Effect of Cataracts on Hydroxychloroquine Retinopathy Screening
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Screening and Monitoring Examinations
2.3. Diagnostic and Classification Criteria
2.4. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics
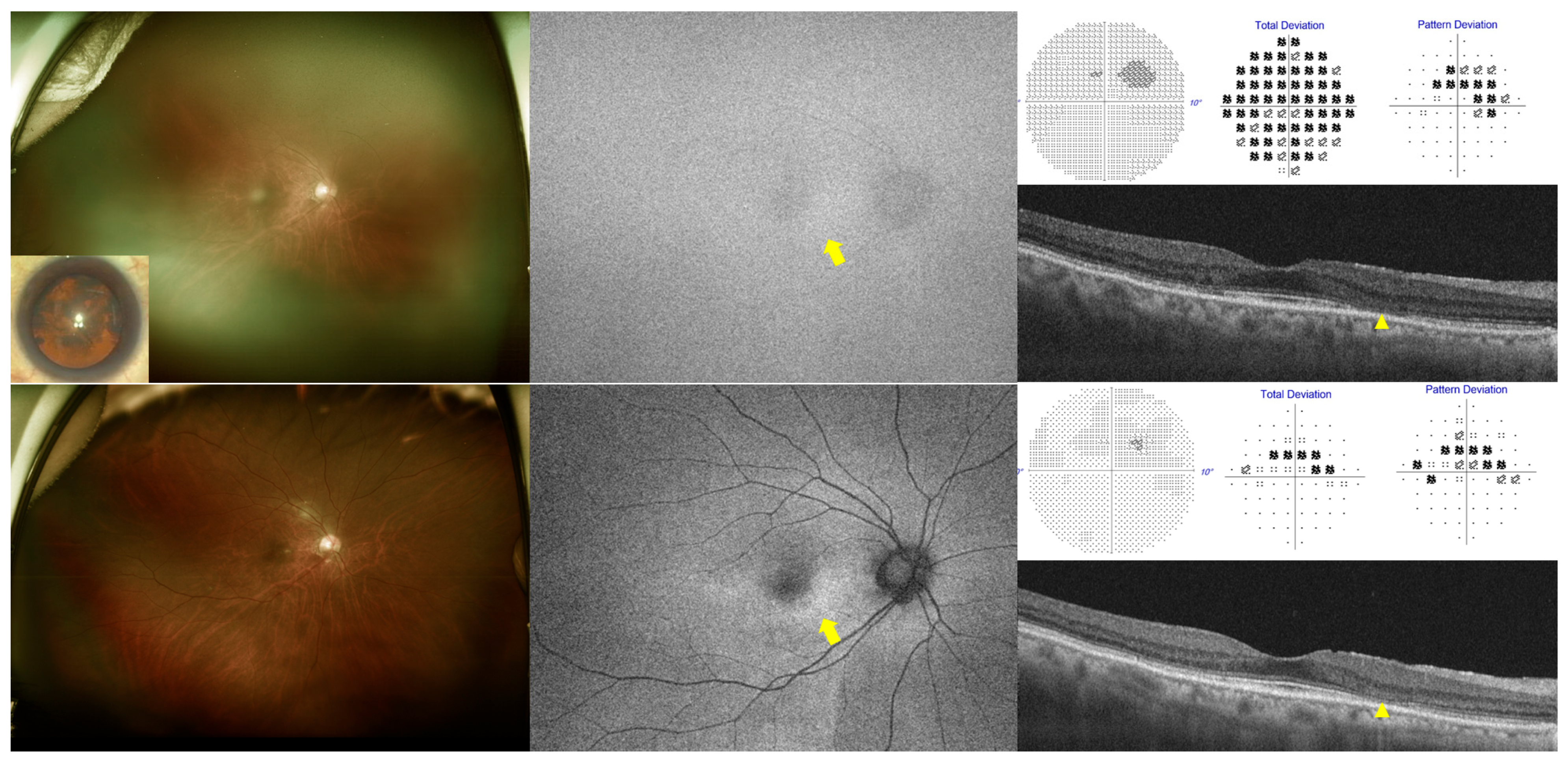
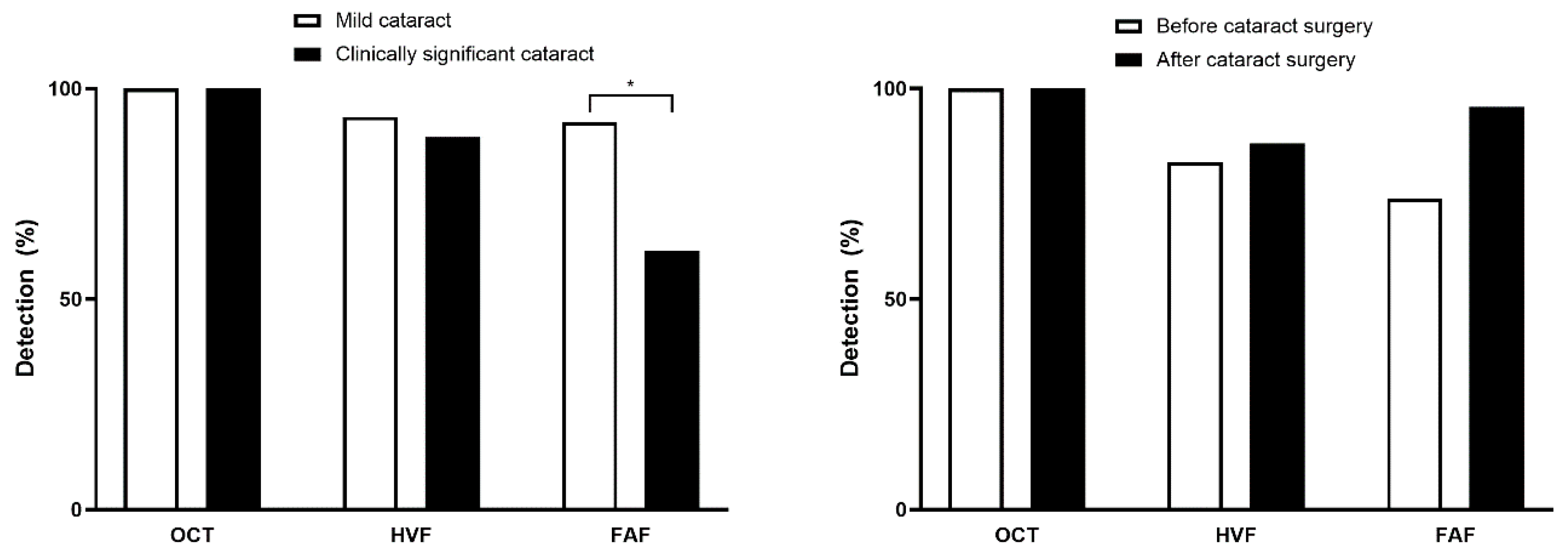
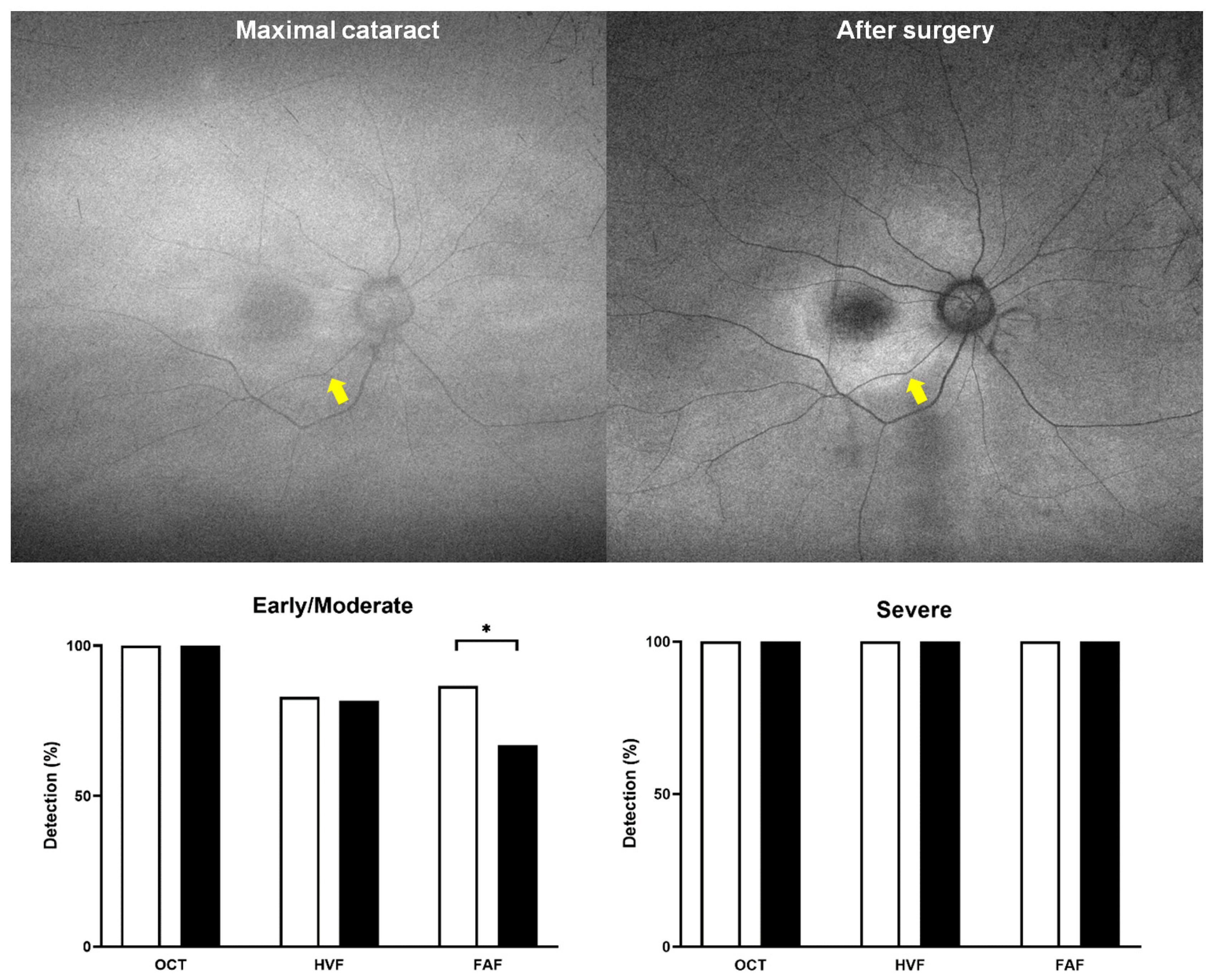
3.2. Presence or Severity of Cataracts and Screening Test Results
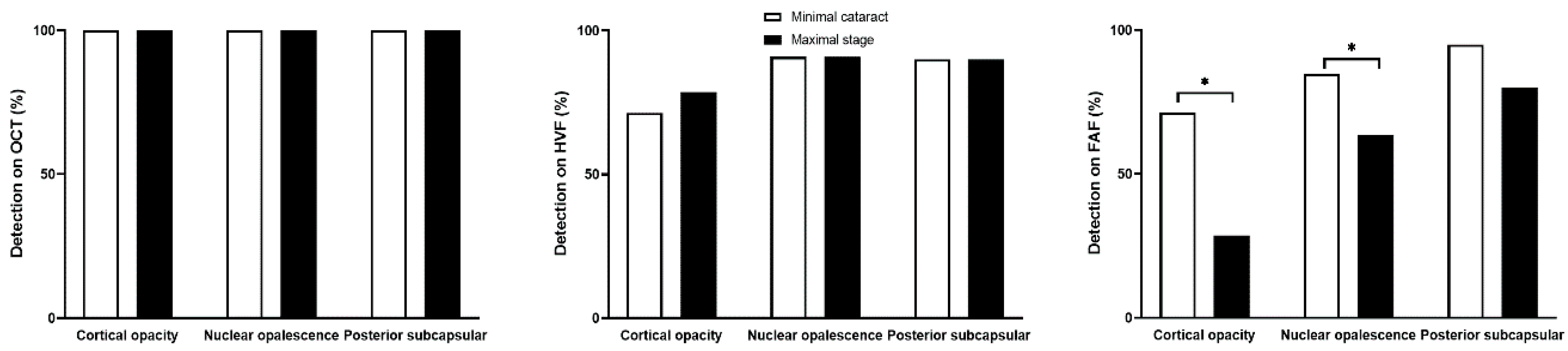
3.3. Cataract Type and Screening Test Results
3.4. Association Between Screening Test Results and HCQ Retinopathy Severity in Cataract Patients
3.5. Factors Associated with Toxicity Detection Among Patients with Cataracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ERG | Electroretinogram |
| FAF | Fundus autofluorescence |
| HCQ | Hydroxychloroquine |
| HVF | Humphrey visual field |
| OCT | Optical coherence tomography |
| RA | Rheumatoid arthritis |
| RPE | Retinal pigment epithelium |
| SLE | Systemic lupus erythematosus |
References
- Marmor, M.F.; Kellner, U.; Lai, T.Y.; Melles, R.B.; Mieler, W.F.; American Academy of Ophthalmology. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 2016, 123, 1386–1394. [Google Scholar] [CrossRef]
- Melles, R.B.; Marmor, M.F. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014, 132, 1453–1460. [Google Scholar] [CrossRef]
- Melles, R.B.; Jorge, A.M.; Marmor, M.F.; Zhou, B.; Conell, C.; Niu, J.; McCormick, N.; Zhang, Y.; Choi, H.K. Hydroxychloroquine Dose and Risk for Incident Retinopathy: A Cohort Study. Ann. Intern. Med. 2023, 176, 166–173. [Google Scholar] [CrossRef]
