Arterial Enhancement Fraction-Spectral CT-Based Model as Part of Prediction Model in BRAFV600E-Positive Papillary Thyroid Carcinoma
Abstract
1. Introduction
2. Materials and Methods
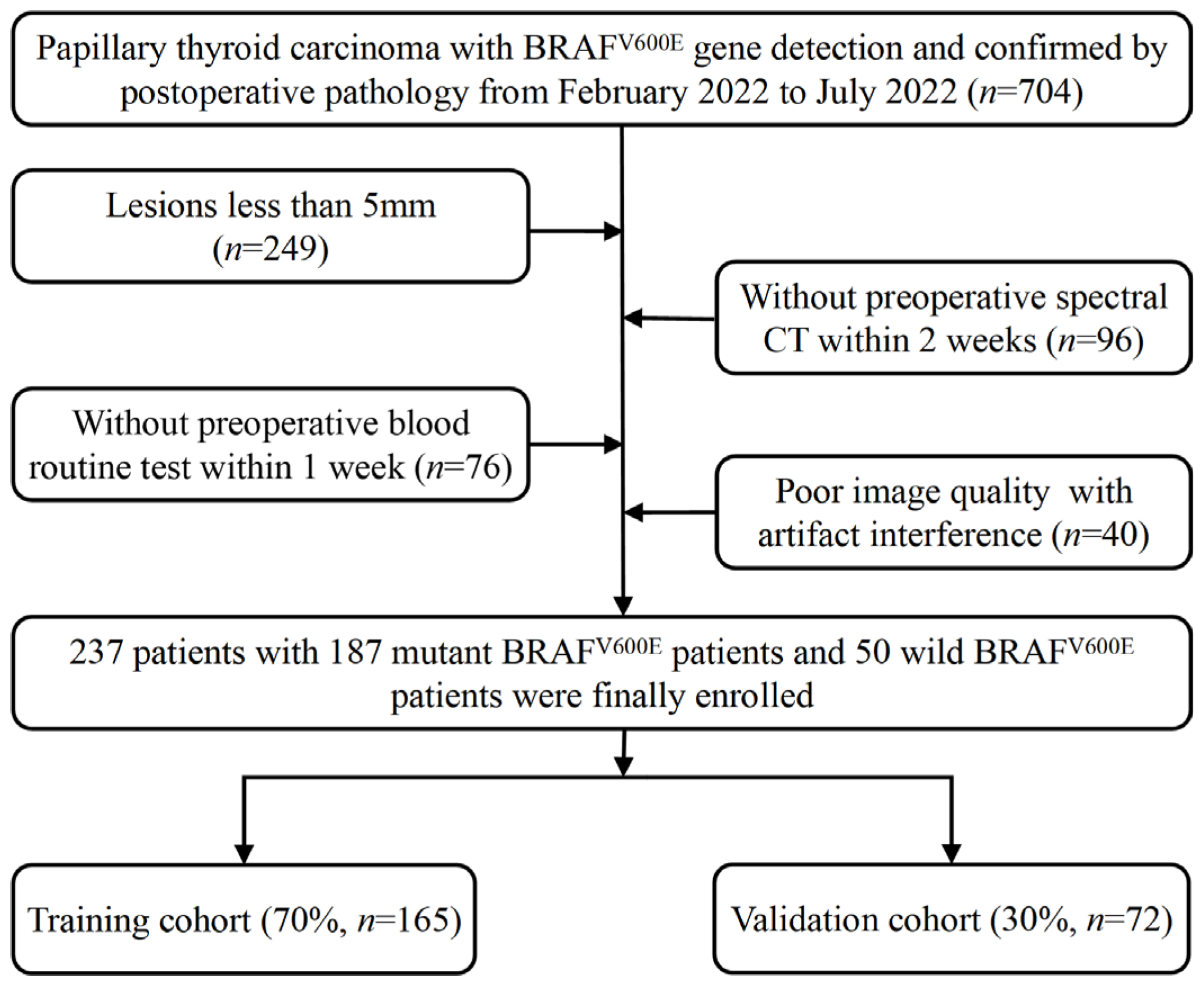
2.1. Patient Selection
2.2. DLCT Imaging Acquisition
2.3. AEF and DLCT Parameters Measurement
2.4. Detection of the BRAFV600E Mutation and Collection of Clinical Information
2.5. Prediction Models Based on Significant Parameters and Construction of Nomogram
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Comparison of the AEF and DLCT Parameters
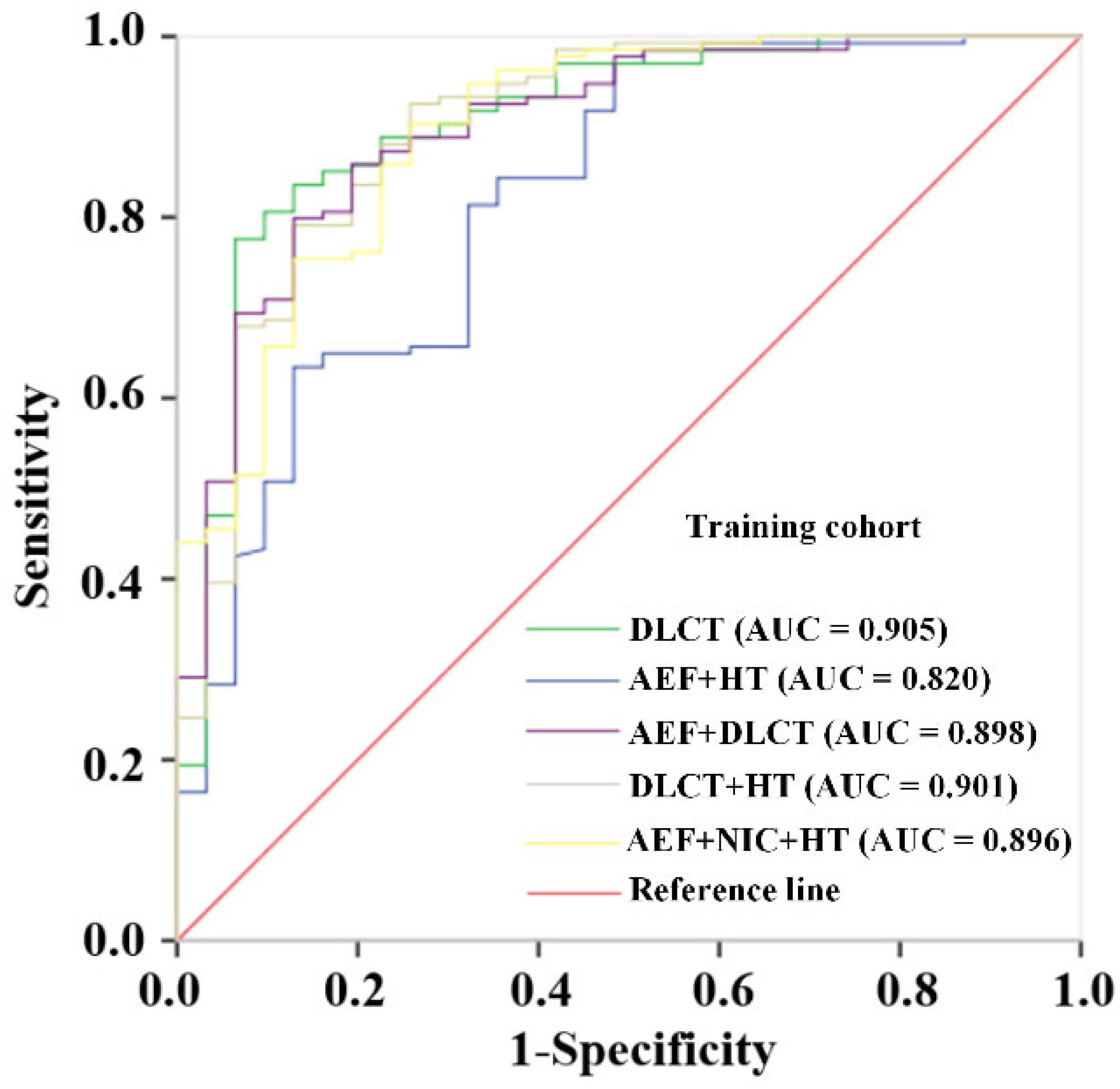
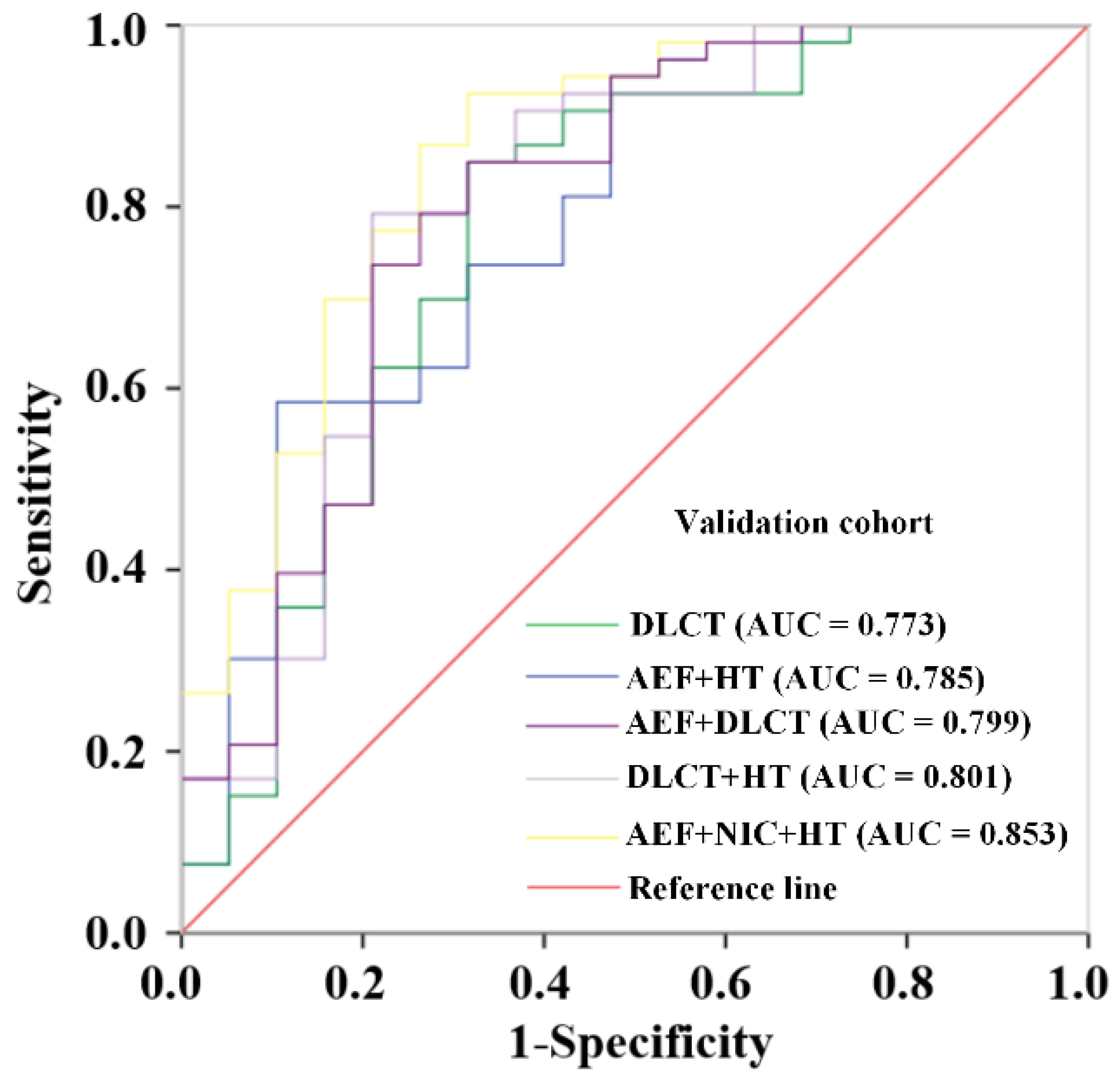
3.3. Construction and Validation of Prediction Models Base on Different Combinations
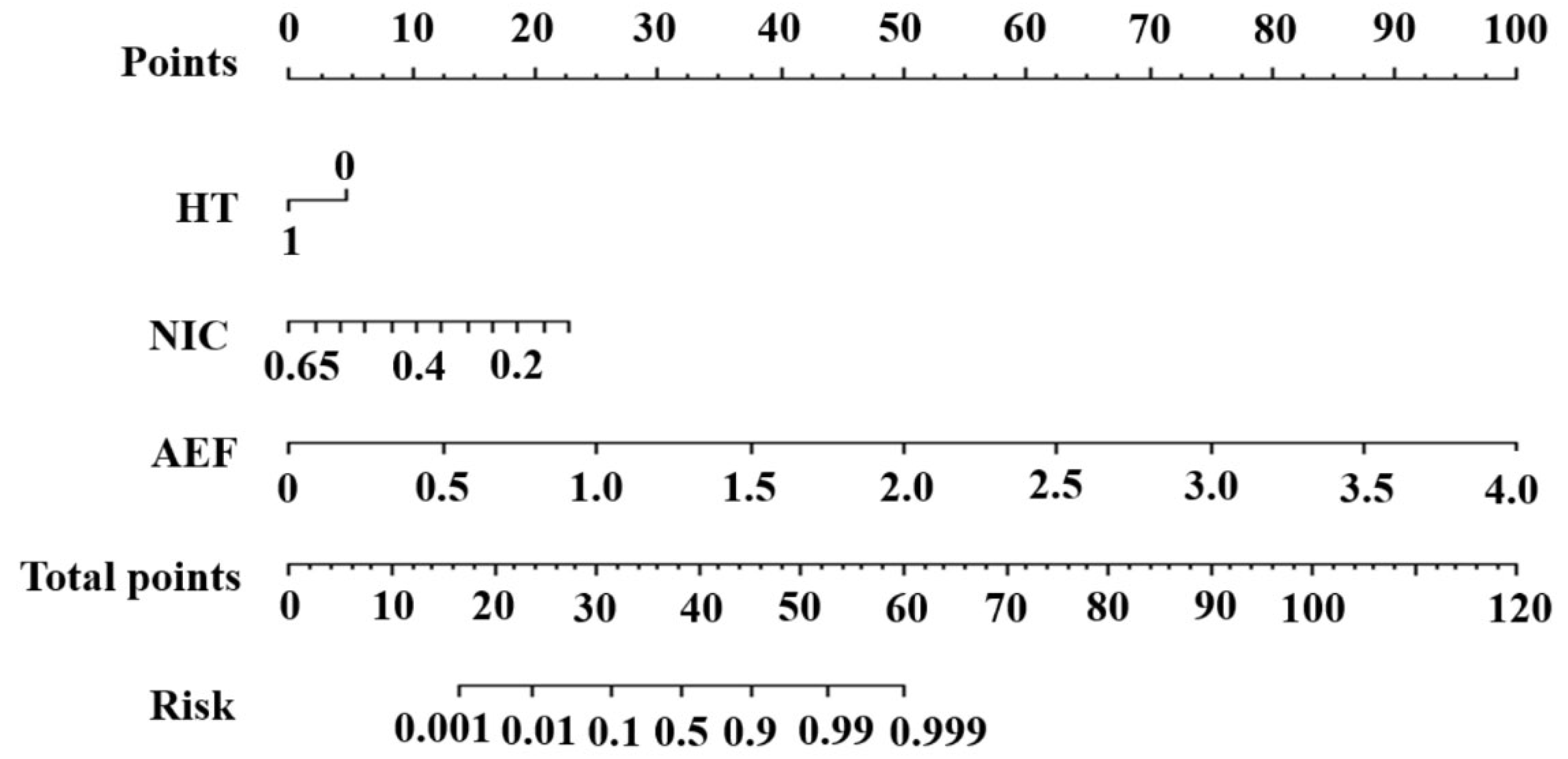
3.4. Construction and Evaluation of the Nomogram
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid 2017, 27, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Jeon, M.J.; Oh, H.-S.; Han, M.; Lee, Y.-M.; Kim, T.Y.; Chung, K.-W.; Kim, W.B.; Shong, Y.K.; Song, D.E.; et al. Do aggressive variants of papillary thyroid carcinoma have worse clinical outcome than classic papillary thyroid carcinoma? Eur. J. Endocrinol. 2018, 179, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.M.; Macerola, E.; Proietti, A.; Vignali, P.; Sparavelli, R.; Torregrossa, L.; Matrone, A.; Basolo, A.; Elisei, R.; Santini, F.; et al. Clinical-Pathological Features and Treatment Outcome of Patients with Hobnail Variant Papillary Thyroid Carcinoma. Front. Endocrinol. 2022, 13, 842424. [Google Scholar] [CrossRef] [PubMed]
