Dynamic Whole-Body FDG PET/CT for Predicting Malignancy in Head and Neck Tumors and Cervical Lymphadenopathy
Abstract
1. Background
2. Methods
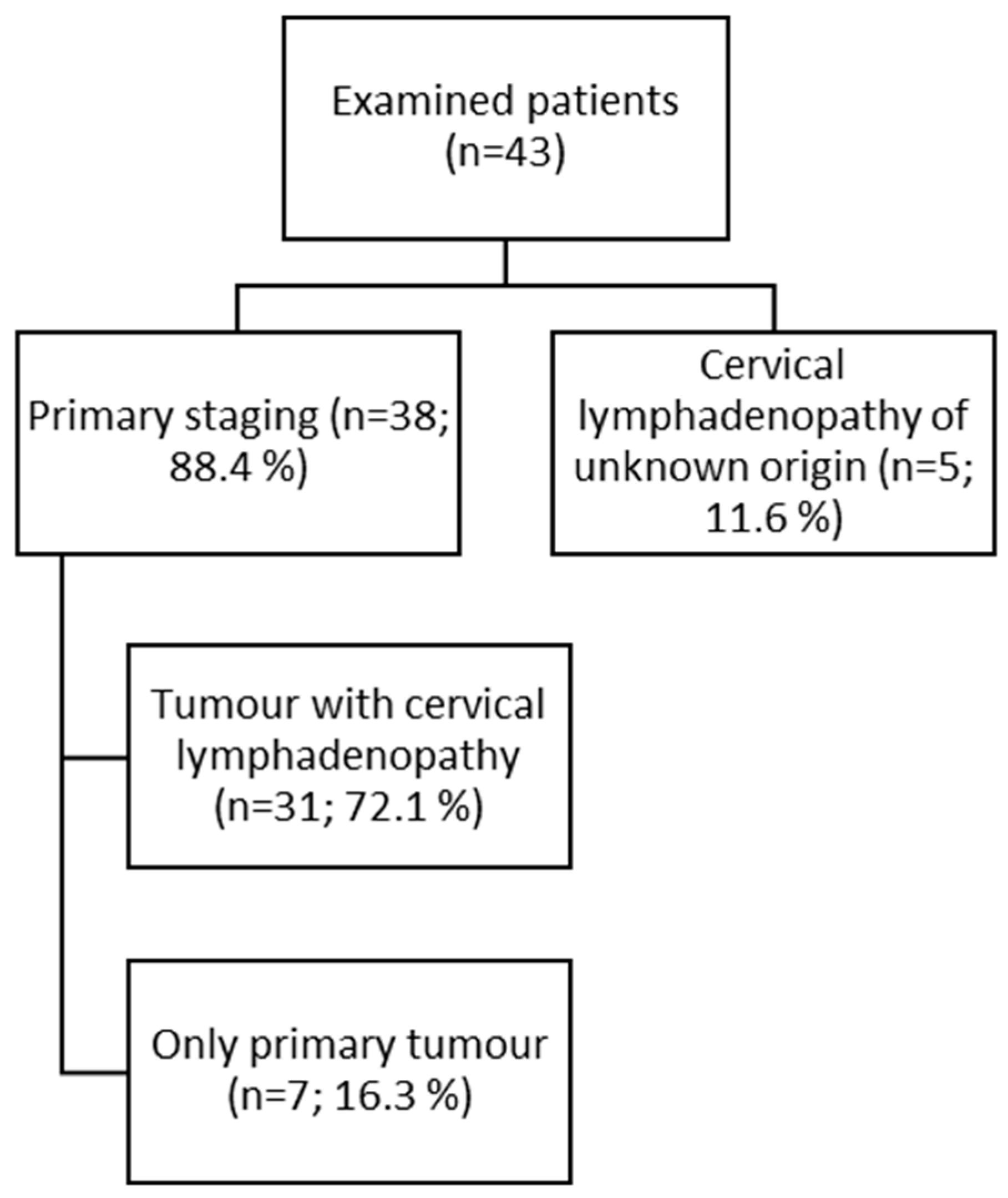
2.1. Patient Population
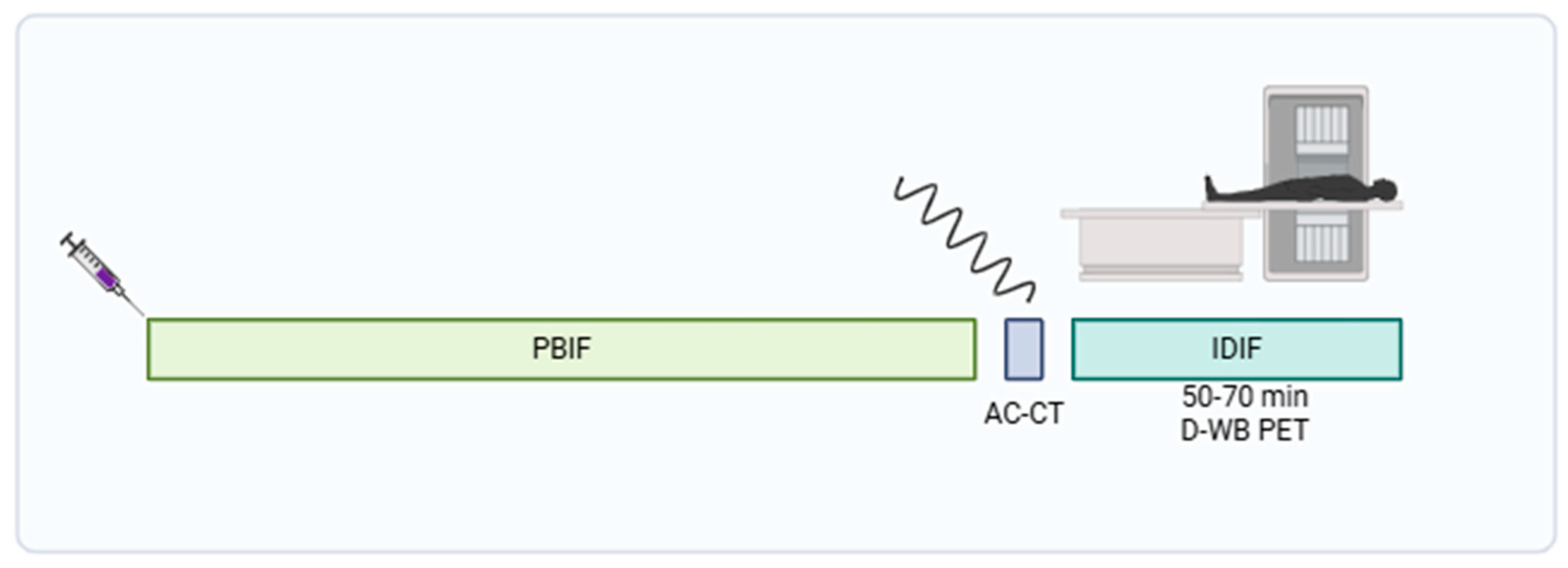
2.2. Data Acquisition and Image Reconstruction
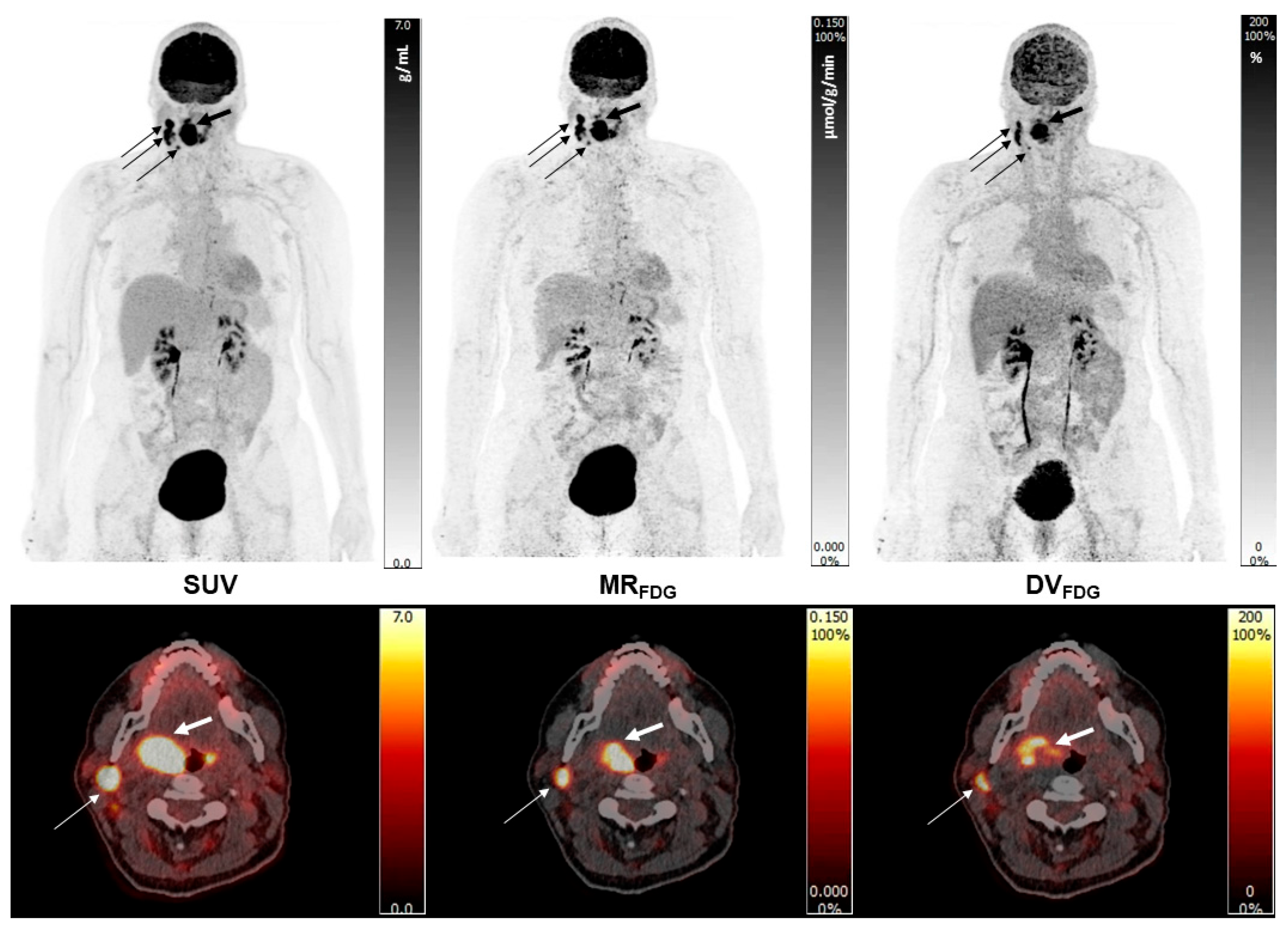
2.3. Image Analysis and VOI Delineation
2.4. Statistical Analysis
3. Results
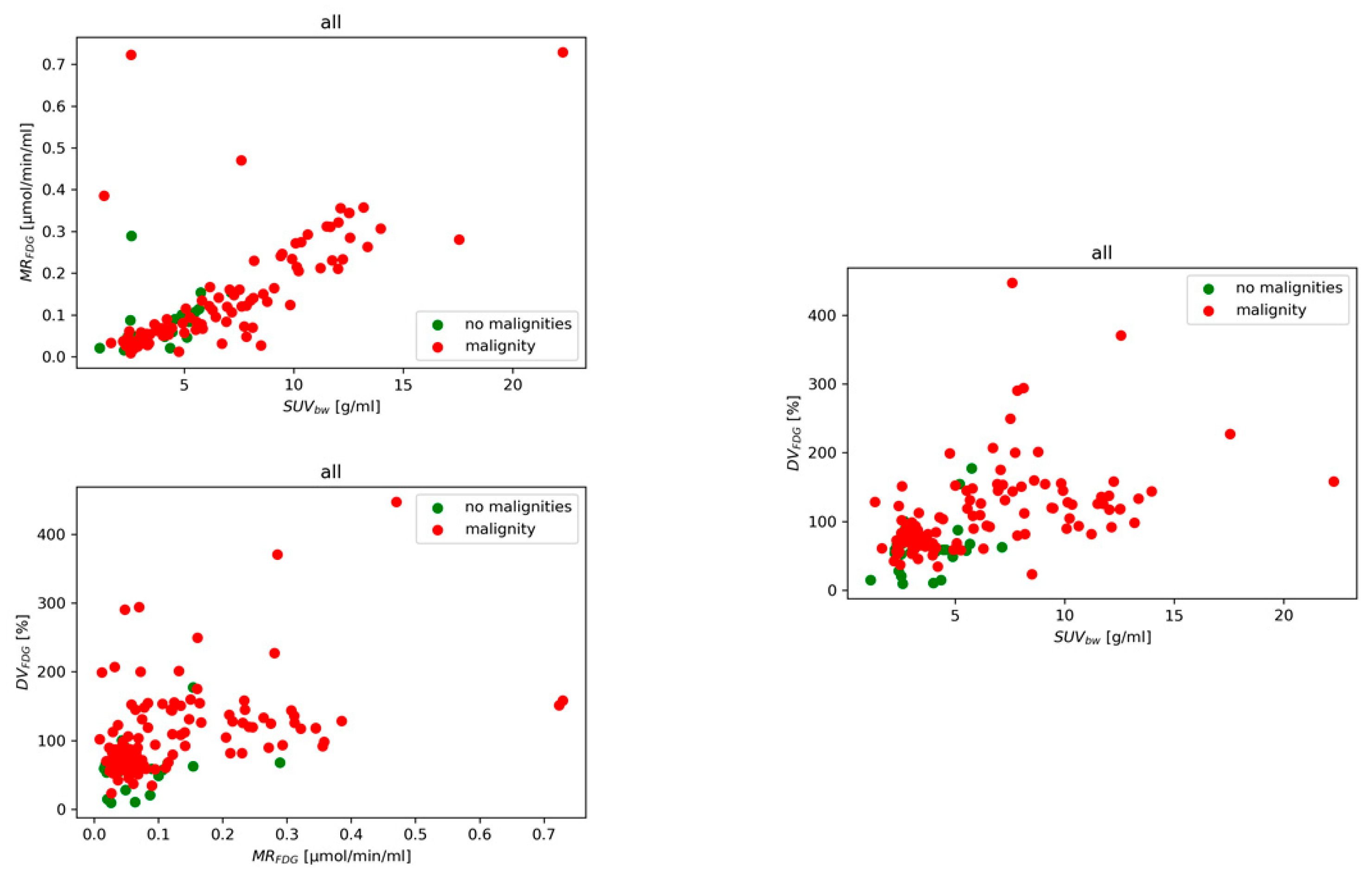
3.1. Diagnostic Performance of Models
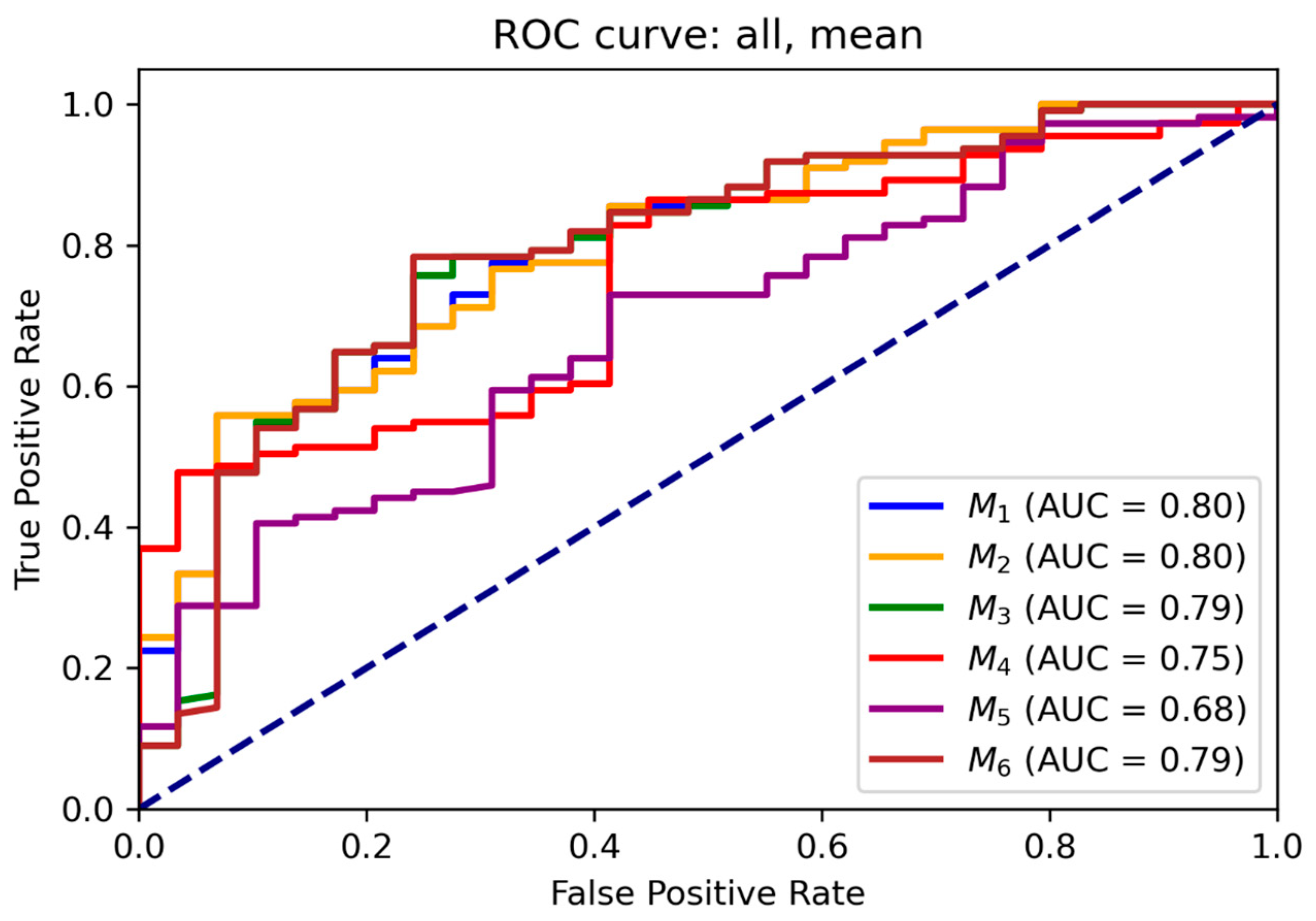
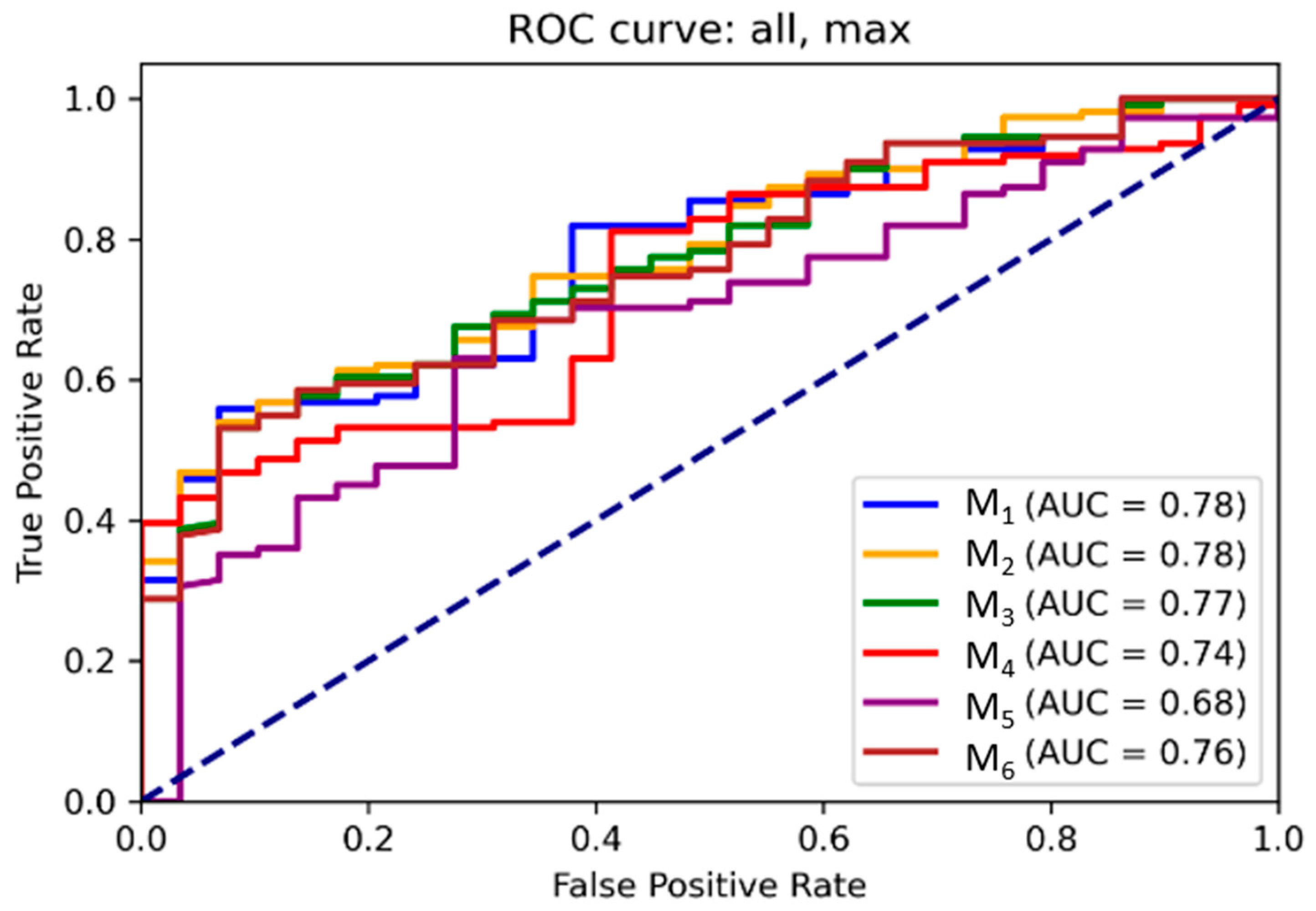
3.2. ROC and Threshold Analysis
3.3. Relative Feature Contributions
3.4. Decision Thresholds and Clinical Translation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hess, S.; Blomberg, B.A.; Zhu, H.J.; Høilund-Carlsen, P.F.; Alavi, A. The Pivotal Role of FDG-PET/CT in Modern Medicine. Acad. Radiol. 2014, 21, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Eskian, M.; Alavi, A.; Khorasanizadeh, M.; Viglianti, B.L.; Jacobsson, H.; Barwick, T.D.; Meysamie, A.; Yi, S.K.; Iwano, S.; Bybel, B.; et al. Effect of blood glucose level on standardized uptake value (SUV) in 18F- FDG PET-scan: A systematic review and meta-analysis of 20,807 individual SUV measurements. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, P.; Minn, H.; Leskinen-Kallio, S.; Bergman, J.; Ruotsalainen, U.; Joensuu, H. Influence of the blood glucose concentration on FDG uptake in cancer—A PET study. J. Nucl. Med. 1993, 34, 1–6. [Google Scholar]
- Boellaard, R.; Krak, N.C.; Hoekstra, O.S.; Lammertsma, A.A. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: A simulation study. J. Nucl. Med. 2004, 45, 1519–1527. [Google Scholar]
- Boellaard, R. Standards for PET image acquisition and quantitative data analysis. J. Nucl. Med. 2009, 50 (Suppl. S1), 11S–20S. [Google Scholar] [CrossRef]
- Huang, S.C. Anatomy of SUV. Standardized uptake value. Nucl. Med. Biol. 2000, 27, 643–646. [Google Scholar] [CrossRef]
- Thie, J.A. Understanding the standardized uptake value, its methods, and implications for usage. J. Nucl. Med. 2004, 45, 1431–1434. [Google Scholar]
- Patlak, C.S.; Blasberg, R.G.; Fenstermacher, J.D. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J. Cereb. Blood Flow Metab. 1983, 3, 1–7. [Google Scholar] [CrossRef]
- Dias, A.H.; Smith, A.M.; Shah, V.; Pigg, D.; Gormsen, L.C.; Munk, O.L. Clinical validation of a population-based input function for 20-min dynamic whole-body 18F-FDG multiparametric PET imaging. EJNMMI Phys. 2022, 9, 60. [Google Scholar] [CrossRef]
- Karakatsanis, N.A.; Lodge, M.A.; Tahari, A.K.; Zhou, Y.; Wahl, R.L.; Rahmim, A. Dynamic whole-body PET parametric imaging: I. Concept, acquisition protocol optimization and clinical application. Phys. Med. Biol. 2013, 58, 7391–7418. [Google Scholar] [CrossRef]
- Dias, A.H.; Pedersen, M.F.; Danielsen, H.; Munk, O.L.; Gormsen, L.C. Clinical feasibility and impact of fully automated multiparametric PET imaging using direct Patlak reconstruction: Evaluation of 103 dynamic whole-body 18F-FDG PET/CT scans. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 837–850. [Google Scholar] [CrossRef]
- Bakhshayesh Karam, M.; Doroudinia, A.; Safavi Nainee, A.; Kaghazchi, F.; Yousefi Koma, A.; Mehrian, P.; Hossein, F.A. Role of FDG PET/CT scan in head and neck cancer patients. Arch. Iran. Med. 2017, 20, 452–458. [Google Scholar] [CrossRef]
