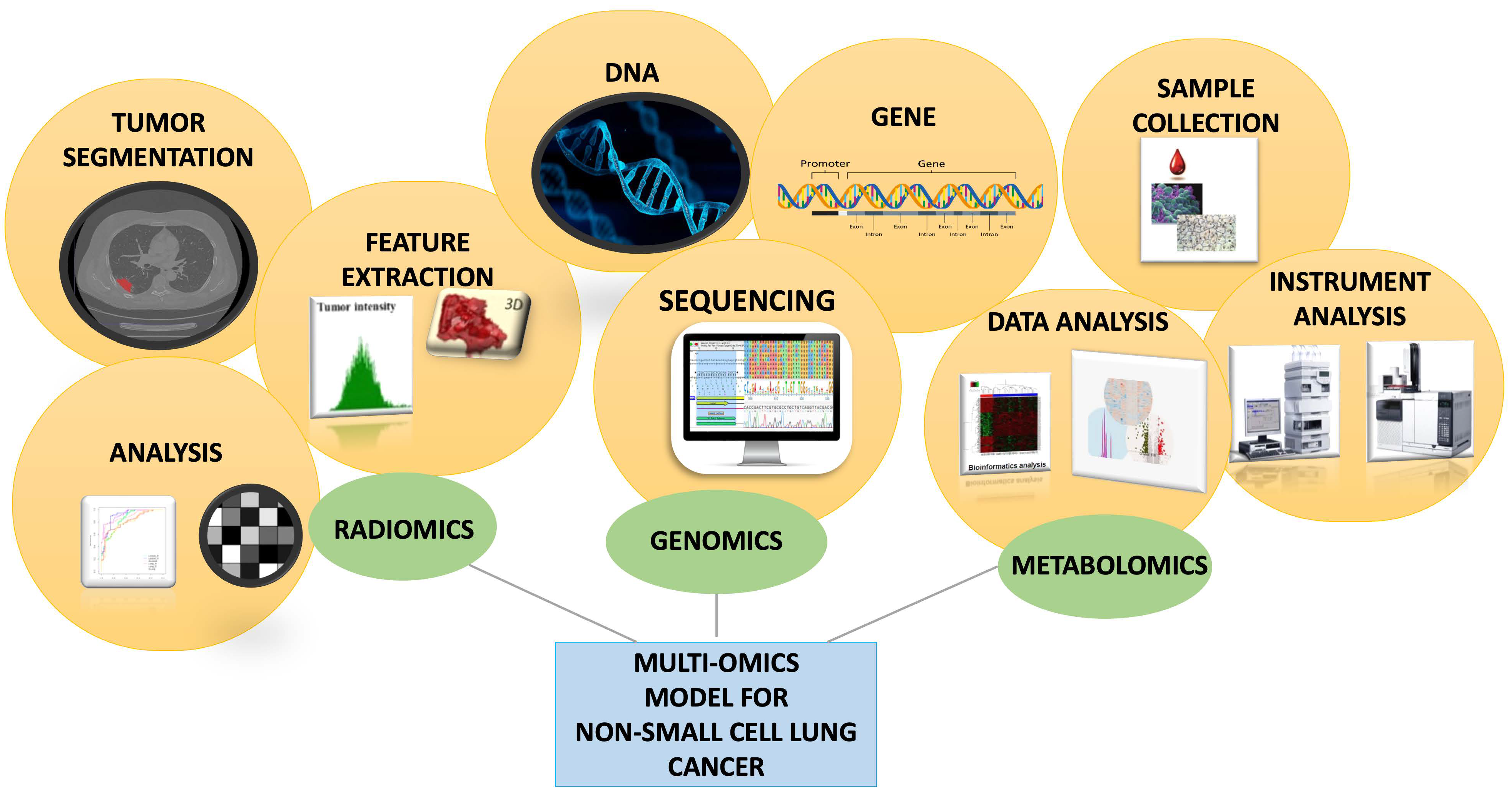
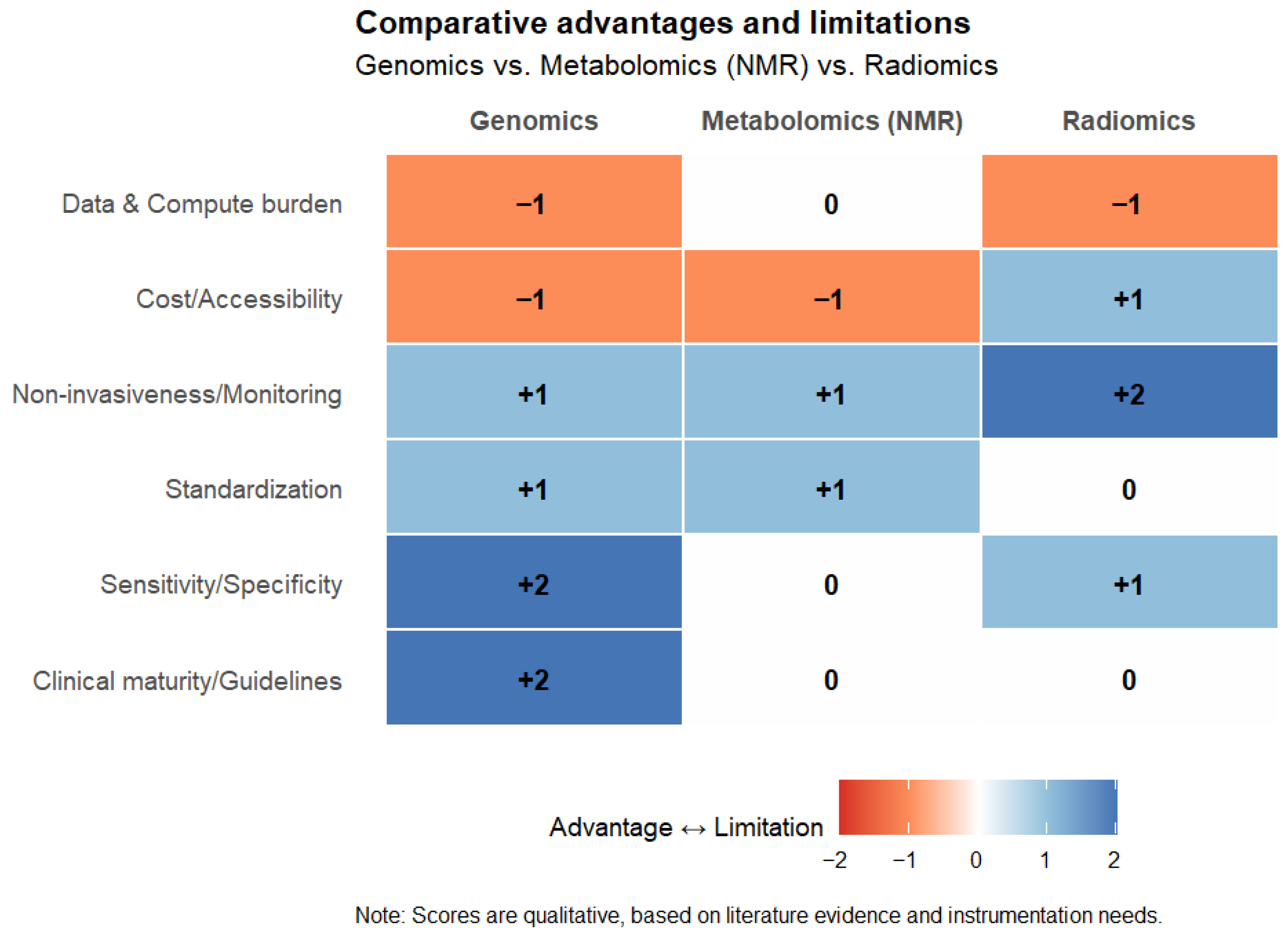
Advancing Non-Small-Cell Lung Cancer Management Through Multi-Omics Integration: Insights from Genomics, Metabolomics, and Radiomics
Abstract
1. Introduction