- Bouzehouane, N.; Roy, P.; Decaussin-Petrucci, M.; Bertholon-Gregoire, M.; Bully, C.; Perrin, A.; Lasolle, H.; Lifante, J.-C.; Borson-Chazot, F.; Bournaud, C. Prognostic Impact of Microscopic Extra-Thyroidal Extension (mETE) on Disease Free Survival in Patients with Papillary Thyroid Carcinoma (PTC). Cancers 2022, 14, 2591. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Chen, Y.; Sadow, P.M.; Suh, H.; Lee, K.E.; Choi, J.Y.; Suh, Y.J.; Wang, T.S.; Lubitz, C.C. BRAF(V600E) Is Correlated with Recurrence of Papillary Thyroid Microcarcinoma: A Systematic Review, Multi-Institutional Primary Data Analysis, and Meta-Analysis. Thyroid 2016, 26, 248–255. [Google Scholar] [CrossRef]
- Chen, D.; Su, X.; Zhu, L.; Jia, H.; Han, B.; Chen, H.; Liang, Q.; Hu, C.; Yang, H.; Liu, L.; et al. Papillary thyroid cancer organoids harboring BRAF(V600E) mutation reveal potentially beneficial effects of BRAF inhibitor-based combination therapies. J. Transl. Med. 2023, 21, 9. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Lesnik, D.; Cunnane, M.E.; Zurakowski, D.; Acar, G.O.; Ecevit, C.; Mace, A.; Kamani, D.; Randolph, G.W. Papillary thyroid carcinoma nodal surgery directed by a preoperative radiographic map utilizing CT scan and ultrasound in all primary and reoperative patients. Head Neck 2014, 36, 191–202. [Google Scholar] [CrossRef]