- Szyszko, T.A.; Cook, G.J.R. PET/CT and PET/MRI in head and neck malignancy. Clin. Radiol. 2018, 73, 60–69. [Google Scholar] [CrossRef]
- Zhuang, S.M.; Wu, X.-F.; Li, J.-J.; Zhang, G.-H. Management of lymph node metastases from an unknown primary site to the head and neck (Review). Mol. Clin. Oncol. 2014, 2, 917–922. [Google Scholar] [CrossRef]
- Liu, Y. FDG PET/CT for metastatic squamous cell carcinoma of unknown primary of the head and neck. Oral Oncol. 2019, 92, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Tsetsos, N.; Poutoglidis, A.; Arsos, G.; Tsentemeidou, A.; Kilmpasanis, A.; Katsampoukas, D.; Fyrmpas, G. 18F-FDG-PET/CT interpretation pitfalls in patients with head and neck cancer. Am. J. Otolaryngol. 2022, 43, 103209. [Google Scholar] [CrossRef] [PubMed]
- Purohit, B.S.; Ailianou, A.; Dulguerov, N.; Becker, C.D.; Ratib, O.; Becker, M. FDG-PET/CT pitfalls in oncological head and neck imaging. Insights Imaging 2014, 5, 585–602. [Google Scholar] [CrossRef] [PubMed]
