Abstract
Background/Objectives: When transitioning from an older surgical technique to a newer one, we expect improved treatment outcomes and fewer complications. However, direct comparative studies to confirm these advantages are often lacking. Tubular minimally invasive transforaminal lumbar interbody fusion (MISTLIF) has been widely used, but limitations in visualization and endplate preparation may compromise fusion quality. Biportal endoscopic TLIF (BETLIF), a more recent alternative, offers enhanced magnification and superior hemostasis. Still, CT-based comparative data on fusion integrity remain limited. To evaluate the clinical and radiological outcomes following a chronological transition from MISTLIF to BETLIF, using thin-slice CT to assess fusion integrity. Methods: This retrospective study analyzed 179 patients treated by a single surgeon between January 2018 and May 2021. The first 90 cases underwent MISTLIF, followed by 89 BETLIF procedures. Clinical outcomes included Visual Analog Scale (VAS), Oswestry Disability Index (ODI), and Japanese Orthopedic Association (JOA) scores. Radiological assessments at one year postoperatively (X-ray and thin-slice CT) included disc height, segmental lordosis, Bridwell fusion grade, cage subsidence, and subchondral osteolysis. Results: BETLIF was associated with significantly shorter hospital stays (5.7 vs. 7.4 days) and fewer transfusions (0% vs. 14.7%). BETLIF showed significantly better ODI (12.7 vs. 23.5), JOA scores (26.4 vs. 20.6), and comparable VAS improvement. Radiologically, BETLIF had significantly higher fusion rates (93.3% vs. 82.4%), greater disc height restoration, and lower rates of cage subsidence (5.0% vs. 13.7%) and osteolysis (13.3% vs. 52.9%). Conclusions: BETLIF demonstrated superior clinical and radiological outcomes, likely due to enhanced endoscopic visualization and precise endplate preparation.
1. Introduction
Lumbar interbody fusion is a well-established surgical procedure for patients with degenerative lumbar conditions. Among various posterior approaches, transforaminal lumbar interbody fusion (TLIF) is widely utilized because of its unilateral access to the disc space and preservation of posterior elements.
Minimally invasive TLIF (MISTLIF), introduced by Foley and Lefkowitz in 2002, utilizes a tubular retractor system to access the surgical field while minimizing soft tissue trauma [1,2]. This technique provides well-known benefits, including reduced blood loss, reduced postoperative pain, shorter hospital stay, and comparable clinical outcomes compared to open TLIF. However, technical challenges, such as a narrow surgical corridor and limited visualization, can affect disc preparation and graft placement, potentially compromising fusion results [1,3,4,5].
Unilateral biportal endoscopic (UBE) spinal surgery has emerged as a promising alternative that offers enhanced visualization, excellent bleeding control, and ergonomic handling of surgical instruments [6,7,8,9]. UBE-based transforaminal lumbar interbody fusion, also known as biportal endoscopic TLIF (BETLIF), has demonstrated promising results in both clinical and radiological domains [10,11,12]. BETLIF allows for radical disc space preparation under direct endoscopic visualization, preservation of the subchondral bony endplate, generous graft placement, and the insertion of large or double interbody cages, all of which may contribute to improved fusion integrity and lower risk of cage subsidence [10,13,14].
Despite these theoretical advantages, comparative studies assessing the fusion quality and clinical efficacy of BETLIF versus MISTLIF remain scarce. The existing literature reports that the treatment outcomes of BETLIF are either compatible or inferior to those of MISTLIF [11,15,16,17]. Implementing new techniques requires investment in equipment and training and involves a learning curve that may initially increase complication rates [18,19,20]. Therefore, it is crucial to determine whether BETLIF yields superior outcomes that justify its broader clinical adoption.
Although plain radiographs are often used to assess fusion, thin-slice computed tomography (CT) with multiplanar reconstruction is currently the most reliable method for evaluating the interbody fusion status [21,22,23]. However, this modality is underutilized in assessing the treatment outcomes of BETLIF. Additionally, key radiographic parameters, such as cage subsidence and subchondral osteolysis, which are potential markers of pseudoarthrosis, are frequently overlooked or misinterpreted as part of the normal fusion process [24,25,26].
This study aims to compare the clinical and radiographic outcomes between MISTLIF and BETLIF using standardized CT-based fusion criteria. We hypothesized that BETLIF results in superior fusion quality and clinical outcomes compared with MISTLIF.
2. Materials and Methods
2.1. Patient Selection
After receiving approval from the Institutional Review Board, this retrospective study included all patients who underwent minimally invasive lumbar interbody fusion performed by the corresponding author at our institution from January 2018 to May 2021. Surgical indications comprised lumbar disc degeneration with radiographic evidence of segmental instability, presenting as mechanical lower back pain, with or without neurological symptoms or radiculopathy.
Segmental instability was defined as one or more of the following criteria: (1) spondylolisthesis exceeding Meyerding grade II; (2) anterior–posterior translation > 4 mm on dynamic lateral radiographs; (3) segmental disc angle change > 10° on flexion-extension views; or (4) presence of a vacuum disc on plain radiographs with corresponding facet effusion on MRI.
Exclusion criteria included: (1) prior lumbar spinal surgery, (2) spinal infection or neoplastic disease, and (3) follow-up duration < 12 months.
A total of 179 patients (45 males, 134 females) were included in this study. The first 90 patients underwent MISTLIF using a tubular retractor system, while the following 89 patients underwent BETLIF via the UBE approach.