- Saldana-Garrido, J.D.; Canto-Cerdan, M.; Gil-Guillen, V.F.; Alfaro-Beltra, M.L.; Sivera, F. Analysis of central corneal thickness in systemic lupus erythematosus. Front. Med. 2025, 12, 1545415. [Google Scholar] [CrossRef]
- Yusuf, I.H.; Charbel Issa, P.; Ahn, S.J. Novel imaging techniques for hydroxychloroquine retinopathy. Front. Med. 2022, 9, 1026934. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Joung, J.; Lee, B.R. Evaluation of Hydroxychloroquine Retinopathy Using Ultra-Widefield Fundus Autofluorescence: Peripheral Findings in the Retinopathy. Am. J. Ophthalmol. 2020, 209, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Cumming, R.G.; Mitchell, P.; Leeder, S.R. Use of inhaled corticosteroids and the risk of cataracts. N. Engl. J. Med. 1997, 337, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Reiter, G.S.; Schwarzenbacher, L.; Schartmuller, D.; Roggla, V.; Leydolt, C.; Menapace, R.; Schmidt-Erfurth, U.; Sacu, S. Influence of lens opacities and cataract severity on quantitative fundus autofluorescence as a secondary outcome of a randomized clinical trial. Sci. Rep. 2021, 11, 12685. [Google Scholar] [CrossRef]
- Eugui, P.; Merkle, C.W.; Gesperger, J.; Lichtenegger, A.; Baumann, B. Investigation of the scattering and attenuation properties of cataracts formed in mouse eyes with 1060-nm and 1310-nm swept-source optical coherence tomography. Biomed. Opt. Express 2021, 12, 6391–6406. [Google Scholar] [CrossRef]
- Ahn, S.J. Classification of Hydroxychloroquine Retinopathy: A Literature Review and Proposal for Revision. Diagnostics 2024, 14, 1803. [Google Scholar] [CrossRef]