- Chai, B.; Xiang, D.; Wang, W.; Ren, Y.; Wang, F.; Wang, J.; Zhou, G.; Zheng, C. Arterial enhancement fraction in evaluating the therapeutic effect and survival for hepatocellular carcinoma patients treated with DEB-TACE. Cancer Imaging 2022, 22, 38. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Y.-K.; Geng, D.; Wang, J.-W.; Chen, X.-B.; Si, Y.; Shen, M.-P.; Su, G.-Y.; Xu, X.-Q.; Wu, F.-Y. Added value of arterial enhancement fraction derived from dual-energy computed tomography for preoperative diagnosis of cervical lymph node metastasis in papillary thyroid cancer: Initial results. Eur. Radiol. 2024, 34, 1292–1301. [Google Scholar] [CrossRef]
- Zhi, J.; Jia, X.-J.; Yan, J.; Wang, H.-C.; Feng, B.; Xing, H.-Y.; Jia, Y.-T. BRAFV600E mutant colorectal cancer cells mediate local immunosuppressive microenvironment through exosomal long noncoding RNAs. World J. Gastrointest. Oncol. 2021, 13, 2129–2148. [Google Scholar] [CrossRef]
- Husain, A.; Hu, N.; Sadow, P.M.; Nucera, C. Expression of angiogenic switch, cachexia and inflammation factors at the crossroad in undifferentiated thyroid carcinoma with BRAFv600E. Cancer Lett. 2016, 380, 577–585. [Google Scholar] [CrossRef]
- Liang, H.; Zhou, Y.; Zheng, Q.; Yan, G.; Liao, H.; Du, S.; Zhang, X.; Lv, F.; Zhang, Z.; Li, Y.M. Dual-energy CT with virtual monoenergetic images and iodine maps improves tumor conspicuity in patients with pancreatic ductal adenocarcinoma. Insights Imaging 2022, 13, 153. [Google Scholar] [CrossRef]