2.2. Surgical Techniques
The two procedures share similar elements in terms of anesthesia, patient positioning, sterilization, neural decompression, instrumentation, and fixation. Both used a double-cage construct, featuring one PEEK cage (Reborn®, Baui, Taipei, Taiwan) and one Ti-PEEK composite cage (Combo-T®, A-Spine, Taipei, Taiwan). The bone graft material includes 3 mL of demineralized bone matrix (SurFuse®, HansBiomed, Daejeon, Republic of Korea), autografts from laminotomy/facetectomy, and β-tricalcium phosphate blocks (Figure 1). Fixation is performed under fluoroscopic guidance with cannulated pedicle screws (Smartloc, A-Spine, Taipei, Taiwan), which have breakable long barrels designed to facilitate rod insertion and spondylolisthesis reduction using the cantilever technique. The key distinctions between these two approaches are summarized in Table 1 and explained below:
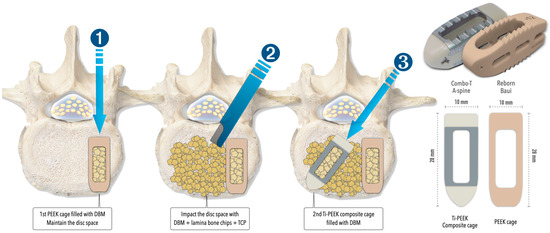
Figure 1.
The illustrations explain the insertion of double cages and the specifications of the cages used in both MISTLIF and BETLIF. DBM = demineralized bone matrix; PEEK = polyetheretherketone; Ti = Titanium; TCP = tricalcium phosphate.

Table 1.
Summary of MISTLIF and BETLIF.
2.2.1. Bleeding Control and Irrigation
BETLIF is performed under continuous saline irrigation to maintain the hydrostatic pressure, facilitate debris clearance, and suppress bleeding. Hemostasis is achieved using radiofrequency wands (ArthroCare®, Austin, TX, USA) and bone wax. Therefore, a waterproof draping and drainage system is essential. In contrast, MISTLIF is performed in air, with hemostasis relying on suction and electrocautery, which often leads to higher intraoperative blood loss.
2.2.2. Working Channel and Soft Tissue Management
MISTLIF utilizes a 26 mm diameter tubular retractor system (METRx X-TUBE®, Medtronic Sofamor Danek, Minneapolis, MN, USA). Soft tissues inside the tube must be removed. In contrast, BETLIF does not require a fixed retractor. The hydrostatic pressure creates a dynamic working space after muscle detachment and coagulation, minimizing tissue trauma (Figure 2).
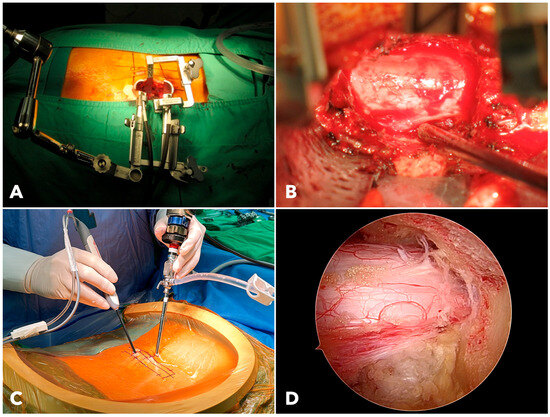
Figure 2.
(A) The setup of the extendable tubular retractor system used for MISTLIF. (B) The surgical field of MISTLIF viewed under the surgical microscope. (C) The operating scene of BETLIF demonstrates the handling of endoscopes and surgical instruments. (D) The clear and magnified surgical field of BETLIF viewed under the endoscope.
2.2.3. Decompression and Visualization
In MISTLIF, visualization is limited by the tubular corridor, which requires table tilting for contralateral access. In contrast, BETLIF allows the surgeon to advance a 4 mm endoscope into the spinal canal. The 30° lens further expands the field of view without ergonomic compromise. The endoscopic system and saline medium provide a clear, magnified image, improving decompression efficiency.
2.2.4. Disc Space Preparation
In BETLIF, direct endoscopic visualization enables radical discectomy while preserving the bony endplate. Sharp disc shavers and curettes are avoided in favor of blunt disc spreaders and specially designed endplate strippers. The endoscope is inserted into the disc space to ensure complete removal of the cartilaginous endplate (Figure 3). In MISTLIF, disc space preparation relies on tactile feedback via serial disc shavers and curettes, increasing the possibility of inadvertent endplate violation.
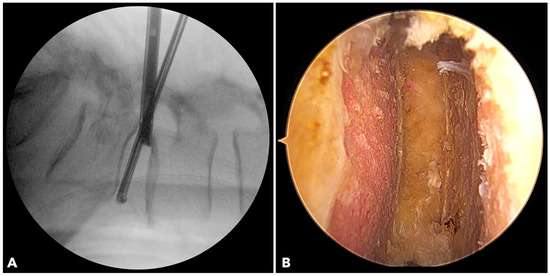
Figure 3.
(A) The fluoroscope image shows the endoscope being inserted into the disc to evaluate the final results of disc space preparation. (B) The endoscopic photo verifies a perfect disc space preparation with no damage to the bony endplate.
2.3. Clinical and Radiological Outcome Assessment
Clinical and demographic data were retrieved from the medical records, including age, sex, body mass index, comorbidities, operative time, intraoperative blood loss, transfusion, hospital stay, and complications. Patient-reported outcomes were evaluated using the Visual Analog Scale (VAS) for back and leg pain, the modified Oswestry Disability Index (ODI), and the Japanese Orthopedic Association (JOA) score. Assessments were performed preoperatively and at 3, 6, and 12 months postoperatively, with annual follow-up.