- Yusuf, I.H.; Foot, B.; Lotery, A.J. The Royal College of Ophthalmologists recommendations on monitoring for hydroxychloroquine and chloroquine users in the United Kingdom (2020 revision): Executive summary. Eye 2021, 35, 1532–1537. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Blaha, G.; Marx, J. Humphrey visual field findings in hydroxychloroquine toxicity. Eye 2011, 25, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Chylack, L.T., Jr.; Wolfe, J.K.; Singer, D.M.; Leske, M.C.; Bullimore, M.A.; Bailey, I.L.; Friend, J.; McCarthy, D.; Wu, S.Y. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch. Ophthalmol. 1993, 111, 831–836. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, Y.; Zhao, L.; Sun, M.; An, L.; Yang, Y.; Jiang, A.; Xu, Y.; Chen, Z.; Li, X. Correlation among Lens Opacities Classification System III grading, the 25-item National Eye Institute Visual Functioning Questionnaire, and Visual Function Index-14 for age-related cataract assessment. Int. Ophthalmol. 2020, 40, 1831–1839. [Google Scholar] [CrossRef]
- Hashemi, H.; Pakzad, R.; Yekta, A.; Aghamirsalim, M.; Pakbin, M.; Ramin, S.; Khabazkhoob, M. Global and regional prevalence of age-related cataract: A comprehensive systematic review and meta-analysis. Eye 2020, 34, 1357–1370. [Google Scholar] [CrossRef]
- Jobling, A.I.; Augusteyn, R.C. What causes steroid cataracts? A review of steroid-induced posterior subcapsular cataracts. Clin. Exp. Optom. 2002, 85, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Sasamoto, Y.; Gomi, F.; Sawa, M.; Sakaguchi, H.; Tsujikawa, M.; Nishida, K. Effect of cataract in evaluation of macular pigment optical density by autofluorescence spectrometry. Investig. Ophthalmol. Vis. Sci. 2011, 52, 927–932. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Obana, A.; Gohto, Y.; Seto, T.; Gellermann, W. Autofluorescence imaging of macular pigment: Influence and correction of ocular media opacities. J. Biomed. Opt. 2014, 19, 96010. [Google Scholar] [CrossRef]
- Simon, R.; Brauer, J.L.; Meller, D.; Hammer, M. Impact of cataract on the spectral measurement of fundus autofluorescence. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 2057–2059. [Google Scholar] [CrossRef]
- Cheong, K.X.; Ong, C.J.T.; Chandrasekaran, P.R.; Zhao, J.; Teo, K.Y.C.; Mathur, R. Review of Retinal Imaging Modalities for Hydroxychloroquine Retinopathy. Diagnostics 2023, 13, 1752. [Google Scholar] [CrossRef]
- Pandya, H.K.; Robinson, M.; Mandal, N.; Shah, V.A. Hydroxychloroquine retinopathy: A review of imaging. Indian J. Ophthalmol. 2015, 63, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Bao, Y.; Chen, Y.; Li, X. Correlation of lens density measured using the Pentacam Scheimpflug system with the Lens Opacities Classification System III grading score and visual acuity in age-related nuclear cataract. Br. J. Ophthalmol. 2008, 92, 1471–1475. [Google Scholar] [CrossRef]
- Kim, Y.N.; Park, J.H.; Tchah, H. Quantitative Analysis of Lens Nuclear Density Using Optical Coherence Tomography (OCT) with a Liquid Optics Interface: Correlation between OCT Images and LOCS III Grading. J. Ophthalmol. 2016, 2016, 3025413. [Google Scholar] [CrossRef] [PubMed]