- Arico, F.M.; Trimarchi, R.; Portaluri, A.; Barilla, C.; Migliaccio, N.; Bucolo, G.M.; Cicero, G.; Sofia, C.; Booz, C.; Vogl, T.J.; et al. Virtual monoenergetic dual-layer dual-energy CT images in colorectal cancer: CT diagnosis could be improved? Radiol. Medica 2023, 128, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Koch, V.; Weitzer, N.; Dos Santos, D.P.; Gruenewald, L.D.; Mahmoudi, S.; Martin, S.S.; Eichler, K.; Bernatz, S.; Gruber-Rouh, T.; Booz, C.; et al. Multiparametric detection and outcome prediction of pancreatic cancer involving dual-energy CT, diffusion-weighted MRI, and radiomics. Cancer Imaging 2023, 23, 38. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, F.; Wang, L.; Du, L.; Shen, H.; Wang, X.; Zeng, Z.; Liu, D.; Tao, J.; Wu, J.; et al. Quantitative parameters derived from dual-energy computed tomography for the preoperative prediction of early recurrence in patients with esophageal squamous cell carcinoma. Eur. Radiol. 2023, 33, 7419–7428. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, Q.; Zhang, D.; Li, X.; Yu, J.; Liu, Q.; Li, Z.; Huang, J.; Zhang, X.; Tang, Z. Nomogram based on spectral CT quantitative parameters and typical radiological features for distinguishing benign from malignant thyroid micro-nodules. Cancer Imaging 2023, 23, 13. [Google Scholar] [CrossRef]
- Zhou, Y.; Geng, D.; Su, G.Y.; Chen, X.B.; Si, Y.; Shen, M.P.; Xu, X.Q.; Wu, F.Y. Extracellular Volume Fraction Derived From Dual-Layer Spectral Detector Computed Tomography for Diagnosing Cervical Lymph Nodes Metastasis in Patients with Papillary Thyroid Cancer: A Preliminary Study. Front. Oncol. 2022, 12, 851244. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Xu, F.-J.; Agyekum, E.A.; Xiang, H.; Wang, Y.