In addition to analyzing mean score differences, we evaluated the proportion of patients achieving the minimum clinically important difference (MCID) for each functional outcome measure. The following MCID thresholds, based on established literature, were applied: 2.1 for VAS for back pain, 2.8 for VAS for leg pain, 14.9 for ODI, and 2.0 for JOA score [27,28].
Radiographic assessments included anteroposterior and lateral lumbar radiographs taken before surgery and at 1, 3, 6, and 12 months postoperatively. Flexion-extension lateral views were obtained preoperatively, at 12 months, and then every one to two years afterward to evaluate fusion status, segmental stability, and adjacent segment degeneration. Disc height was defined as the mean of anterior and posterior disc space measurements. Segmental lordosis refers to the angle between the superior endplates of the upper and lower vertebrae of the fusion segment [29]. All patients underwent magnetic resonance imaging (MRI) of the lumbar spine before the surgery.
Lumbar spine computed tomography (CT) with 3 mm thin-slice coronal and sagittal reconstructions was performed one year after the surgery. Fusion status was assessed using the Bridwell grading system based on bone window images (Figure 4). Grades I and II were defined as successful fusion. Cage subsidence was defined as sinking > 2 mm beyond the bony endplate [24,30]. The presence of subchondral osteolysis was also recorded as a potential indicator of pseudoarthrosis (Figure 5).
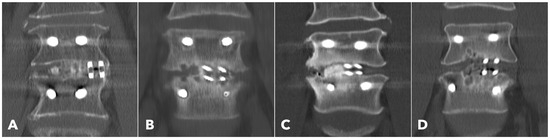
Figure 4.
Postoperative 1-year CT reconstruction to evaluate the Bridwell fusion grade. (A) Grade I solid fusion with remodeling of the trabeculae. (B) Grade II fusion with bridging bone formation and no radiolucency between the cages and the endplates. (C) Grade III fusion with radiolucency between the bone graft and endplate. (D) Grade IV fusion with resorption of the bone graft.
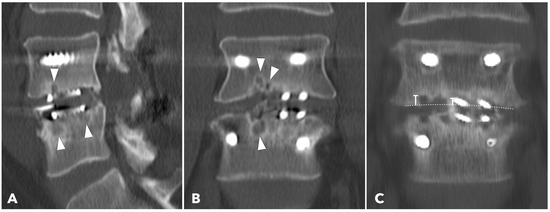
Figure 5.
(A,B) 1-year CT reconstruction revealed the presence of subchondral osteolysis or endplate cyst formation (white arrowheads). (C) The measurement of cage subsidence on the coronal CT reconstruction image.
2.4. Statistical Analysis
Continuous variables are expressed as mean ± standard deviation. Between-group comparisons were performed using Student’s t-test for continuous data and the chi-square test for categorical data. The null hypothesis is that BETLIF does not yield superior outcomes compared to MISTLIF. To address the issue of multiple hypothesis testing, p-values were adjusted using the Benjamini–Hochberg procedure to control the false discovery rate (FDR). Both raw and FDR-adjusted p-values (q-values) were reported to enhance interpretability and maintain statistical rigor. Adjusted p-values (q-values) < 0.05 were considered statistically significant.
3. Results
There were 90 patients in the MISTLIF group and 89 in the BETLIF group who fulfilled the selection criteria, with 114 fused segments per group. There were no statistically significant differences between groups in demographic characteristics, follow-up duration, preoperative diagnoses, or surgical levels (Table 2).

Table 2.
Demographic characteristics between groups.
The rate of intraoperative or postoperative blood transfusion was significantly higher in the MISTLIF group than in the BETLIF group (14.7% vs. 0%, q < 0.001). The mean hospital stay was also significantly longer for MISTLIF patients (7.4 ± 2.3 days) than for BETLIF patients (5.7 ± 1.1 days, q < 0.001) (Table 3).

Table 3.
Comparison of clinical outcomes between groups.
Preoperative clinical parameters, including back and leg VAS scores, ODI, and JOA scores, were comparable between the groups (all q > 0.05), indicating similar baseline functional status. Both groups experienced significant postoperative improvement in back and leg pain, with no statistically significant intergroup differences at the final follow-up (VAS back pain, q = 0.850; VAS leg pain, q = 0.653). However, the BETLIF group demonstrated superior postoperative functional recovery, as evidenced by a significantly lower ODI score (12.7 ± 16.1 vs. 23.5 ± 14.4, q < 0.001) and higher JOA score (26.4 ± 3.2 vs. 20.6 ± 2.5, q < 0.001) (Table 3 and Table 4).

Table 4.
Comparison of the functional and radiological results before and after the surgery.
The proportion of patients achieving MCID was generally higher in the BETLIF group. Specifically, MCID attainment rates for ODI (83.1% vs. 73.3%) and JOA score (97.7% vs. 88.9%) were notably higher compared to MISTLIF. After applying the Benjamini–Hochberg correction, MCID attainment for JOA score remained statistically significant (q = 0.042), while the differences in VAS (back), VAS (leg), and ODI did not reach statistical significance (q-values = 0.140, 0.272, and 0.179, respectively). This suggests that while both groups showed functional improvement, BETLIF achieved clinically meaningful recovery more consistently, especially in neurological function, as reflected by the JOA score.
