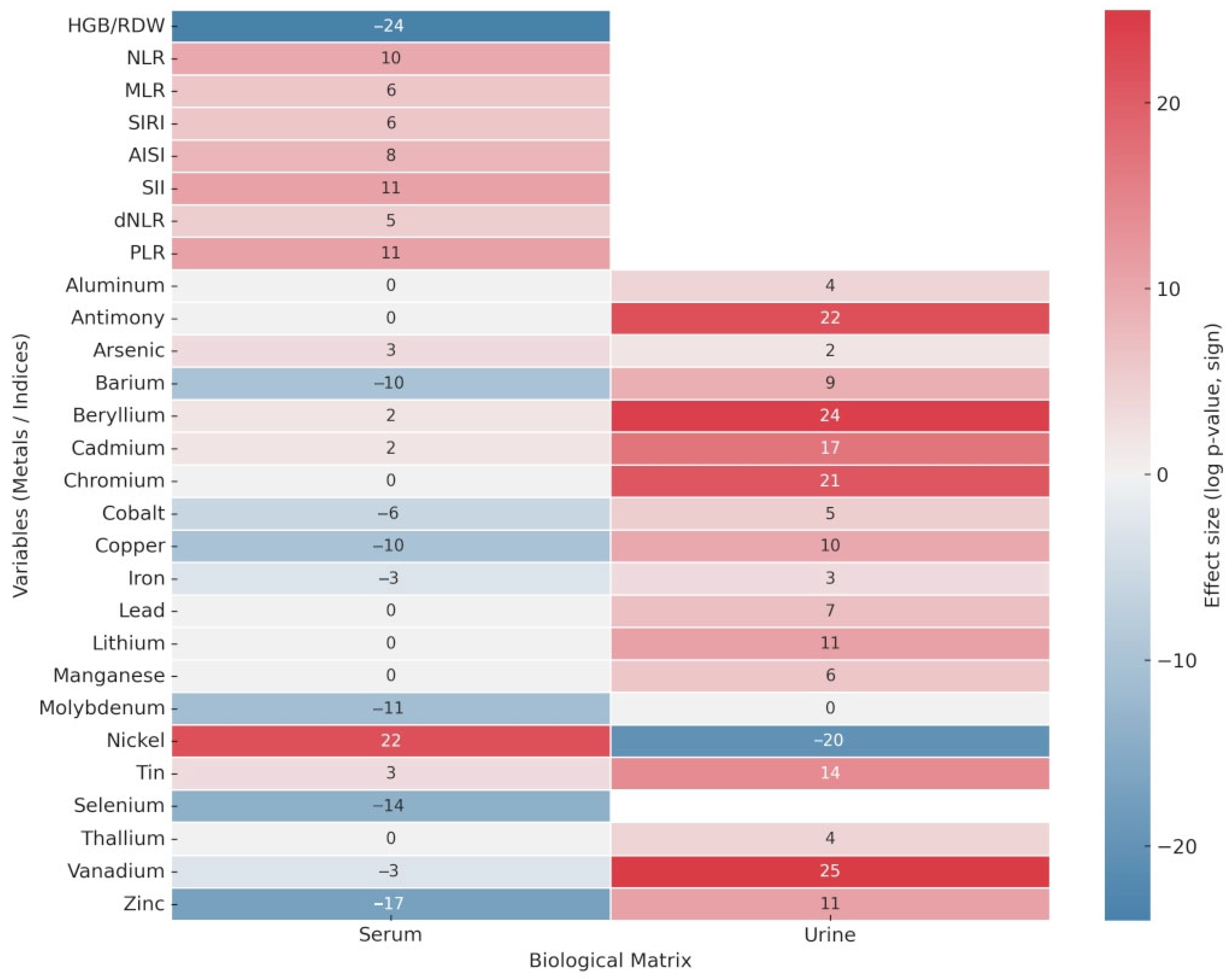
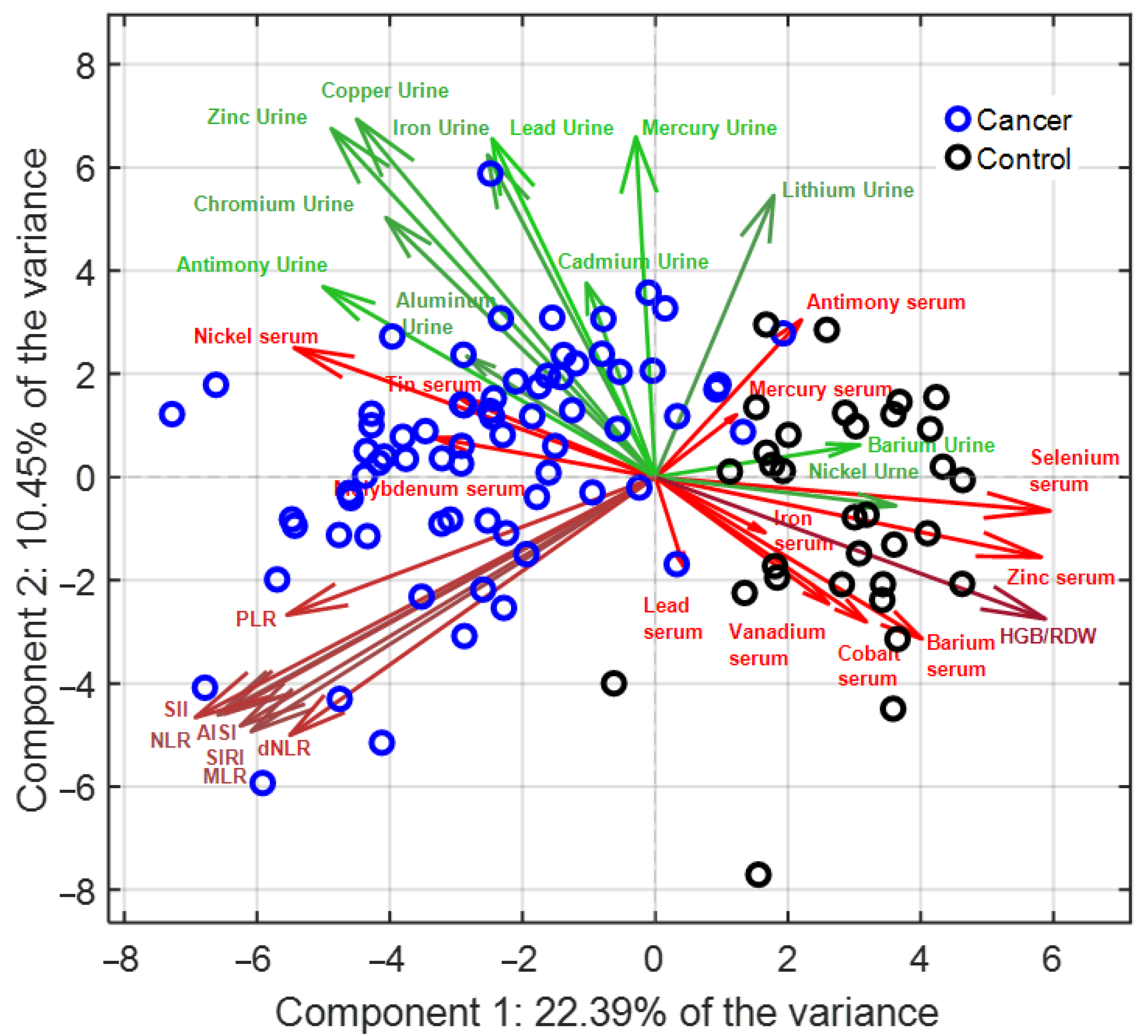
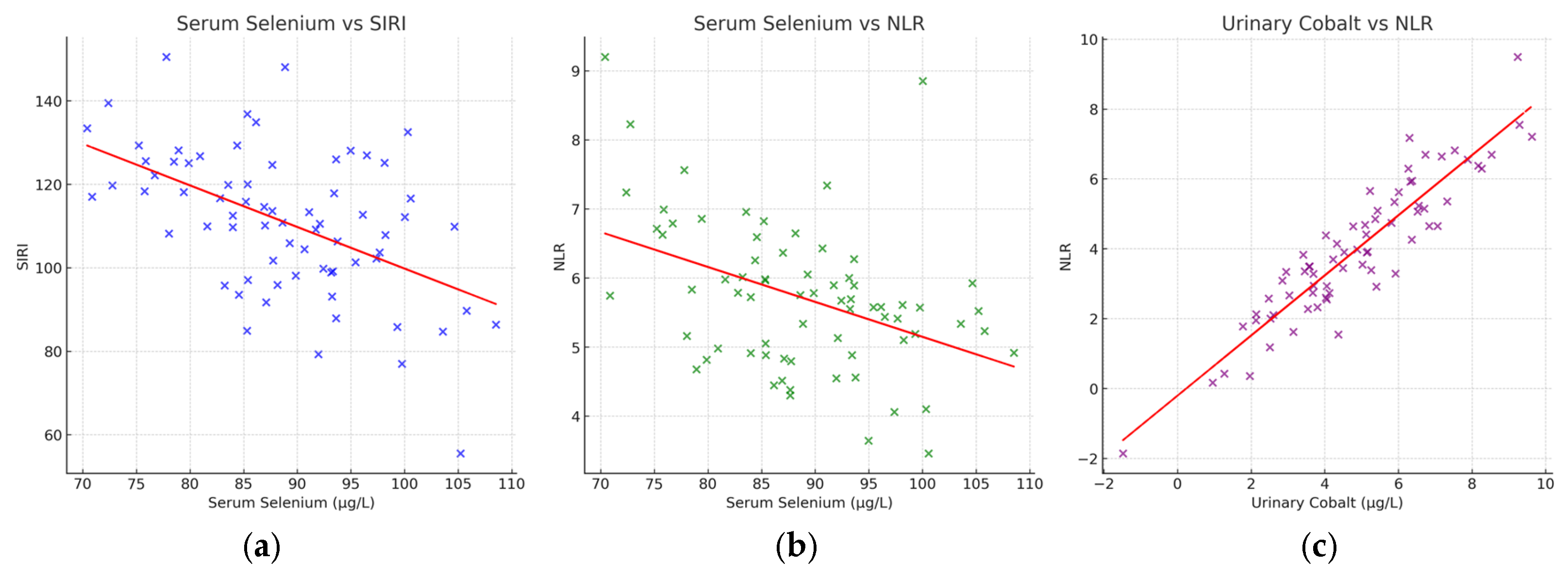
| Haemotological Data | p-Values | |
|---|---|---|
| HGB/RDW | 6.28 × 10−24 | |
| NLR | 1.13 × 10−10 | |
| MLR | 2.65 × 10−6 | |
| SIRI | 1.59 × 10−6 | |
| AISI | 8.51 × 10−8 | |
| SII | 1.73 × 10−11 | |
| dNLR | 1.02 × 10−5 | |
| PLR | 9.35 × 10−11 | |
| Metals | Serum p-values | Urine p-values |
| Aluminum | 1.96 × 10−4 | |
| Antimony | 2.54 × 10−22 | |
| Arsenic | 7.46 × 10−3 | 4.45 × 10−2 |
| Barium | 8.08 × 10−10 | 2.07 × 10−9 |
| Beryllium | 3.53 × 10−2 | 1.04 × 10−24 |
| Cadmium | 3.87 × 10−2 | 1.99 × 10−17 |
| Chromium | 9.39 × 10−21 | |
| Cobalt | 5.37 × 10−6 | 8.11 × 10−5 |
| Copper | 1.53 × 10−10 | |
| Iron | 3.82 × 10−3 | 1.22 × 10−3 |
| Lead | 4.92 × 10−7 | |
| Lithium | 1.29 × 10−11 | |
| Manganese | 9.46 × 10−6 | |
| Molybdenum | 5.02 × 10−11 | |
| Nickel | 2.31 × 10−22 | 5.17 × 10−20 |