-D.; Zhang, J.; Sun, H.; Zhang, G.-L.; Bo, X.-S.; Lv, W.-Z.; et al. Radiomic Model for Determining the Value of Elasticity and Grayscale Ultrasound Diagnoses for Predicting BRAF(V600E) Mutations in Papillary Thyroid Carcinoma. Front. Endocrinol. 2022, 13, 872153. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, J.M.; Kim, J.H.; Klotz, E.; Kim, H.C.; Han, J.K.; Choi, B.I. CT color mapping of the arterial enhancement fraction of VX2 carcinoma implanted in rabbit liver: Comparison with perfusion CT. AJR Am. J. Roentgenol. 2011, 196, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Sadow, P.M.; Priolo, C.; Nanni, S.; Karreth, F.A.; Duquette, M.; Martinelli, R.; Husain, A.; Clohessy, J.; Kutzner, H.; Mentzel, T.; et al. Role of BRAFV600E in the first preclinical model of multifocal infiltrating myopericytoma development and microenvironment. J. Natl. Cancer Inst. 2014, 106, dju182. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Tallini, G.; Puxeddu, E.; Sponziello, M.; Moretti, S.; Ligorio, C.; Cavaliere, A.; Rhoden, K.J.; Verrienti, A.; Maranghi, M.; et al. BRAFV600E mutation and expression of proangiogenic molecular markers in papillary thyroid carcinomas. Eur. J. Endocrinol. 2011, 165, 455–463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Durante, C.; Puxeddu, E.; Ferretti, E.; Morisi, R.; Moretti, S.; Bruno, R.; Barbi, F.; Avenia, N.; Scipioni, A.; Verrienti, A.; et al. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J. Clin. Endocrinol. Metab. 2007, 92, 2840–2843. [Google Scholar] [CrossRef]