The overall complication rate was low and comparable between groups. Dural tears, epidural hematomas, and transient neurological deficits occurred at similar frequencies without a significant difference. Pedicle screw malposition was observed only in the BETLIF group (2.25%, q = 0.230), whereas screw loosening occurred only in the MISTLIF group (3.33%, q = 0.149). The reoperation rates were also low and similar between groups (1.1% vs. 2.25%, q = 0.673). Three patients required a re-operation. One patient in the BETLIF group required revision for screw malposition-induced motor weakness. One patient in each group required a reoperation for postoperative epidural hematoma and motor weakness. All the affected patients achieved near-complete recovery following revision surgery (Table 3).
Radiographic analysis revealed comparable preoperative disc heights between the groups (5.9 ± 1.0 mm vs. 6.1 ± 1.7 mm, q = 0.445). Both groups exhibited significant postoperative increases in disc height; however, the BETLIF group achieved a significantly greater postoperative disc height (10.5 ± 0.9 mm vs. 9.6 ± 1.0 mm, p < 0.001) and greater disc height restoration (4.4 ± 1.5 mm vs. 3.7 ± 1.5 mm, q = 0.005).
Segmental lordosis improved significantly in both groups postoperatively, with no statistically significant differences between groups in the magnitude of change (6.2 ± 3.7° vs. 6.1 ± 2.0°, q = 0.937) or in the pre- and postoperative angles (q = 0.339 and 0.173, respectively).
Among the segments evaluated by CT at one year (56.7% in MISTLIF vs. 67.4% in BETLIF, q = 0.216), the BETLIF group showed significantly higher rates of bridging bone in the sagittal (96.6% vs. 88.2%, p = 0.027) and coronal planes (93.3% vs. 82.4%, p = 0.025). The overall fusion success rate (Bridwell Grade I or II) was significantly higher in the BETLIF group (93.3% vs. 82.4%, p = 0.025). However, these statistical significances were eliminated after applying the Benjamini–Hochberg correction to control the FDR. Furthermore, a substantially higher proportion of BETLIF cases were classified as Bridwell Grade I (73.3% vs. 19.6%, q < 0.001), indicating superior fusion quality (Figure 6 and Figure 7).
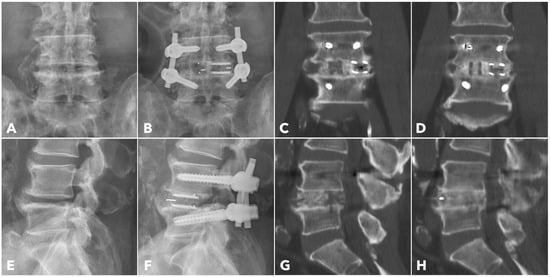
Figure 6.
A 67-year-old male patient underwent BETLIF at L4-5 due to recurrent lumbar disc herniation and painful disc degeneration (A,E). The postoperative X-rays showed restoration of the disc height with double cages in the disc space (B,F). The immediate postoperative CT scan demonstrated the cage positions and no violation of the bony endplates (C,G). The 1-year CT scan revealed Bridwell grade I solid fusion with remodeling of the bone graft (D,H).
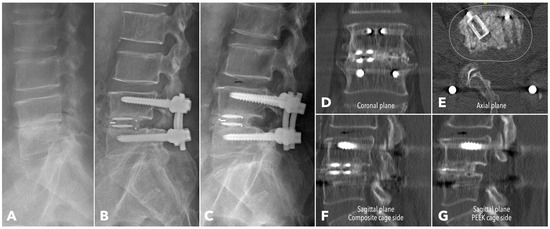
Figure 7.
A 62-year-old male patient underwent BETLIF at L4-5 due to painful disc degeneration with restoration of the disc height after the surgery (A,B). The postoperative 6-month X-ray showed consolidation of the bone graft (C). The 1-year CT scan revealed Bridwell grade I solid fusion with remodeling of the bone graft at both coronal plane (D) and sagittal plane (F,G) reconstructions. The axial plane reconstruction (E) demonstrated the cage footprint and the fusion bed. The white dashed line indicates the margin of the vertebral body.
Subchondral osteolysis was significantly more frequent in the MISTLIF group (52.9% vs. 13.3%, q < 0.001). Cage subsidence was also significantly more prevalent in the MISTLIF group (13.7% vs. 5.0%, p = 0.023), although most patients in both groups exhibited no evidence of subsidence (Table 5). Again, the statistical significance was no longer significant after applying the Benjamini–Hochberg correction to control the FDR.

Table 5.
Comparison of radiological outcomes between groups.
4. Discussion
This study provides a comprehensive comparison of biportal endoscopic TLIF (BETLIF) and minimally invasive tubular TLIF (MISTLIF) in terms of clinical and radiological outcomes, with a particular emphasis on fusion quality assessed using thin-slice CT scans, which is currently considered the gold standard for evaluating interbody fusion [21,23]. Our findings suggest that BETLIF yields superior results to MISTLIF in several critical aspects, including blood loss, hospitalization duration, functional recovery, and radiographic evidence of fusion.
BETLIF was associated with significantly lower rates of intraoperative or postoperative blood transfusion, with none of the BETLIF patients requiring transfusion compared with 14.7% in the MISTLIF group. Continuous saline irrigation intrinsic to the UBE technique enables superior hemostasis by maintaining hydrostatic pressure and suppressing venous oozing. This facilitates a clear visual field for safe tissue dissection and reduces transfusion-related risks such as immunologic reactions or infections [31].