- Metser, U.; Miller, E.; Lerman, H.; Even-Sapir, E. Benign nonphysiologic lesions with increased 18F-FDG uptake on PET/CT: Characterization and incidence. Am. J. Roentgenol. 2007, 189, 1203–1210. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Keyes, J.W., Jr. SUV: Standard uptake or silly useless value? J. Nucl. Med. 1995, 36, 1836–1839. [Google Scholar]
- Laffon, E.; Marthan, R. Is Patlak y-intercept a relevant metrics? Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1287–1290. [Google Scholar] [CrossRef]
- Guedj, D.; Neveü, S.; Becker, M.; Mermod, M. FDG PET-CT for the Detection of Occult Nodal Metastases in Head and Neck Cancer: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 2954. [Google Scholar] [CrossRef]
- Shum, W.Y.; Hsieh, T.C.; Yeh, J.J.; Chen, J.H.; Su, C.C.; Liang, J.A.; Kao, C.H. Clinical usefulness of dual-time FDG PET-CT in assessment of esophageal squamous cell carcinoma. Eur. J. Radiol. 2012, 81, 1024–1028. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, X.; Lu, Y.; Liu, C.; Gong, K.; Li, Y.; Cherry, S.R. High-temporal-resolution kinetic modeling of lung tumors with dual-blood input function using total-body dynamic PET. J. Nucl. Med. 2024, 65, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.; Jeong, H.J.; Shin, J.H.; Kim, H.W.; Park, J.H. Dual-time-point 18F-FDG PET/CT for differentiation of malignancy and inflammation in head and neck cancer. Nucl. Med. Mol. Imaging 2008, 42, 18–23. [Google Scholar]
- Koo, P.J.; Lin, F.I.; Lin, M.; Alavi, A. Dual-time-point 18F-FDG PET imaging of head and neck squamous cell carcinoma: Comparison of early and delayed images. Clin. Nucl. Med. 2009, 34, 774–778. [Google Scholar]
- Soffers, F.; Helsen, N.; Van den Wyngaert, T.; Carp, L.; Hoekstra, O.S.; Goethals, L.; Martens, M.; Deben, K.; Spaepen, K.; De Bree, R.; et al. Dual time point imaging in locally advanced head and neck cancer to assess residual nodal disease after chemoradiotherapy. EJNMMI Res. 2022, 12, 34. [Google Scholar] [CrossRef]
- Silvoniemi, A.; Laine, J.; Aro, K.; Nissi, L.; Bäck, L.; Schildt, J.; Hirvonen, J.; Hagström, J.; Irjala, H.; Aaltonen, L.M.; et al. Circulating Tumor DNA in Head and Neck Squamous Cell Carcinoma: Association with Metabolic Tumor Burden Determined with FDG-PET/CT. Cancers 2023, 15, 3970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef]

