Resolved Central Serous Chorioretinopathy Mimicking Hydroxychloroquine Toxicity: A Case Series and Literature Review
Abstract
1. Introduction
2. Case Series Description
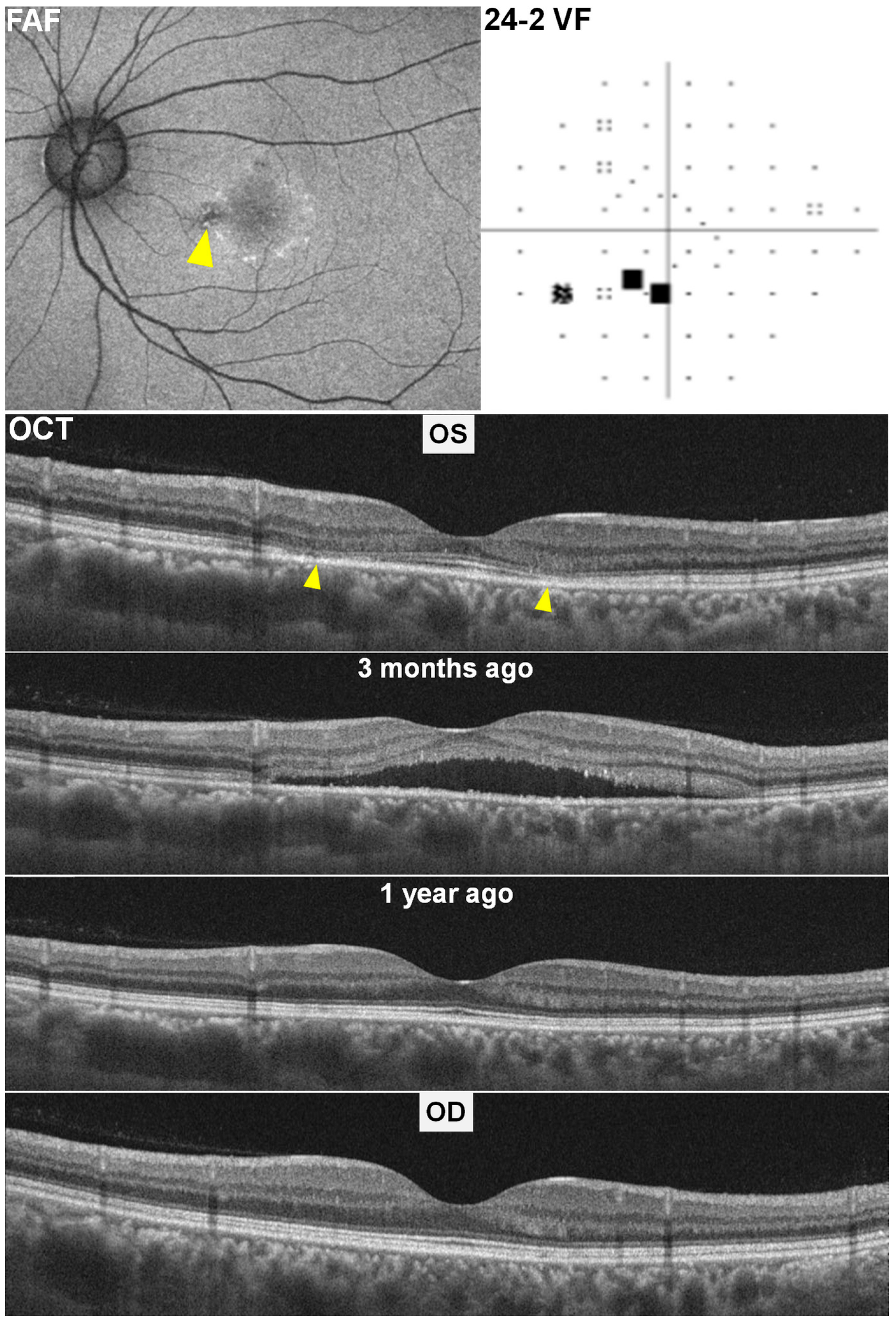
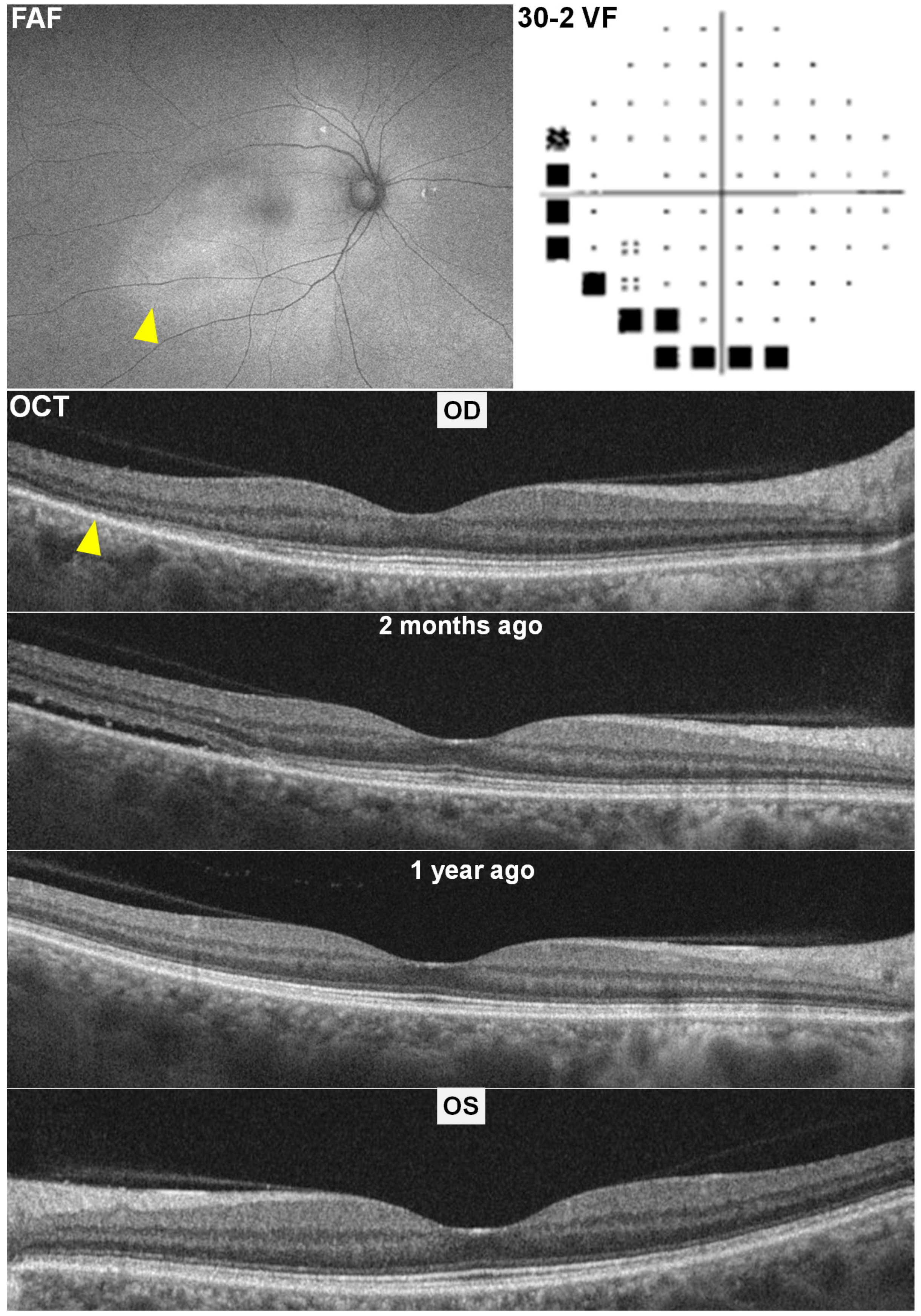
2.1. Case 1
2.2. Case 2
2.3. Case 3
3. Literature Review of Potential Mimickers of Hydroxychloroquine Retinopathy
3.1. Paraneoplastic and Autoimmune Retinopathies
3.2. Macular Dystrophies
3.3. Macular Telangiectasia Type 2 (MacTel 2)
3.4. Age-Related Macular Degeneration (AMD)
3.5. Solar and Photic Retinopathy
3.6. Drug-Induced Mimickers Beyond HCQ
3.7. Summary of Differentiating Strategies
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABW | Actual body weight |
| BCVA | Best-corrected visual acuity |
| CSCR | Central serous chorioretinopathy |
| eGFR | Estimated glomerular filtration rate |
| ERG | Electroretinogram |
| FAF | Fundus autofluorescence |
| HCQ | Hydroxychloroquine |
| HVF | Humphrey visual field |
| OCT | Optical coherence tomography |
| PED | Pigment epithelial detachment |
| RPE | Retinal pigment epithelium |
| SLE | Systemic lupus erythematosus |
References
- Nicholson, B.; Noble, J.; Forooghian, F.; Meyerle, C. Central serous chorioretinopathy: Update on pathophysiology and treatment. Surv. Ophthalmol. 2013, 58, 103–126. [Google Scholar] [CrossRef]
- Daruich, A.; Matet, A.; Dirani, A.; Bousquet, E.; Zhao, M.; Farman, N.; Jaisser, F.; Behar-Cohen, F. Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog. Retin. Eye Res. 2015, 48, 82–118. [Google Scholar] [CrossRef]
- Kido, A.; Miyake, M.; Tamura, H.; Hiragi, S.; Kimura, T.; Ohtera, S.; Takahashi, A.; Ooto, S.; Kawakami, K.; Kuroda, T.; et al. Incidence of central serous chorioretinopathy (2011–2018): A nationwide population-based cohort study of Japan. Br. J. Ophthalmol. 2022, 106, 1748–1753. [Google Scholar] [CrossRef]
- Kitzmann, A.S.; Pulido, J.S.; Diehl, N.N.; Hodge, D.O.; Burke, J.P. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980–2002. Ophthalmology 2008, 115, 169–173. [Google Scholar] [CrossRef]
- Rai, B.B.; Morley, M.G.; Bernstein, P.S.; Maddess, T. Pattern of vitreo-retinal diseases at the national referral hospital in Bhutan: A retrospective, hospital-based study. BMC Ophthalmol. 2020, 20, 51. [Google Scholar] [CrossRef]
- Haimovici, R.; Koh, S.; Gagnon, D.R.; Lehrfeld, T.; Wellik, S.; Central Serous Chorioretinopathy Case-Control Study, G. Risk factors for central serous chorioretinopathy: A case-control study. Ophthalmology 2004, 111, 244–249. [Google Scholar] [CrossRef]