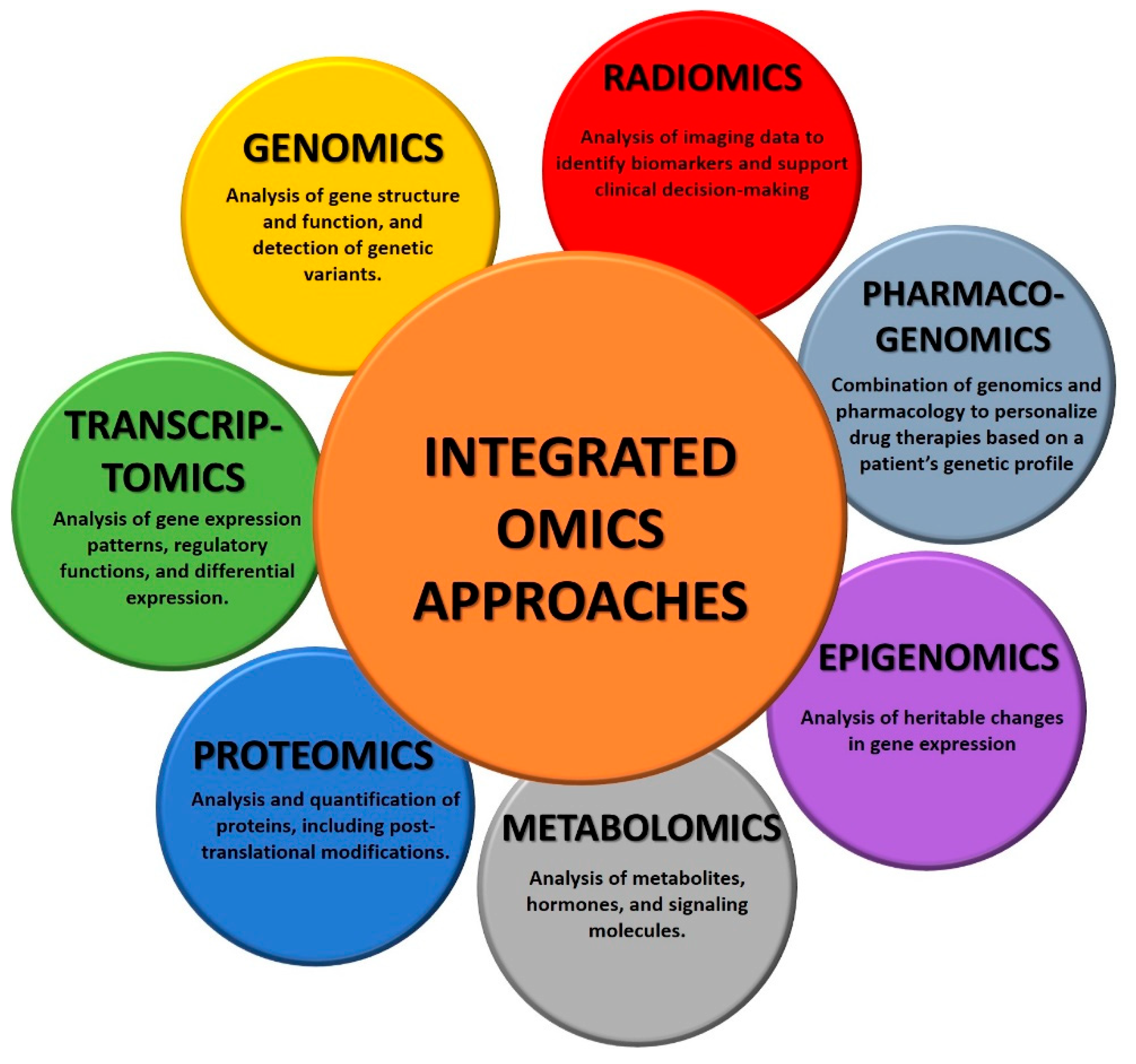
- Genomics is the study of the entire genome. It involves sequencing, mapping, and analyzing genetic material to understand the function of specific genes in diseases, identify genetic variations, and explore genetic relationships. Specifically in cancer, genomics analysis focuses on identifying mutations, copy number alterations, and structural variations in the genome. This information helps to identify driver mutations and potential therapeutic targets [6].