- Liu, D.; Hu, S.; Hou, P.; Jiang, D.; Condouris, S.; Xing, M. Suppression of BRAF/MEK/MAP kinase pathway restores expression of iodide-metabolizing genes in thyroid cells expressing the V600E BRAF mutant. Clin. Cancer Res. 2007, 13, 1341–1349. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Y.; Shen, Y.; Tian, R.; Sheng, Y.; Que, H. Global prevalence and epidemiological trends of Hashimoto’s thyroiditis in adults: A systematic review and meta-analysis. Front. Public Health 2022, 10, 1020709. [Google Scholar] [CrossRef]
- Shangguan, R.; Hu, Y.P.; Huang, J.; Yang, S.J.; Ye, L.; Lin, R.X.; Zhu, J.; Zhang, T.L.; Ying, L.; Li, P. Association Between BRAFV600E Mutation and the American College of Radiology Thyroid Imaging, Reporting and Data System in Solitary Papillary Thyroid Carcinoma. Acad. Radiol. 2019, 26, 154–160. [Google Scholar] [CrossRef]
- Xu, J.; Ding, K.; Mu, L.; Huang, J.; Ye, F.; Peng, Y.; Guo, C.; Ren, C. Hashimoto’s Thyroiditis: A “Double-Edged Sword” in Thyroid Carcinoma. Front. Endocrinol. 2022, 13, 801925. [Google Scholar] [CrossRef]
- Zeng, R.C.; Jin, L.P.; Chen, E.D.; Dong, S.Y.; Cai, Y.F.; Huang, G.L.; Li, Q.; Jin, C.; Zhang, X.H.; Wang, O.C. Potential relationship between Hashimoto’s thyroiditis and BRAF(V600E) mutation status in papillary thyroid cancer. Head Neck 2016, 38 (Suppl. S1), E1019–E1025. [Google Scholar] [CrossRef]