- Carvalho-Recchia, C.A.; Yannuzzi, L.A.; Negrao, S.; Spaide, R.F.; Freund, K.B.; Rodriguez-Coleman, H.; Lenharo, M.; Iida, T. Corticosteroids and central serous chorioretinopathy. Ophthalmology 2002, 109, 1834–1837. [Google Scholar] [CrossRef]
- Marmor, M.F.; Carr, R.E.; Easterbrook, M.; Farjo, A.A.; Mieler, W.F.; American Academy of, O. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: A report by the American Academy of Ophthalmology. Ophthalmology 2002, 109, 1377–1382. [Google Scholar] [CrossRef]
- Marmor, M.F.; Kellner, U.; Lai, T.Y.; Lyons, J.S.; Mieler, W.F.; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 2011, 118, 415–422. [Google Scholar] [CrossRef]
- Marmor, M.F.; Kellner, U.; Lai, T.Y.; Melles, R.B.; Mieler, W.F.; American Academy of Ophthalmology. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 2016, 123, 1386–1394. [Google Scholar] [CrossRef]
- Carter, E.E.; Barr, S.G.; Clarke, A.E. The global burden of SLE: Prevalence, health disparities and socioeconomic impact. Nat. Rev. Rheumatol. 2016, 12, 605–620. [Google Scholar] [CrossRef]
- Nikolaidou, A.; Gianni, T.; Sandali, A.; Toumasis, P.; Benekos, K.; Tsina, E. Ocular manifestations of Juvenile Systemic Lupus Erythematosus: A systematic review. Eye 2025, 39, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
- Silpa-archa, S.; Lee, J.J.; Foster, C.S. Ocular manifestations in systemic lupus erythematosus. Br. J. Ophthalmol. 2016, 100, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.Y.; Ahn, S.J. Advances and outlook for lupus retinopathy. Expert Rev. Ophthalmol. 2024, 19, 105–118. [Google Scholar] [CrossRef]
- Canadian Hydroxychloroquine Study, G. A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N. Engl. J. Med. 1991, 324, 150–154. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Kostopoulou, M.; Alunno, A.; Aringer, M.; Bajema, I.; Boletis, J.N.; Cervera, R.; Doria, A.; Gordon, C.; Govoni, M.; et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 736–745. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Kostopoulou, M.; Andersen, J.; Aringer, M.; Arnaud, L.; Bae, S.C.; Boletis, J.; Bruce, I.N.; Cervera, R.; Doria, A.; et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann. Rheum. Dis. 2024, 83, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Canamary, A.M., Jr.; Takahashi, W.Y.; Sallum, J.M.F. Autoimmune retinopathy: A Review. Int. J. Retin. Vitr. 2018, 4, 1. [Google Scholar] [CrossRef]