- Transcriptomics involves the study of all the RNA transcripts of a cell, hence focusing on the study of gene expression patterns. This technology is particularly useful to understand which genes are actively expressed in a specific disease and how gene expression patterns change under different conditions. Transcriptomics is particularly useful in cancer since it provides insights into tumor cells, helping to identify aberrant transcriptions and the corresponding genes that are actively involved in the disease. This information helps researchers to understand molecular pathways involved in cancer development and progression, but also in the discovery of new molecular targets for anticancer therapies [7].
- Proteomics involves the study of the complete set of proteins in a cell or tissue. It involves the identification, quantification, and characterization of proteins to understand their functions, interactions, and modifications. More specifically, in cancer research, proteomics can identify changes in protein expression levels, post-translational modifications, and protein–protein interactions. This information is valuable for understanding the functional consequences of genomic alterations that lead to tumor growth and metastasis. Importantly, proteomics contributed to the identification of clinical biomarkers as well as new therapeutic targets [8].
- Metabolomics analyzes small-molecule metabolites within a cell, and it can provide critical information about the state. In fact, changes in metabolic pathways are often associated with cancer, and metabolites in biological samples can help to identify new biomarkers for cancer diagnosis, monitoring, and therapy [9].
- Epigenomics investigates changes due to the intricate set of epigenetic modifications, such as DNA methylation and histone modification. A biological picture given by epigenomics can help scientists correlate epigenetic changes in cancer development and progression [10].
- Pharmacogenomics combines genomics and pharmacology to study how an individual’s genetic makeup influences the response to drugs. Especially in cancer, it helps in personalized medicine by tailoring treatments for a specific patient’s genetic profile, making therapies safer and more effective. In fact, although cancers may have specific disease-defining mutations, a patient’s genetic variation could affect drug response [11].
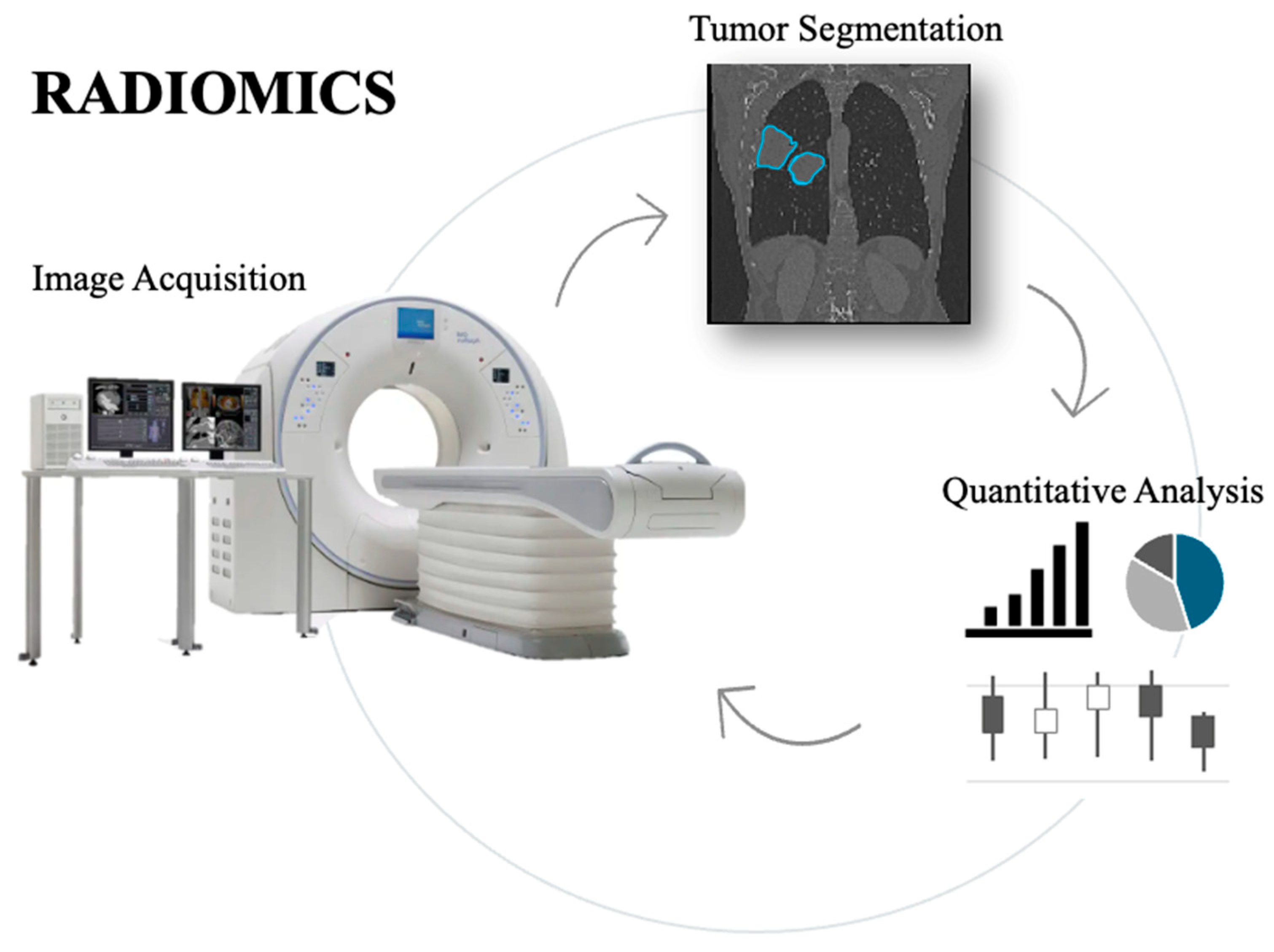