Hospitalization time was also significantly reduced in the BETLIF group. Although both approaches utilize the Wiltse intermuscular corridor to minimize tissue disruption, BETLIF eliminates the need for a tubular retractor and relies on dynamic saline irrigation to maintain working space. This allows for smaller incisions, less muscle dissection, and potentially faster postoperative recovery [3,10,16,17].
Pain relief and improved functional status were observed in both groups after surgery. However, BETLIF demonstrated significantly better results at the final follow-up, indicated by lower ODI and higher JOA scores. These improvements are likely due to the minimal soft tissue injury from the endoscopic approach and minimal bleeding during surgery [9,10,12,32]. Unlike the significant improvements in ODI and JOA scores, there was no notable difference in VAS scores for leg and back pain between the groups at final follow-up. ODI and JOA scores assess broader functional disability and are likely more sensitive to the benefits of BETLIF. In contrast, VAS scores for back and leg pain are more influenced by segmental stabilization and effective neural decompression, which was effective in both techniques.
Our analysis of MCID attainment highlights the clinical relevance of the observed outcome differences. Although the mean ODI and JOA scores significantly improved in the BETLIF group, only the JOA MCID attainment rate remained statistically significant after FDR correction. This finding supports the notion that BETLIF may provide a more robust improvement in neurological recovery, which may be attributed to better endoscopic visualization and more precise decompression techniques. The application of the Benjamini–Hochberg method further strengthens the validity of our results by mitigating the risk of false-positive findings from multiple comparisons.
Radiographic analysis further favored BETLIF. CT-based evaluation demonstrated significantly higher fusion rates in the BETLIF group (93.3% vs. 82.4%), with a markedly greater proportion achieving Bridwell Grade I fusion (73.3% vs. 19.6%). This improvement is especially notable, given that all other surgical parameters (i.e., cage material, bone graft composition, and fixation technique) were identical between the groups. Thus, the superior outcomes of BETLIF can be reasonably attributed to enhanced disc space preparation and preservation of the bony endplate under direct endoscopic visualization.
In MISTLIF, disc preparation is performed in air, with visualization often hindered by bleeding and constrained by the retractor’s corridor. Surgeons must rely on tactile feedback, which may unintentionally damage the bony endplate and increase the risk of cage subsidence or pseudoarthrosis. Some studies have attempted to reduce endplate damage by using fluoroscopy. However, when damage was visible on the fluoroscope, it had already occurred. In a cadaveric study, Tatsuma et al. demonstrated that 48% of the endplate area was damaged after MISTLIF [33]. In contrast, BETLIF allows clear visualization of the entire disc space under saline medium, enabling precise cartilage removal without damage to the subchondral bone, a critical factor for promoting fusion integrity [34,35].
Cage subsidence was significantly less frequent in BETLIF (5.0%) than in MISTLIF (13.7%, p = 0.023), although such significance was mitigated after applying the Benjamini–Hochberg correction. While some authors argue that mild subsidence may represent a physiological adaptation during fusion, excessive subsidence can lead to loss of disc height and segmental lordosis, recurrent stenosis, and complications related to hardware [24,30,36,37]. Increasing the cage footprint using double cages is a reasonable approach to enhance initial segmental stability, improve load distribution, and ensure graft-to-endplate contact [10,36,38,39,40,41]. In posterior approaches, neural elements often limit the size of the cage. However, the UBE technique facilitates safe and effective neural decompression, including the release of epidural adhesions, thereby expanding the accessible corridor. The use of “sentinel pins” to shield neural structures allows for the insertion of larger cages even in severely collapsed disc spaces [10,42]. The BETLIF’s emphasis on endplate preservation, combined with the ability to insert larger cages via safe neural retraction and annular release, may explain the lower subsidence rate and improved disc height restoration in this group.
Although both groups showed significant postoperative increases in segmental lordosis, no intergroup differences were observed. This finding is consistent with previous studies that have demonstrated posterior fusion techniques to be less effective in restoring global sagittal alignment than anterior approaches [29,43]. In our cohort, spinal fusion was primarily indicated for instability and neural compression, rather than correction of deformity. Techniques such as anterior cage positioning and cantilever reduction may account for the segmental lordosis gains observed; however, assessing their impact on global alignment requires a prospective long-term follow-up study, which is beyond the scope of this study [38,44,45,46].
This study has several limitations. First, as a retrospective study, it was subject to inherent selection and follow-up biases, particularly in the subgroup of patients (56.7% and 67.4% in each group) who underwent CT evaluation. Second, the sample size may be insufficient to detect rare or long-term complications. Third, the follow-up period might not fully reflect the durability of outcomes or long-term alignment effects. BETLIF, by minimizing muscle detachment and preserving posterior ligamentous structures, may reduce abnormal biomechanical stress at adjacent levels. Long-term follow-up is necessary to verify its protective effect against adjacent segment degeneration. Nevertheless, standardized surgical techniques, consistent instrumentation and fusion materials, and uniform postoperative protocols minimize confounding factors, enabling a focused comparison of the two approaches.
5. Conclusions
Both MISTLIF and BETLIF are safe and effective minimally invasive techniques for lumbar interbody fusion that yield favorable clinical and radiological outcomes. However, this study demonstrates that BETLIF provides significantly improved functional recovery, higher fusion rates, and better fusion quality than MISTLIF. These advantages are primarily attributable to the inherent strengths of the biportal endoscopic approach, including enhanced visualization, superior hemostasis, and the ability to perform meticulous disc space preparation under direct endoscopic guidance. Given these findings, BETLIF represents a promising advancement in minimally invasive spine surgery and may serve as a preferred technique for achieving reliable and high-quality lumbar fusion.