- Weppelmann, T.A.; Khalil, S.; Zafrullah, N.; Amir, S.; Margo, C.E. Ocular Paraneoplastic Syndromes: A Critical Review of Diffuse Uveal Melanocytic Proliferation and Autoimmune Retinopathy. Cancer Control 2022, 29, 10732748221144458. [Google Scholar] [CrossRef]
- Boeck, K.; Hofmann, S.; Klopfer, M.; Ian, U.; Schmidt, T.; Engst, R.; Thirkill, C.E.; Ring, J. Melanoma-associated paraneoplastic retinopathy: Case report and review of the literature. Br. J. Dermatol. 1997, 137, 457–460. [Google Scholar] [CrossRef]
- Brossard-Barbosa, N.; Dezard, V.; Margolin, E. Treatment of Cancer-Associated Retinopathy: A Systematic Literature Review. Ophthalmol. Retina 2023, 7, 819–828. [Google Scholar] [CrossRef]
- Miyake, Y.; Tsunoda, K. Occult macular dystrophy. Jpn. J. Ophthalmol. 2015, 59, 71–80. [Google Scholar] [CrossRef]
- Heath Jeffery, R.C.; Chen, F.K. Stargardt disease: Multimodal imaging: A review. Clin. Exp. Ophthalmol. 2021, 49, 498–515. [Google Scholar] [CrossRef]
- Shroyer, N.F.; Lewis, R.A.; Lupski, J.R. Analysis of the ABCR (ABCA4) gene in 4-aminoquinoline retinopathy: Is retinal toxicity by chloroquine and hydroxychloroquine related to Stargardt disease? Am. J. Ophthalmol. 2001, 131, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Pinckers, A. Patterned dystrophies of the retinal pigment epithelium. A review. Ophthalmic Paediatr. Genet. 1988, 9, 77–114. [Google Scholar] [CrossRef] [PubMed]
- Tanner, A.; Chan, H.W.; Pulido, J.S.; Arno, G.; Ba-Abbad, R.; Jurkute, N.; Robson, A.G.; Egan, C.A.; Knight, H.; Calcagni, A.; et al. Clinical and Genetic Findings in CTNNA1-Associated Macular Pattern Dystrophy. Ophthalmology 2021, 128, 952–955. [Google Scholar] [CrossRef]
- Chew, E.Y.; Peto, T.; Clemons, T.E.; Sallo, F.B.; Pauleikhoff, D.; Leung, I.; Jaffe, G.J.; Heeren, T.F.C.; Egan, C.A.; Charbel Issa, P.; et al. Macular Telangiectasia Type 2: A Classification System Using MultiModal Imaging MacTel Project Report Number 10. Ophthalmol. Sci. 2023, 3, 100261. [Google Scholar] [CrossRef]
- Jones, P.; Kalra, G.; Al-Sheikh, M.; Chhablani, J. Mimickers of hydroxychloroquine retinal toxicity. Clin. Exp. Ophthalmol. 2024, 52, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Steinberg, J.S.; Gobel, A.; Fleckenstein, M.; Schmitz-Valckenberg, S. Fundus autofluorescence imaging in dry AMD: 2014 Jules Gonin lecture of the Retina Research Foundation. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 7–16. [Google Scholar] [CrossRef]
