A Multi-Modality Approach to the Assessment of a Right Atrium Mass in a Female Patient with Breast Cancer Undergoing Neoadjuvant Chemotherapy
Abstract
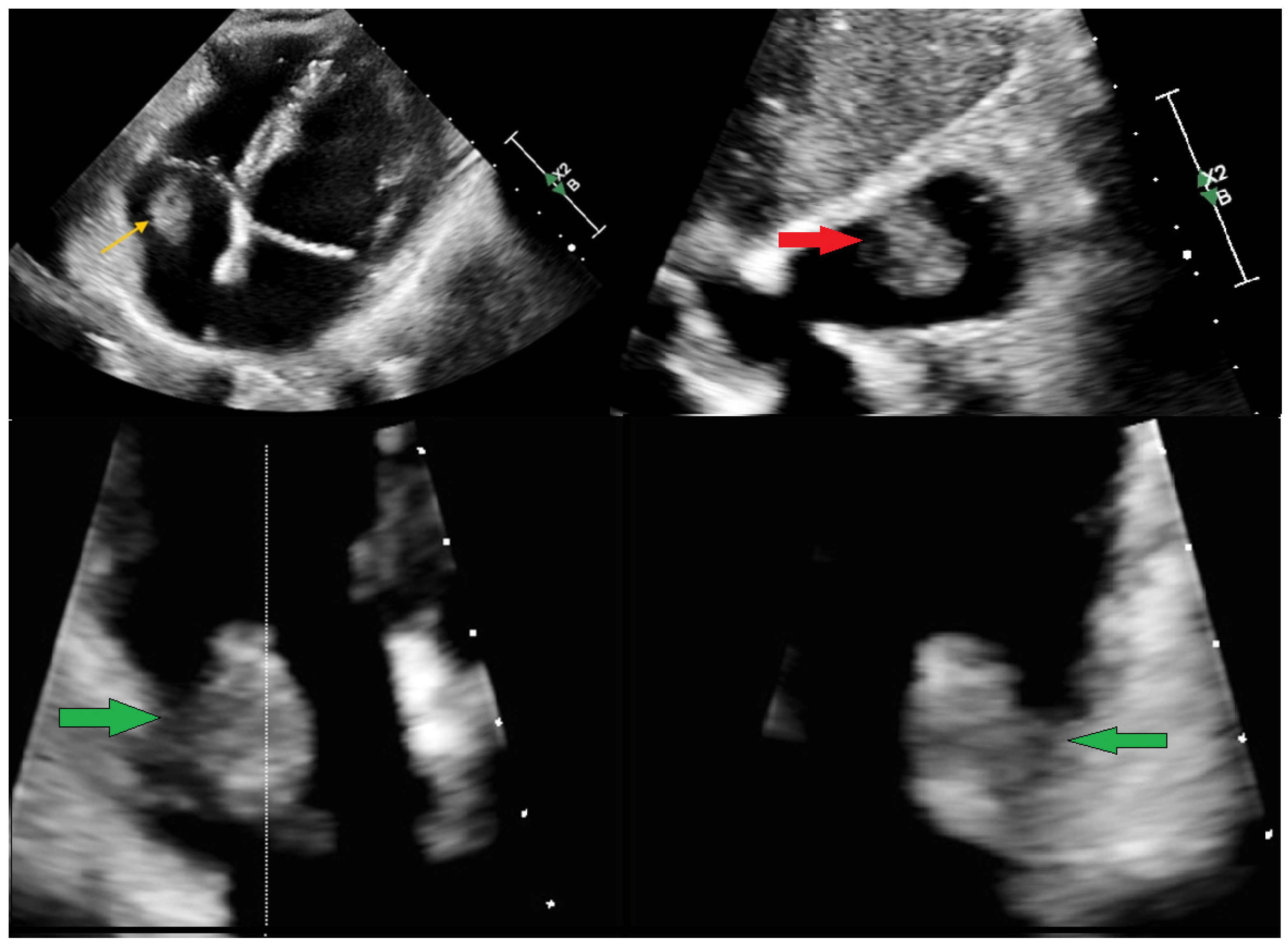
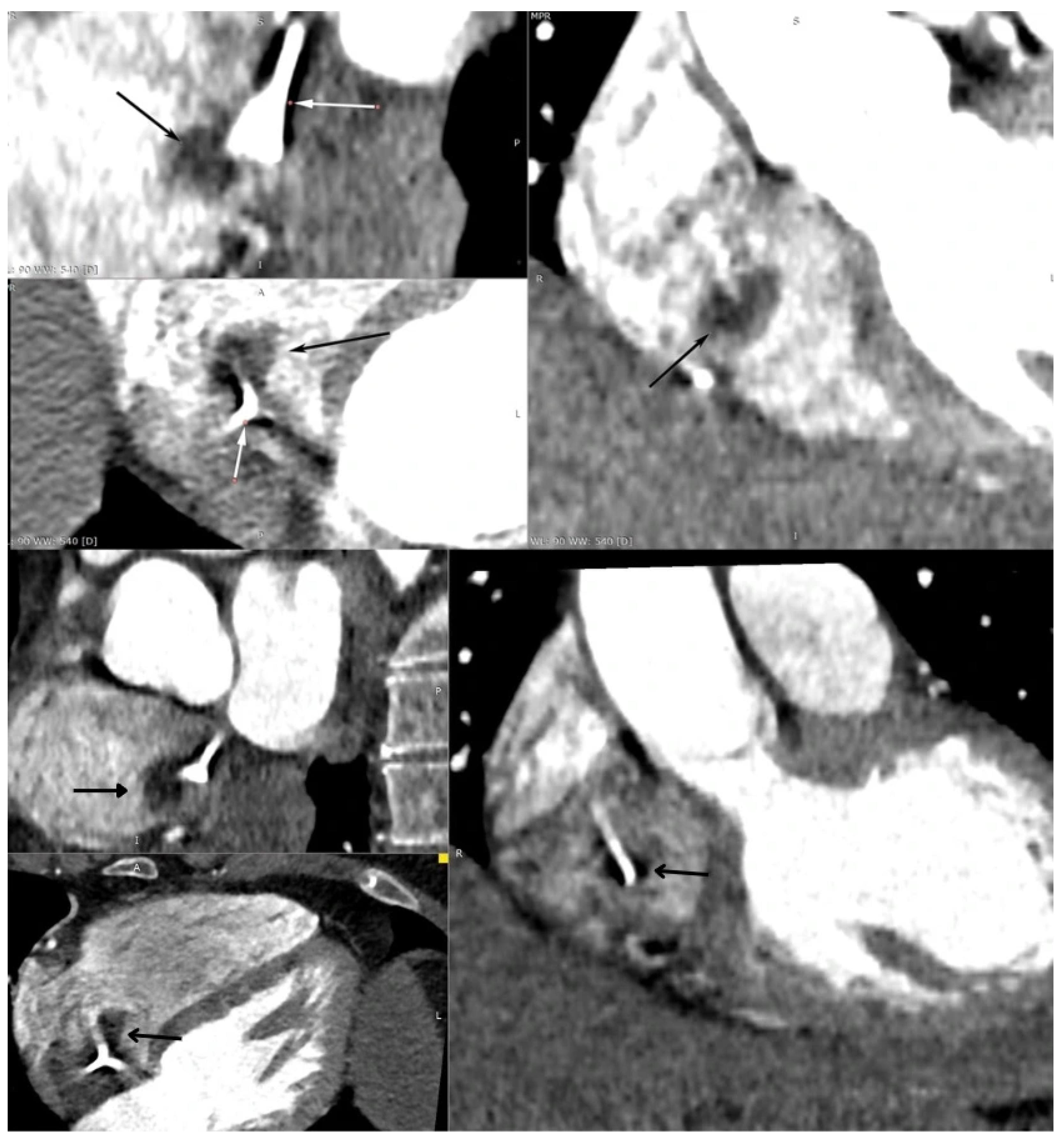

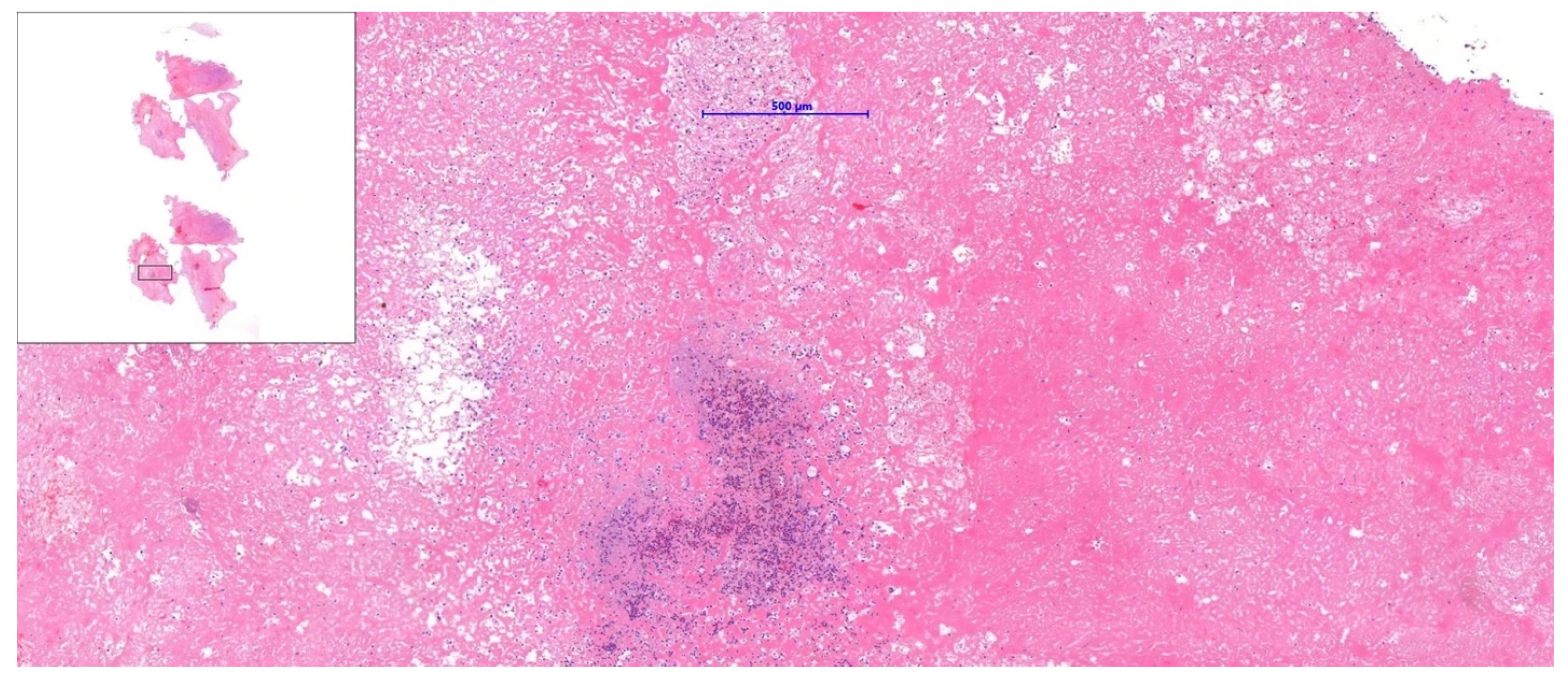
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pradella, S.; Grazzini, G.; Letteriello, M.; De Amicis, C.; Grassi, R.; Maggialetti, N.; Carbone, M.; Palumbo, P.; Carotti, M.; Di Cesare, E.; et al. Masses in right side of the heart: Spectrum of imaging findings. Acta Biomed. 2020, 91, 60–70. [Google Scholar] [CrossRef]
- Giusca, S.; Mereles, D.; Ochs, A.; Buss, S.; André, F.; Seitz, S.; Riffel, J.; Fortner, P.; Andrulis, M.; Schönland, S.; et al. Incremental value of cardiac magnetic resonance for the evaluation of cardiac tumors in adults: Experience of a high volume tertiary cardiology centre. Int. J. Cardiovasc. Imaging 2017, 33, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- De Martino, A.; Pattuzzi, C.; Garis, S.; Bosco, F.; Virgone, V.M.; Salsano, A.; Santini, F.; Pucci, A. A Comprehensive Review of Cardiac Tumors: Imaging, Pathology, Treatment, and Challenges in the Third Millennium. Diagnostics 2025, 15, 1390. [Google Scholar] [CrossRef] [PubMed]