| Tin | 8.83 × 10−3 | 2.25 × 10−14 |
| Selenium | 3.73 × 10−14 | NA |
| Thallium | 1.03 × 10−4 | |
| Vanadium | 4.25 × 10−3 | 6.03 × 10−25 |
| Zinc | 2.69 × 10−17 | 1.24 × 10−11 |
| No statistically significant difference (p ≥ 0.05) | ||
| Significantly higher in the cancer group (p < 10−5) | ||
| Significantly higher in the cancer group (p < 0.05) | ||
| Significantly higher in the control group (p < 0.05) | ||
| Significantly higher in the control group (p < 10−5) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coradduzza, D.; Perra, T.; Sibono, L.; Sanna, A.; Cossu, M.; Azara, E.G.; Petracca, F.; Madeddu, R.B.; De Miglio, M.R.; Carru, C.; et al. Diagnostic Stratification of Pancreatic Ductal Adenocarcinoma via Metallomics and Blood-Based Biomarkers. Diagnostics 2025, 15, 2818. https://doi.org/10.3390/diagnostics15212818
Coradduzza D, Perra T, Sibono L, Sanna A, Cossu M, Azara EG, Petracca F, Madeddu RB, De Miglio MR, Carru C, et al. Diagnostic Stratification of Pancreatic Ductal Adenocarcinoma via Metallomics and Blood-Based Biomarkers. Diagnostics. 2025; 15(21):2818. https://doi.org/10.3390/diagnostics15212818
Chicago/Turabian StyleCoradduzza, Donatella, Teresa Perra, Leonardo Sibono, Andrea Sanna, Maurizio Cossu, Emanuela G. Azara, Francesco Petracca, Roberto Beniamino Madeddu, Maria Rosaria De Miglio, Ciriaco Carru, and et al. 2025. "Diagnostic Stratification of Pancreatic Ductal Adenocarcinoma via Metallomics and Blood-Based Biomarkers" Diagnostics 15, no. 21: 2818. https://doi.org/10.3390/diagnostics15212818
APA StyleCoradduzza, D., Perra, T., Sibono, L., Sanna, A., Cossu, M., Azara, E. G., Petracca, F., Madeddu, R. B., De Miglio, M. R., Carru, C., Grosso, M., Cossu, M. L., & Medici, S. (2025). Diagnostic Stratification of Pancreatic Ductal Adenocarcinoma via Metallomics and Blood-Based Biomarkers. Diagnostics, 15(21), 2818. https://doi.org/10.3390/diagnostics15212818