- Radiomics is the analysis of the numerical features extracted by radiological images. It converts the qualitative information from the images into numbers, which are invisible to the naked eye view. Radiomics feature extraction can be performed from all imaging modalities such as Computed Tomography (CT), Magnetic Resonance Imaging (MRI), and ultrasound (US), among others. Feature selection and radiomic analysis, instead, require a machine learning model or a formal analysis [12,13].
2. Role of Genomics in Cancer
3. Role of Metabolomics in Cancer
3.1. Metabolomics in the Discovery of Cancer Biomarkers in Lung Cancer
3.2. Metabolomics: Methodology and Instrumentation
3.3. Nuclear Magnetic Resonance (NMR) Metabolomics Applications in NSCLC
4. Role of Radiomics in Lung Cancer
- First-Order Features: These features analyze the distribution of intensities within the ROI, without considering the spatial relationship between voxels. They provide information on the variability of tumor intensity and its homogeneity. Common first-order features include:
- Mean: the average intensity of the voxels within the ROI.
- Standard Deviation: a measure of the dispersion of intensities.
- Skewness: the symmetry of the intensity distribution.
- Kurtosis: a measure of the shape of a distribution.
- Entropy: a measure of the complexity and disorder of the intensity.
- Second-Order Features (Texture Features): These features focus on the spatial relationships between voxels, describing the texture and heterogeneity of the tumor. Common techniques used to extract these features include gray-level co-occurrence matrices (GLCMs) and gray-level run-length matrices (GLRLMs). Key second-order features include:
- Contrast: a measure of the difference in intensity between adjacent voxels.
- Correlation: a measure of the correlation between the intensities of voxels.
- Energy: a measure of the uniformity of intensity distribution.
- Homogeneity: a measure of the uniformity of intensities.
- Third-Order Features (Shape Features): These features describe the geometry of the ROI, such as the shape and size of the tumor. Some examples include:
- Volume: the total volume of the region of interest.
- Sphericity: describes how close the shape of the ROI is to a sphere.
- Surface Area: the surface area of the ROI.
- Compactness: the ratio of volume to surface area, describing the regularity of the shape.
- Higher-Order Statistics: These features focus on advanced measures, such as the fractal dimension of the tumor surface, which provides information on its irregularity and geometric complexity. An example is:
- Fractal Dimension: a measure of the complexity of the tumor’s geometry, useful for characterizing more irregular tumors.