Author Contributions
Conceptualization, J.-L.P.; methodology, J.-L.P.; validation, Y.-H.H. and J.-L.P.; formal analysis, Y.-H.H. and J.-L.P.; investigation, Y.-H.H. and J.-L.P.; resources, J.-L.P.; data curation, Y.-H.H. and J.-L.P.; writing—original draft preparation, Y.-H.H.; writing—review and editing, J.-L.P.; visualization, J.-L.P.; supervision, J.-L.P.; project administration, J.-L.P.; funding acquisition, J.-L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Far Eastern Memorial Hospital, grant number FEMH-2025-C-053.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Research Ethics Review Committee of Far Eastern Memorial Hospital (protocol code 114137-E, approved on 15 July 2025).
Informed Consent Statement
Patient’s informed consent was waived due to the study’s retrospective design.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MISTLIF | Minimally invasive transforaminal lumbar interbody fusion |
| BETLIF | Biportal endoscopic transforaminal lumbar interbody fusion |
| CT | Computed tomography |
| MRI | Magnetic resonance image |
| VAS | Visual analog scale |
| ODI | Oswestry disability index |
| JOA | Japanese orthopedic association |
| UBE | Unilateral biportal endoscopy |
| MCID | Minimum clinically important difference |
| FDR | False discovery rate |
| PEEK | Polyetheretherketone |
| Ti | Titanium |
| DBM | Demineralized bone matrix |
| TCP | Tricalcium phosphate |
References
- Prabhu, M.C.; Jacob, K.C.; Patel, M.R.; Pawlowski, H.; Vanjani, N.N.; Singh, K. History and Evolution of the Minimally Invasive Transforaminal Lumbar Interbody Fusion. Neurospine 2022, 19, 479–491. [Google Scholar] [CrossRef]
- Foley, K.T.; Lefkowitz, M.A. Advances in minimally invasive spine surgery. Clin. Neurosurg. 2002, 49, 499–517. [Google Scholar] [PubMed]
- Lener, S.; Wipplinger, C.; Hernandez, R.N.; Hussain, I.; Kirnaz, S.; Navarro-Ramirez, R.; Schmidt, F.A.; Kim, E.; Hartl, R. Defining the MIS-TLIF: A Systematic Review of Techniques and Technologies Used by Surgeons Worldwide. Global Spine J. 2020, 10, 151S–167S. [Google Scholar] [CrossRef]
- Vazan, M.; Gempt, J.; Meyer, B.; Buchmann, N.; Ryang, Y.M. Minimally invasive transforaminal lumbar interbody fusion versus open transforaminal lumbar interbody fusion: A technical description and review of the literature. Acta Neurochir. 2017, 159, 1137–1146. [Google Scholar] [CrossRef]
- Lee, M.J.; Mok, J.; Patel, P. Transforaminal Lumbar Interbody Fusion: Traditional Open Versus Minimally Invasive Techniques. J. Am. Acad. Orthop. Surg. 2018, 26, 124–131. [Google Scholar] [CrossRef]
- Song, K.S.; Kim, P. Assessment of Clinical and Radiologic Outcomes of Biportal Endoscopic Posterior Cervical Inclinatory Foraminotomy: A Retrospective Cohort Study. J. Korean Neurosurg. Soc. 2025, 68, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Murillo, M.; Castro-Toral, J.; Bonome-Gonzalez, C.; de Mon-Montoliu, J.A. Endoscopic surgery for multilevel spinal stenosis: A comprehensive meta-analysis and subgroup analysis of uniportal and biportal approaches. Asian Spine J. 2025, 19, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Upfill-Brown, A.; Curtin, N.; Hamad, C.D.; Shah, A.; Kwon, B.; Kim, Y.H.; Heo, D.H.; Park, C.W.; Sheppard, W.L. Clinical outcomes and complications after biportal endoscopic spine surgery: A comprehensive systematic review and meta-analysis of 3673 cases. Eur. Spine J. 2023, 32, 2637–2646. [Google Scholar] [CrossRef]
- Pao, J.L.; Lin, S.M.; Chen, W.C.; Chang, C.H. Unilateral biportal endoscopic decompression for degenerative lumbar canal stenosis. J. Spine Surg. 2020, 6, 438–446. [Google Scholar] [CrossRef]
- Pao, J.L. Biportal Endoscopic Transforaminal Lumbar Interbody Fusion Using Double Cages: Surgical Techniques and Treatment Outcomes. Neurospine 2023, 20, 80–91. [Google Scholar] [CrossRef]
- Kang, M.S.; You, K.H.; Choi, J.Y.; Heo, D.H.; Chung, H.J.; Park, H.J. Minimally invasive transforaminal lumbar interbody fusion using the biportal endoscopic techniques versus microscopic tubular technique. Spine J. 2021, 21, 2066–2077. [Google Scholar] [CrossRef]
- Heo, D.H.; Lee, D.C.; Kim, H.S.; Park, C.K.; Chung, H. Clinical Results and Complications of Endoscopic Lumbar Interbody Fusion for Lumbar Degenerative Disease: A Meta-Analysis. World Neurosurg. 2021, 145, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.H. Biportal endoscopic transforaminal lumbar interbody fusion using a large cage for degenerative spondylolisthesis with stenosis. Neurosurg. Focus Video 2024, 10, V15. [Google Scholar] [CrossRef]