- Chirapapaisan, C.; Srivannaboon, S.; Chonpimai, P. Efficacy of Swept-source Optical Coherence Tomography in Axial Length Measurement for Advanced Cataract Patients. Optom. Vis. Sci. 2020, 97, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Cheung, G.; Lee, S.Y. Comparison of spectral domain and swept-source optical coherence tomography in pathological myopia. Eye 2014, 28, 488–491. [Google Scholar] [CrossRef]
| Characteristics | Mean (SD) or Number |
|---|---|
| Sex, female (%) | 68 (94.4) |
| Age, yrs | 60.8 ± 12.4 (range: 20–81) |
| Diagnosis, SLE:RA:others * | 31:37:4 |
| Best-corrected visual acuity, logMAR | 0.26 ± 0.41 |
| Spherical equivalent, diopter (D) | −1.4 ± 3.0 |
| Daily dose, mg | 261.8 ± 78.5 |
| Daily dose/real body weight (RBW), mg/kg | 5.0 ± 1.5 |
| Daily dose/RBW > 5 mg/kg (%) | 30 (41.7) |
| Duration of hydroxychloroquine use, months | 190.6 ± 84.3 (range: 24–372) |
| Cumulative dose, g | 1487 ± 775 |
| Systemic corticosteroid use (%) | 58 (80.6) |
| Severity of retinopathy, eyes | |
| Early:moderate:severe (%) | 50:32:59 (35.5:22.7:41.8) |
| Pattern of retinopathy, eyes | |
| Parafoveal:pericentral:mixed (%) | 15:83:43 (10.6:58.9:30.5) |
| Characteristics (n = 141 Eyes) | Number (%) |
|---|---|
| Nuclear opalescence (LOCS III) | |
| None or Gr 1 | 57 (40.4%) |
| Grade 2 | 51 (36.2%) |
| Grade 3 | 28 (19.9%) |
| Grade 4 | 3 (2.1%) |
| Grade 5 | 2 (1.4%) |
| Grade 6 | 0 (0%) |
| Cortical opacity (LOCS III) | |
| None | 53 (37.6%) |
| Grade 1 | 31 (22.0%) |
| Grade 2 | 43 (30.5%) |
| Grade 3 | 10 (7.1%) |
| Grade 4 | 4 (2.8%) |
| Grade 5 | 0 (0%) |
| Posterior subcapsular cataract (LOCS III) | |
| None | 112 (79.4%) |
| Grade 1 | 2 (1.4%) |
| Grade 2 | 7 (5.0%) |
| Grade 3 | 16 (11.3%) |
| Grade 4 | 4 (2.8%) |
| Grade 5 | 0 (0%) |