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSCLC | Non-small-cell lung cancer |
| SCLC | Small-Cell Lung Cancer |
| LC | Lung Cancer |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| US | Ultrasound |
| NGS | Next-generation Sequencing |
| CNS | Central nervous system |
| ctDNA | Circulating tumor DNA |
| FDG-PET | 18F-deoxyglucose-positron emission tomography |
| GLUT | Glucose transporter |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| HIF | Hypoxia-inducible factor |
| HK | Hexokinase |
| PDAC | Pancreatic ductal adenocarcinoma |
| TCA | Tricarboxylic acid |
| GSH | Glutathione |
| S1P | Sphingosine-1-phosphate |
| LPE | Lysophosphatidylethanolamine |
| LDCT | Low-dose computed tomography |
| NMR | Nuclear magnetic resonance |
| MS | Mass spectrometry |
| MALDI-MSI | Matrix-assisted laser desorption/ionization mass spectrometric imaging |
| MRSI | Magnetic resonance spectroscopic imaging |
| HRMAS MRS | High-resolution magic angle spinning magnetic resonance spectroscopy |
| RRLC | Rapid resolution liquid chromatography |
| TMAO | Trimethylamine N-oxide |
| MWA | Microwave ablation |
| MVDA | Multivariate data analysis |
| ADMA | Asymmetric dimethylarginine |
| HC | Healthy control |
| PLC | Primary lung cancer |
| SLC | Secondary lung cancer |
| ROI | Region of interest |
| IBSI | Imaging Biomarker Standardization Initiative |
| GLCM | Gray-level co-occurrence matrices |
| GLRLM | Gray-level run-length matrices |
References
- Kiechle, F.L.; Zhang, X.; Holland-Staley, C.A. The -omics Era and Its Impact. Arch. Pathol. Lab. Med. 2004, 128, 1337–1345. [Google Scholar] [CrossRef]
- Lay, J.O.; Liyanage, R.; Borgmann, S.; Wilkins, C.L. Problems with the “omics”. Trends Anal. Chem. 2006, 25, 1046–1056. [Google Scholar] [CrossRef]
- Subramanian, I.; Verma, S.; Kumar, S.; Jere, A.; Anamika, K. Multi-omics Data Integration, Interpretation, and Its Application. Bioinform. Biol. Insights 2020, 14, 1177932219899051. [Google Scholar] [CrossRef]
- Schneider, M.V.; Orchard, S. Omics Technologies, Data and Bioinformatics Principles. Bioinform. Omics Data Methods Protoc. 2011, 719, 3–30. [Google Scholar]
- Chakraborty, S.; Hosen, M.I.; Ahmed, M.; Shekhar, H.U. Onco-Multi-OMICS Approach: A New Frontier in Cancer Research. Biomed. Res. Int. 2018, 2018, 9836256. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.F.; Mardis, E.R. The emerging clinical relevance of genomics in cancer medicine. Nat. Rev. Clin. Oncol. 2018, 15, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Supplitt, S.; Karpinski, P.; Sasiadek, M.; Laczmanska, I. Current Achievements and Applications of Transcriptomics in Personalized Cancer Medicine. Int. J. Mol. Sci. 2021, 22, 1422. [Google Scholar] [CrossRef]
- Kwon, Y.W.; Jo, H.-S.; Bae, S.; Seo, Y.; Song, P.; Song, M.; Yoon, J.H. Application of Proteomics in Cancer: Recent Trends and Approaches for Biomarkers Discovery. Front. Med. 2021, 8, 747333. [Google Scholar] [CrossRef]
- Schmidt, D.R.; Patel, R.; Kirsch, D.G.; Lewis, C.A.; Vander Heiden, M.G.; Locasale, J.W. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J. Clin. 2021, 71, 333–358. [Google Scholar] [CrossRef]
- Sandoval, J.; Esteller, M. Cancer epigenomics: Beyond genomics. Curr. Opin. Genet. Dev. 2012, 22, 50–55. [Google Scholar] [CrossRef]