- Park, D.Y.; Heo, D.H. The Use of Dual Direction Expandable Titanium Cage with Biportal Endoscopic Transforaminal Lumbar Interbody Fusion: A Technical Consideration with Preliminary Results. Neurospine 2023, 20, 110–118. [Google Scholar] [CrossRef]
- Park, M.K.; Park, S.A.; Son, S.K.; Park, W.W.; Choi, S.H. Clinical and radiological outcomes of unilateral biportal endoscopic lumbar interbody fusion (ULIF) compared with conventional posterior lumbar interbody fusion (PLIF): 1-year follow-up. Neurosurg. Rev. 2019, 42, 753–761. [Google Scholar] [CrossRef]
- Heo, D.H.; Park, C.K. Clinical results of percutaneous biportal endoscopic lumbar interbody fusion with application of enhanced recovery after surgery. Neurosurg. Focus 2019, 46, E18. [Google Scholar] [CrossRef]
- Yang, H.; Cheng, F.; Hai, Y.; Liu, Y.; Pan, A. Unilateral biportal endoscopic lumbar interbody fusion enhanced the recovery of patients with the lumbar degenerative disease compared with the conventional posterior procedures: A systematic review and meta-analysis. Front. Neurol. 2022, 13, 1089981. [Google Scholar] [CrossRef]
- Brusko, G.D.; Wang, M.Y. Endoscopic Lumbar Interbody Fusion. Neurosurg. Clin. N. Am. 2020, 31, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.J.; Choi, C.M.; Jung, J.T.; Lee, S.J.; Kim, Y.S. Learning Curve Associated with Complications in Biportal Endoscopic Spinal Surgery: Challenges and Strategies. Asian Spine J. 2016, 10, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Kim, H.J.; Kim, G.U.; Choi, M.H.; Chang, B.S.; Lee, C.K.; Yeom, J.S. Learning Curve for Lumbar Decompressive Laminectomy in Biportal Endoscopic Spinal Surgery Using the Cumulative Summation Test for Learning Curve. World Neurosurg. 2019, 122, e1007–e1013. [Google Scholar] [CrossRef]
- Benson, J.C.; Lehman, V.T.; Sebastian, A.S.; Larson, N.A.; Nassr, A.; Diehn, F.E.; Wald, J.T.; Murthy, N.S. Successful fusion versus pseudarthrosis after spinal instrumentation: A comprehensive imaging review. Neuroradiology 2022, 64, 1719–1728. [Google Scholar] [CrossRef]
- Choudhri, T.F.; Mummaneni, P.V.; Dhall, S.S.; Eck, J.C.; Groff, M.W.; Ghogawala, Z.; Watters, W.C., 3rd; Dailey, A.T.; Resnick, D.K.; Sharan, A.; et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 4: Radiographic assessment of fusion status. J. Neurosurg. Spine 2014, 21, 23–30. [Google Scholar] [CrossRef]
- Duits, A.A.A.; van Urk, P.R.; Lehr, A.M.; Nutzinger, D.; Reijnders, M.R.L.; Weinans, H.; Foppen, W.; Oner, F.C.; van Gaalen, S.M.; Kruyt, M.C. Radiologic Assessment of Interbody Fusion: A Systematic Review on the Use, Reliability, and Accuracy of Current Fusion Criteria. JBJS Rev. 2024, 12, e23.00065. [Google Scholar] [CrossRef] [PubMed]
- Parisien, A.; Wai, E.K.; ElSayed, M.S.A.; Frei, H. Subsidence of Spinal Fusion Cages: A Systematic Review. Int. J. Spine Surg. 2022, 16, 1103–1118. [Google Scholar] [CrossRef]
- Fujibayashi, S.; Takemoto, M.; Izeki, M.; Takahashi, Y.; Nakayama, T.; Neo, M. Does the formation of vertebral endplate cysts predict nonunion after lumbar interbody fusion? Spine 2012, 37, E1197–E1202. [Google Scholar] [CrossRef]
- Sasaki, M.; Umegaki, M.; Fukunaga, T.; Hijikata, Y.; Banba, Y.; Matsumoto, K.; Miyao, Y. Vertebral Endplate Cyst Formation in Relation to Properties of Interbody Cages. Neurospine 2021, 18, 170–176. [Google Scholar] [CrossRef]
- Goh, G.S.; Liow, M.H.L.; Ling, Z.M.; Soh, R.C.C.; Guo, C.M.; Yue, W.M.; Tan, S.B.; Chen, J.L. Severity of Preoperative Myelopathy Symptoms Affects Patient-reported Outcomes, Satisfaction, and Return to Work After Anterior Cervical Discectomy and Fusion for Degenerative Cervical Myelopathy. Spine 2020, 45, 649–656. [Google Scholar] [CrossRef]
- Parker, S.L.; Adogwa, O.; Paul, A.R.; Anderson, W.N.; Aaronson, O.; Cheng, J.S.; McGirt, M.J. Utility of minimum clinically important difference in assessing pain, disability, and health state after transforaminal lumbar interbody fusion for degenerative lumbar spondylolisthesis. J. Neurosurg. Spine 2011, 14, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Champagne, P.O.; Walsh, C.; Diabira, J.; Plante, M.E.; Wang, Z.; Boubez, G.; Shedid, D. Sagittal Balance Correction Following Lumbar Interbody Fusion: A Comparison of the Three Approaches. Asian Spine J. 2019, 13, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.A.R.; Aguirre, A.O.; Kuo, C.C.; Ruggiero, N.; Azmy, S.; Khan, A.; Ghannam, M.M.; Almeida, N.D.; Jowdy, P.K.; Mullin, J.P.; et al. Vertebral bone quality score independently predicts cage subsidence following transforaminal lumbar interbody fusion. Spine J. 2022, 22, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Ackfeld, T.; Schmutz, T.; Guechi, Y.; Le Terrier, C. Blood Transfusion Reactions-A Comprehensive Review of the Literature including a Swiss Perspective. J. Clin. Med. 2022, 11, 2859. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.H.; Hong, Y.H.; Lee, D.C.; Chung, H.J.; Park, C.K. Technique of biportal endoscopic transforaminal lumbar interbody fusion. Neurospine 2020, 17, S129–S137. [Google Scholar] [CrossRef]