- Agyekum, E.A.; Wang, Y.G.; Xu, F.J.; Akortia, D.; Ren, Y.Z.; Chambers, K.H.; Wang, X.; Taupa, J.O.; Qian, X.Q. Predicting BRAFV600E mutations in papillary thyroid carcinoma using six machine learning algorithms based on ultrasound elastography. Sci. Rep. 2023, 13, 12604. [Google Scholar] [CrossRef]


| Variables | Wild Set (n = 50) | Mutant Set (n = 187) | p-Value |
|---|---|---|---|
| Gender | 0.976 | ||
| male | 9 | 34 | |
| female | 41 | 153 | |
| Age | 0.491 | ||
| <40 | 26 | 87 | |
| ≥40 | 24 | 100 | |
| Laterality | 0.124 | ||
| solitary lesions unilaterally | 39 | 122 | |
| multiple lesions unilaterally | 6 | 20 | |
| multiple lesions bilaterally | 5 | 45 | |
| HT | 0.002 | ||
| negative | 21 | 124 | |
| positive | 29 | 63 | |
| Calcification | 0.034 | ||
| negative | 28 | 134 | |
| positive | 22 | 53 | |
| Diamater (mm) | 8.00 (6.00, 12.25) | 9.00 (7.00, 13.00) | 0.39 |
| AEF | 0.90 (0.70, 1.09) | 1.17 (1.02, 1.39) | <0.001 |
| DLCT parameters | |||
| λHU | 5.27 ± 3.12 | 4.12 ± 1.25 | 0.013 |
| NIC | 0.30 (0.24, 0.48) | 0.27 (0.21, 0.34) | 0.010 |
| Zeff | 8.79 ± 0.61 | 8.54 ± 0.36 | 0.006 |
| ICa | 2.78 ± 1.21 | 3.11 ± 0.96 | 0.082 |
| ICv | 3.02 ± 0.97 | 2.54 ± 0.75 | <0.001 |
| Tumor-associated inflammatory parameters | |||
| NLR | 2.29 (1.75, 3.01) | 2.06 (1.67, 2.81) | 0.395 |
| SIRI | 0.67 (0.52, 0.92) | 0.64 (0.43, 0.83) | 0.760 |
| PNI | 51.50 ± 3.88 | 51.33 ± 4.04 | 0.789 |
| Models | Training Cohort | Validation Cohort | ||||
|---|---|---|---|---|---|---|
| AUC (95% CI) | Sensitivity | Specificity | AUC (95% CI) | Sensitivity | Specificity | |
| DLCT | 0.905 (0.841–0.968) | 0.806 | 0.903 | 0.773 (0.633–0.912) | 0.849 | 0.684 |