- Wheeler, H.E.; Maitland, M.L.; Dolan, M.E.; Cox, N.J.; Ratain, M.J. Cancer pharmacogenomics: Strategies and challenges. Nat. Rev. Genet. 2013, 14, 23–34. [Google Scholar]
- Brunese, M.C.; Fantozzi, M.R.; Fusco, R.; De Muzio, F.; Gabelloni, M.; Danti, G.; Borgheresi, A.; Palumbo, P.; Bruno, F.; Gandolfo, N.; et al. Update on the Applications of Radiomics in Diagnosis, Staging, and Recurrence of Intrahepatic Cholangiocarcinoma. Diagnostics 2023, 13, 1488. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Brunese, M.C.; Setola, S.V.; Ottaiano, A.; Cardone, C.; Avallone, A.; Patrone, R.; Pradella, S.; et al. Radiomics and machine learning analysis by computed tomography and magnetic resonance imaging in colorectal liver metastases prognostic assessment. Radiol. Med. 2023, 128, 1310–1332. [Google Scholar] [CrossRef]
- Leiter, A.; Veluswamy, R.R.; Wisnivesky, J.P. The global burden of lung cancer: Current status and future trends. Nat. Rev. Clin. Oncol. 2023, 20, 624–639. [Google Scholar] [CrossRef]
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2025, 156, 1336–1346. [Google Scholar] [CrossRef]
- Zheng, X.; Tian, H.; Li, W.; Li, J.; Xu, K.; Jin, C.; Pang, Y. The diagnosis value of dual-energy computed tomography (DECT) multi-parameter imaging in lung adenocarcinoma and squamous cell carcinoma. BMC Pulm. Med. 2024, 24, 545. [Google Scholar] [CrossRef]
- Goldstraw, P.; Crowley, J.; Chansky, K.; Giroux, D.J.; Groome, P.A.; Rami-Porta, R.; Postmus, P.E.; Rusch, V.; Sobin, L. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the TNM Stage Groupings in the Forthcoming (Seventh) Edition of the TNM Classification of Malignant Tumours. J. Thorac. Oncol. 2007, 2, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Brunese, L.; Mercaldo, F.; Reginelli, A.; Santone, A. Neural Networks for Lung Cancer Detection through Radiomic Features. In Proceedings of the 2019 International Joint Conference on Neural Networks (IJCNN), Budapest, Hungary, 14–19 July 2019; pp. 1–10. [Google Scholar]
- Malik, P.S.; Pathak, N.; Sharma, A.; Birla, M.; Rastogi, A.; Sharma, A.; Khurana, S.; Pushpam, D.; Gupta, I.; Rathore, A.; et al. Young Onset Lung Cancer in India: Insights Into Clinical, Demographic, and Genomic Profiles. Clin. Lung Cancer 2025, 26, 420–428.e4. [Google Scholar] [CrossRef] [PubMed]
- Siddique, F.; Shehata, M.; Ghazal, M.; Contractor, S.; El-Baz, A. Lung Cancer Subtyping: A Short Review. Cancers 2024, 16, 2643. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, Y.; Zhang, Y. Identification of prognostic signature of non–small cell lung cancer based on TCGA methylation data. Sci. Rep. 2020, 10, 8575. [Google Scholar] [CrossRef] [PubMed]
- Borczuk, A.C.; Toonkel, R.L.; Powell, C.A. Genomics of lung cancer. Proc. Am. Thorac. Soc. 2009, 6, 152–158. [Google Scholar] [CrossRef]
- Abbasian, M.H.; Ardekani, A.M.; Sobhani, N.; Roudi, R. The Role of Genomics and Proteomics in Lung Cancer Early Detection and Treatment. Cancers 2022, 14, 5144. [Google Scholar] [CrossRef]
- Toruner, G.A.; Tang, Z.; Tang, G.; Medeiros, L.J.; Hu, S. Low ALK FISH positive metastatic non-small cell lung cancer (NSCLC) patients have shorter progression-free survival after treatment with ALK inhibitors. Cancer Genet. 2020, 241, 57–60. [Google Scholar]