| Clinically significant cataract | 52 (26.9%) |
| Received cataract surgery after retinopathy diagnosis | 23 (16.3%) |
| Characteristics | FAF | HVF | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | |
| Age | 0.732 | 0.132 | ||||||
| Sex | 0.162 | 0.797 | ||||||
| Medical diagnosis | 0.957 | 2.56 (0.87–7.53) | 0.087 | 0.154 | ||||
| Severity of retinopathy | 3.35 (1.84–6.13) | <0.001 | 4.85 (1.40–16.9) | 0.013 | 4.00 (1.60–10.00) | 0.003 | 3.37 (1.17–9.71) | 0.024 |
| Pattern of retinopathy *, Pericentral | 0.060 (0.008–0.46) | 0.007 | 0.785 | 0.13 (0.016–1.01) | 0.051 | 0.472 | ||
| Cortical opacity grade | 0.45 (0.29–0.68) | <0.001 | 0.43 (0.22–0.85) | 0.015 | 0.131 | |||
| Nuclear opalescence grade | 0.69 (0.46–1.05) | 0.084 | 0.21 (0.07–0.66) | 0.007 | 0.314 | |||
| Posterior subcapsular opacity grade | 0.624 | 0.182 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.S.; Ahn, S.J.; Kim, Y.J. Effect of Cataracts on Hydroxychloroquine Retinopathy Screening. Diagnostics 2025, 15, 2736. https://doi.org/10.3390/diagnostics15212736
Kang JS, Ahn SJ, Kim YJ. Effect of Cataracts on Hydroxychloroquine Retinopathy Screening. Diagnostics. 2025; 15(21):2736. https://doi.org/10.3390/diagnostics15212736
Chicago/Turabian StyleKang, Ji Soo, Seong Joon Ahn, and Yu Jeong Kim. 2025. "Effect of Cataracts on Hydroxychloroquine Retinopathy Screening" Diagnostics 15, no. 21: 2736. https://doi.org/10.3390/diagnostics15212736
APA StyleKang, J. S., Ahn, S. J., & Kim, Y. J. (2025). Effect of Cataracts on Hydroxychloroquine Retinopathy Screening. Diagnostics, 15(21), 2736. https://doi.org/10.3390/diagnostics15212736