| AEF + HT | 0.820 (0.733–0.906) | 0.634 | 0.871 | 0.785 (0.656–0.913) | 0.943 | 0.526 |
| AEF + DLCT | 0.898 (0.837–0.959) | 0.799 | 0.871 | 0.799 (0.669–0.929) | 0.849 | 0.684 |
| DLCT + HT | 0.901 (0.835–0.967) | 0.925 | 0.742 | 0.801 (0.669–0.934) | 0.792 | 0.789 |
| AEF + NIC + HT | 0.896 (0.833–0.959) | 0.903 | 0.742 | 0.853 (0.746–0.961) | 0.925 | 0.684 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Lv, L.; Zou, Y.; Song, Z.; Yu, J.; Zhang, X.; Zhang, D. Arterial Enhancement Fraction-Spectral CT-Based Model as Part of Prediction Model in BRAFV600E-Positive Papillary Thyroid Carcinoma. Diagnostics 2025, 15, 2817. https://doi.org/10.3390/diagnostics15212817
Zhou B, Lv L, Zou Y, Song Z, Yu J, Zhang X, Zhang D. Arterial Enhancement Fraction-Spectral CT-Based Model as Part of Prediction Model in BRAFV600E-Positive Papillary Thyroid Carcinoma. Diagnostics. 2025; 15(21):2817. https://doi.org/10.3390/diagnostics15212817
Chicago/Turabian StyleZhou, Bi, Liang Lv, Ya Zou, Zuhua Song, Jiayi Yu, Xiaodi Zhang, and Dan Zhang. 2025. "Arterial Enhancement Fraction-Spectral CT-Based Model as Part of Prediction Model in BRAFV600E-Positive Papillary Thyroid Carcinoma" Diagnostics 15, no. 21: 2817. https://doi.org/10.3390/diagnostics15212817
APA StyleZhou, B., Lv, L., Zou, Y., Song, Z., Yu, J., Zhang, X., & Zhang, D. (2025). Arterial Enhancement Fraction-Spectral CT-Based Model as Part of Prediction Model in BRAFV600E-Positive Papillary Thyroid Carcinoma. Diagnostics, 15(21), 2817. https://doi.org/10.3390/diagnostics15212817