| Histology | Tumor (n = 38) | Lymph Nodes (n = 104) |
|---|---|---|
| Malignant | 30 (78.9%) | 82 (78.8%) |
| Squamous cell carcinoma | 25 (65.8%) | 62 (50.6%) |
| Other malignities (lymphoma, adenocarcinoma, verucosic carcinoma, epithelial-myoepithelial carcinoma, epitheloid sarcoma, sebocellular carcinoma) | 5 (13.2%) | 20 (19.2%) |
| Non-malignant (e.g., inflammation, physiological finding) | 8 (21%) | 22 (21.1%) |
| M3 | Weight for MRFDG | Youden Index | Distance | 95% Sensitivity |
|---|---|---|---|---|
| mean | 7.2 | 70 | 70 | 52 |
| max | −29 | 194 | 147 | 94 |
| Unit | Youden Index | Distance | 95.0% Sensitivity | |
|---|---|---|---|---|
| mean | SUVbw (g/mL) | 5.8 | 3.0 | 2.4 |
| MRFDG (µmol/mL/min) | 0.050 | 0.050 | 0.026 | |
| DVFDG (%) | 68 | 68 | 51 | |
| max | SUVbw (g/mL) | 8.7 | 4.4 | 3.4 |
| MRFDG (µmol/mL/min) | 0.110 | 0.110 | 0.051 | |