- Tagliati, C.; Fogante, M.; Palmisano, A.; Catapano, F.; Lisi, C.; Monti, L.; Lanni, G.; Cerimele, F.; Bernardini, A.; Procaccini, L.; et al. Cardiac Masses and Pseudomasses: An Overview about Diagnostic Imaging and Clinical Background. Medicina 2024, 60, 70. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Bodega, F.; Bergamaschi, L.; Armillotta, M.; Amicone, S.; Canton, L.; Fedele, D.; Suma, N.; Cavallo, D.; Foà, A.; et al. Multimodality Imaging in the Diagnostic Work-Up of Patients With Cardiac Masses. JACC: CardioOncology 2024, 6, 847–862. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, C.; Grizzard, J.D.; Shah, D.J.; Kassi, M.; Reardon, M.J.; Zagurovskaya, M.; Kim, H.W.; Parker, M.A.; Kim, R.J. Cardiovascular magnetic resonance imaging in suspected cardiac tumour: A multicentre outcomes study. Eur. Heart J. 2021, 43, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Shimoi, T.; Kawai, A.; Yonemori, K. Diagnosis and treatment of cardiac tumors. Med. Oncol. 2025, 42, 110. [Google Scholar] [CrossRef] [PubMed]
- Enezate, T.; Alkhatib, D.; Raja, J.; Chinta, V.; Patel, M.; Omran, J. AngioVac for Minimally Invasive Removal of Intravascular and Intracardiac Masses: A Systematic Review. Curr. Cardiol. Rep. 2022, 24, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, J.M.; Rueda, V.; Liao, M.; Kim, G.H.J.; Rochon, P.J.; Zayed, M.A.; Lasorda, D.; Golowa, Y.S.; Shavelle, D.M.; Dexter, D.J. Endovascular Removal of Thrombus and Right Heart Masses Using the AngioVac System: Results of 234 Patients from the Prospective, Multicenter Registry of AngioVac Procedures in Detail (RAPID). J. Vasc. Interv. Radiol. 2021, 32, 549–557.e543. [Google Scholar] [CrossRef] [PubMed]
- Dandu, C.; Alamzaib, S.M.; Patel, D.; Naughton, R.; Polina, A.; Najam, M.; Alhusain, R.; Patel, N.; Sattar, Y.; Alraies, M.C. Investigating the Complications and Causes of Failure of the AngioVac System: A Post-Marketing Surveillance From the MAUDE Database. Cureus 2023, 15, e43720. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chlabicz, M.; Muszyński, P.; Kruszyńska, J.; Kazberuk, P.; Róg-Makal, M.; Lipowicz, M.; Matys, U.; Tomaszuk-Kazberuk, A.; Kożuch, M.; Dobrzycki, S. A Multi-Modality Approach to the Assessment of a Right Atrium Mass in a Female Patient with Breast Cancer Undergoing Neoadjuvant Chemotherapy. Diagnostics 2025, 15, 2683. https://doi.org/10.3390/diagnostics15212683