- Tatsumi, R.; Lee, Y.P.; Khajavi, K.; Taylor, W.; Chen, F.; Bae, H. In vitro comparison of endplate preparation between four mini-open interbody fusion approaches. Eur. Spine J. 2015, 24 (Suppl. 3), 372–377. [Google Scholar] [CrossRef]
- Wu, H.; Shan, Z.; Zhao, F.; Cheung, J.P.Y. Poor Bone Quality, Multilevel Surgery, and Narrow and Tall Cages Are Associated with Intraoperative Endplate Injuries and Late-onset Cage Subsidence in Lateral Lumbar Interbody Fusion: A Systematic Review. Clin. Orthop. Relat. Res. 2022, 480, 163–188. [Google Scholar] [CrossRef]
- Yao, Y.C.; Chou, P.H.; Lin, H.H.; Wang, S.T.; Liu, C.L.; Chang, M.C. Risk Factors of Cage Subsidence in Patients Received Minimally Invasive Transforaminal Lumbar Interbody Fusion. Spine 2020, 45, E1279–E1285. [Google Scholar] [CrossRef]
- Levy, H.A.; Pinter, Z.W.; Reed, R.; Harmer, J.R.; Raftery, K.; Nathani, K.R.; Katsos, K.; Bydon, M.; Fogelson, J.L.; Elder, B.D.; et al. Transforaminal lumbar interbody fusion subsidence: Computed tomography analysis of incidence, associated risk factors, and impact on outcomes. J. Neurosurg. Spine 2024, 41, 463–472. [Google Scholar] [CrossRef]
- Zhao, L.; Xie, T.; Wang, X.; Yang, Z.; Pu, X.; Lu, Y.; Zeng, J. Clinical and radiological evaluation of cage subsidence following oblique lumbar interbody fusion combined with anterolateral fixation. BMC Musculoskelet. Disord. 2022, 23, 214. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Ma, C.; Li, B.; Ren, B.; Liu, J.; Huang, Y.; Qiao, L.; Mao, K. Biomechanical studies of different numbers and positions of cage implantation on minimally invasive transforaminal interbody fusion: A finite element analysis. Front. Surg. 2022, 9, 1011808. [Google Scholar] [CrossRef]
- Yu, Y.; Robinson, D.L.; Ackland, D.C.; Yang, Y.; Lee, P.V.S. Influence of the geometric and material properties of lumbar endplate on lumbar interbody fusion failure: A systematic review. J. Orthop. Surg. Res. 2022, 17, 224. [Google Scholar] [CrossRef]
- Yuan, W.; Kaliya-Perumal, A.K.; Chou, S.M.; Oh, J.Y. Does Lumbar Interbody Cage Size Influence Subsidence? A Biomechanical Study. Spine 2020, 45, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Ushirozako, H.; Hasegawa, T.; Ebata, S.; Ohba, T.; Oba, H.; Mukaiyama, K.; Shimizu, S.; Yamato, Y.; Ide, K.; Shibata, Y.; et al. Impact of sufficient contact between the autograft and endplate soon after surgery to prevent nonunion at 12 months following posterior lumbar interbody fusion. J. Neurosurg. Spine 2020, 33, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.C. “Pin Method” for Endoscopic Lumbar Interbody Fusion. J. Neurol. Surg. Cent. Eur. Neurosurg. 2022, 83, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Leveque, J.A.; Drolet, C.E.; Nemani, V.; Krause, K.L.; Shen, J.; Rathore, A.; Baig, Y.; Louie, P.K. The Impact of Surgical Approach on Sagittal Plane Alignment in Patients Undergoing One- or Two-Level Fusions for Degenerative Pathology: A Multicenter Radiographic Evaluation 6 Months Following Surgery. World Neurosurg. 2022, 164, e311–e317. [Google Scholar] [CrossRef]
- Hu, Y.H.; Niu, C.C.; Hsieh, M.K.; Tsai, T.T.; Chen, W.J.; Lai, P.L. Cage positioning as a risk factor for posterior cage migration following transforaminal lumbar interbody fusion—An analysis of 953 cases. BMC Musculoskelet. Disord. 2019, 20, 260. [Google Scholar] [CrossRef]
- Kim, J.T.; Shin, M.H.; Lee, H.J.; Choi, D.Y. Restoration of lumbopelvic sagittal alignment and its maintenance following transforaminal lumbar interbody fusion (TLIF): Comparison between straight type versus curvilinear type cage. Eur. Spine J. 2015, 24, 2588–2596. [Google Scholar] [CrossRef]
- Deng, L.; Wang, C.; Sun, H.; Lv, N.; Shen, Y.; Qian, Z.; Liu, H. Effects of Cage Implantation Depth on Sagittal Parameters and Functional Outcomes in Posterior Lumbar Interbody Fusion for the Treatment of L4–L5 Lumbar Degenerative Spondylolisthesis. Orthop. Surg. 2024, 16, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).