- Kellner, U.; Renner, A.B.; Tillack, H. Fundus autofluorescence and mfERG for early detection of retinal alterations in patients using chloroquine/hydroxychloroquine. Invest. Ophthalmol. Vis. Sci. 2006, 47, 3531–3538. [Google Scholar] [CrossRef]
- Comander, J.; Gardiner, M.; Loewenstein, J. High-resolution optical coherence tomography findings in solar maculopathy and the differential diagnosis of outer retinal holes. Am. J. Ophthalmol. 2011, 152, 413–419.e6. [Google Scholar] [CrossRef]
- Tenney, S.; Oboh-Weilke, A.; Wagner, D.; Chen, M.Y. Tamoxifen retinopathy: A comprehensive review. Surv. Ophthalmol. 2024, 69, 42–50. [Google Scholar] [CrossRef]
- Lindeke-Myers, A.; Hanif, A.M.; Jain, N. Pentosan polysulfate maculopathy. Surv. Ophthalmol. 2022, 67, 83–96. [Google Scholar] [CrossRef]
- Jorge, A.M.; Melles, R.B.; Marmor, M.F.; Zhou, B.; Zhang, Y.; Choi, H.K. Risk Factors for Hydroxychloroquine Retinopathy and Its Subtypes. JAMA Netw. Open 2024, 7, e2410677. [Google Scholar] [CrossRef] [PubMed]
- Melles, R.B.; Marmor, M.F. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014, 132, 1453–1460. [Google Scholar] [CrossRef]
- Lee, J.M.; Kwon, H.Y.; Ahn, S.J. Atypical Presentations of Hydroxychloroquine Retinopathy: A Case Series Study. J. Clin. Med. 2024, 13, 3411. [Google Scholar] [CrossRef]
- Spaide, R.F.; Campeas, L.; Haas, A.; Yannuzzi, L.A.; Fisher, Y.L.; Guyer, D.R.; Slakter, J.S.; Sorenson, J.A.; Orlock, D.A. Central serous chorioretinopathy in younger and older adults. Ophthalmology 1996, 103, 2070–2079, discussion 2079–2080. [Google Scholar] [CrossRef]
- van Rijssen, T.J.; van Dijk, E.H.C.; Yzer, S.; Ohno-Matsui, K.; Keunen, J.E.E.; Schlingemann, R.O.; Sivaprasad, S.; Querques, G.; Downes, S.M.; Fauser, S.; et al. Central serous chorioretinopathy: Towards an evidence-based treatment guideline. Prog. Retin. Eye Res. 2019, 73, 100770. [Google Scholar] [CrossRef]
- Nicholson, B.P.; Atchison, E.; Idris, A.A.; Bakri, S.J. Central serous chorioretinopathy and glucocorticoids: An update on evidence for association. Surv. Ophthalmol. 2018, 63, 1–8. [Google Scholar] [CrossRef]
- Alarcon, G.S.; McGwin, G.; Bertoli, A.M.; Fessler, B.J.; Calvo-Alen, J.; Bastian, H.M.; Vila, L.M.; Reveille, J.D.; Group, L.S. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: Data from LUMINA, a multiethnic US cohort (LUMINA L). Ann. Rheum. Dis. 2007, 66, 1168–1172. [Google Scholar] [CrossRef]
- Ahn, S.J.; Seo, E.J.; Kim, K.E.; Kim, Y.J.; Lee, B.R.; Kim, J.G.; Yoon, Y.H.; Lee, J.Y. Long-Term Progression of Pericentral Hydroxychloroquine Retinopathy. Ophthalmology 2021, 128, 889–898. [Google Scholar] [CrossRef]