Chlabicz M, Muszyński P, Kruszyńska J, Kazberuk P, Róg-Makal M, Lipowicz M, Matys U, Tomaszuk-Kazberuk A, Kożuch M, Dobrzycki S. A Multi-Modality Approach to the Assessment of a Right Atrium Mass in a Female Patient with Breast Cancer Undergoing Neoadjuvant Chemotherapy. Diagnostics. 2025; 15(21):2683. https://doi.org/10.3390/diagnostics15212683
Chicago/Turabian StyleChlabicz, Małgorzata, Paweł Muszyński, Joanna Kruszyńska, Piotr Kazberuk, Magdalena Róg-Makal, Magdalena Lipowicz, Urszula Matys, Anna Tomaszuk-Kazberuk, Marcin Kożuch, and Sławomir Dobrzycki. 2025. "A Multi-Modality Approach to the Assessment of a Right Atrium Mass in a Female Patient with Breast Cancer Undergoing Neoadjuvant Chemotherapy" Diagnostics 15, no. 21: 2683. https://doi.org/10.3390/diagnostics15212683
APA StyleChlabicz, M., Muszyński, P., Kruszyńska, J., Kazberuk, P., Róg-Makal, M., Lipowicz, M., Matys, U., Tomaszuk-Kazberuk, A., Kożuch, M., & Dobrzycki, S. (2025). A Multi-Modality Approach to the Assessment of a Right Atrium Mass in a Female Patient with Breast Cancer Undergoing Neoadjuvant Chemotherapy. Diagnostics, 15(21), 2683. https://doi.org/10.3390/diagnostics15212683