| DVFDG (%) | 202 | 168 | 96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horňák, G.; Dias, A.H.; Munk, O.L.; Gormsen, L.C.; Ptáček, J.; Karhan, P. Dynamic Whole-Body FDG PET/CT for Predicting Malignancy in Head and Neck Tumors and Cervical Lymphadenopathy. Diagnostics 2025, 15, 2651. https://doi.org/10.3390/diagnostics15202651
Horňák G, Dias AH, Munk OL, Gormsen LC, Ptáček J, Karhan P. Dynamic Whole-Body FDG PET/CT for Predicting Malignancy in Head and Neck Tumors and Cervical Lymphadenopathy. Diagnostics. 2025; 15(20):2651. https://doi.org/10.3390/diagnostics15202651
Chicago/Turabian StyleHorňák, Gregor, André H. Dias, Ole L. Munk, Lars C. Gormsen, Jaroslav Ptáček, and Pavel Karhan. 2025. "Dynamic Whole-Body FDG PET/CT for Predicting Malignancy in Head and Neck Tumors and Cervical Lymphadenopathy" Diagnostics 15, no. 20: 2651. https://doi.org/10.3390/diagnostics15202651
APA StyleHorňák, G., Dias, A. H., Munk, O. L., Gormsen, L. C., Ptáček, J., & Karhan, P. (2025). Dynamic Whole-Body FDG PET/CT for Predicting Malignancy in Head and Neck Tumors and Cervical Lymphadenopathy. Diagnostics, 15(20), 2651. https://doi.org/10.3390/diagnostics15202651