| Condition | Overlap with HCQ Retinopathy | Key Differentiators |
|---|---|---|
| Paraneoplastic/autoimmune retinopathies | Outer nuclear layer thinning, EZ disruption; ring scotomas on visual fields | Rapid onset/progression (weeks–months); positive antiretinal antibodies or malignancy; diffuse ERG changes |
| Macular dystrophy (e.g., occult macular dystrophy, Stargardt disease) | Parafoveal EZ disruption on OCT | Childhood/adolescent onset; foveal involvement; very slow progression; confirmatory genetic variant |
| Macular telangiectasia type 2 (MacTel 2) | Outer retinal cavitations; parafoveal FAF changes | Inner-retinal cavitations, right-angle venules on OCT; late-phase FA leakage; preserved central acuity longer |
| Age-related macular degeneration | Photoreceptor attenuation; FAF irregularities | Drusen lesions on color fundus; discrete FAF drusen margins vs. continuous HCQ ring |
| Solar retinopathy | Focal EZ disruptions; central scotomas on VF | Acute onset linked to known light exposure; bilateral symmetric central lesions; partial recovery over weeks |
| Tamoxifen/thioridazine/pentosan polysulfate toxicity | Photoreceptor loss on OCT; patchy FAF hypoautofluorescence | Presence of macular crystals (tamoxifen); high-dose phenothiazine history; lack of classic HCQ autofluorescent ring |
| Central serous chorioretinopathy | Photoreceptor defects ± RPE changes following resolution of subretinal fluid | Documentation of prior subretinal fluid in the affected area on historical OCT scans |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.J. Resolved Central Serous Chorioretinopathy Mimicking Hydroxychloroquine Toxicity: A Case Series and Literature Review. Diagnostics 2025, 15, 2154. https://doi.org/10.3390/diagnostics15172154
Ahn SJ. Resolved Central Serous Chorioretinopathy Mimicking Hydroxychloroquine Toxicity: A Case Series and Literature Review. Diagnostics. 2025; 15(17):2154. https://doi.org/10.3390/diagnostics15172154
Chicago/Turabian StyleAhn, Seong Joon. 2025. "Resolved Central Serous Chorioretinopathy Mimicking Hydroxychloroquine Toxicity: A Case Series and Literature Review" Diagnostics 15, no. 17: 2154. https://doi.org/10.3390/diagnostics15172154
APA StyleAhn, S. J. (2025). Resolved Central Serous Chorioretinopathy Mimicking Hydroxychloroquine Toxicity: A Case Series and Literature Review. Diagnostics, 15(17), 2154. https://doi.org/10.3390/diagnostics15172154