- Rothe, A.; Bauer, N.; Dietze, L.; Mainka, D.; Lehnert, S.; Scheffler, M. Targeted therapy for non-small cell lung cancer (NSCLC) in a real-world setting: A single practice experience. Cancer Treat. Res. Commun. 2025, 43, 100891. [Google Scholar] [PubMed]
- Farago, A.F.; Azzoli, C.G. Beyond ALK and ROS1: RET, NTRK, EGFR and BRAF gene rearrangements in non-small cell lung cancer. Transl. Lung Cancer Res. 2017, 6, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Osman, D.; Gan, G.N.; Simon, G.R.; Boumber, Y. Recent Advances in Targetable Therapeutics in Metastatic Non-Squamous NSCLC. Front. Oncol. 2016, 6, 112. [Google Scholar] [CrossRef]
- Shah, M.; Noronha, V.; Patil, V.; Singh, A.K.; Menon, N.; Goud, S.; Shah, S.; More, S.; Kapoor, A.; Mishra, B.K.; et al. Genomic Profiling of Driver Gene Alterations in Patients With Non–Small Cell Lung Cancer, Patterns of Treatment and Impact on Survival Outcomes: A Single Center Experience of More Than 1200 Patients. Clin. Lung Cancer 2025, 26, e270–e283. [Google Scholar] [CrossRef]
- Satoh, H.; Okuma, Y.; Shinno, Y.; Masuda, K.; Matsumoto, Y.; Yoshida, T.; Goto, Y.; Horinouchi, H.; Yamamoto, N.; Ohe, Y. Evolving treatments and prognosis in Stage IV non-small cell lung cancer: 20 years of progress of novel therapies. Lung Cancer 2025, 202, 108453. [Google Scholar] [CrossRef]
- Baik, C.S.; Chamberlain, M.C.; Chow, L.Q. Targeted Therapy for Brain Metastases in EGFR-Mutated and ALK-Rearranged Non-Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1268–1278. [Google Scholar] [PubMed]
- Lim, J.U.; Kim, K.Y.; Kim, H.C.; Kim, T.J.; Kim, H.K.; Moon, M.H.; Beck, K.S.; Suh, Y.G.; Song, C.H.; Ahn, J.S.; et al. Comparison of metastasis and treatment patterns among different histopathologic types of lung cancer: Analysis of 6 years of nationwide lung cancer cohort data in Korea. Transl. Lung Cancer Res. 2025, 14, 363–384. [Google Scholar] [CrossRef]
- Nardone, V.; Romeo, C.; D’Ippolito, E.; Pastina, P.; D’Apolito, M.; Pirtoli, L.; Caraglia, M.; Mutti, L.; Bianco, G.; Falzea, A.C.; et al. The role of brain radiotherapy for EGFR- and ALK-positive non-small-cell lung cancer with brain metastases: A review. Radiol. Med. 2023, 128, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Ren, J.; Wei, S.; Yang, Z.; Li, C.; Wang, Z.; Li, M.; Wei, Z.; Liu, Y.; Wang, X.; et al. Integrated analyses of multi-omic data derived from paired primary lung cancer and brain metastasis reveal the metabolic vulnerability as a novel therapeutic target. Genome Med. 2024, 16, 138. [Google Scholar] [CrossRef]
- Hockemeyer, K.G.; Rusthoven, C.G.; Pike, L.R.G. Advances in the Management of Lung Cancer Brain Metastases. Cancers 2024, 16, 3780. [Google Scholar] [CrossRef] [PubMed]
- Ottaiano, A.; Grassi, F.; Sirica, R.; Genito, E.; Ciani, G.; Patanè, V.; Monti, R.; Belfiore, M.P.; Urraro, F.; Santorsola, M.; et al. Associations between Radiomics and Genomics in Non-Small Cell Lung Cancer Utilizing Computed Tomography and Next-Generation Sequencing: An Exploratory Study. Genes 2024, 15, 803. [Google Scholar]
- Rina, A.; Maffeo, D.; Minnai, F.; Esposito, M.; Palmieri, M.; Serio, V.B.; Rosati, D.; Mari, F.; Frullanti, E.; Colombo, F. The Genetic Analysis and Clinical Therapy in Lung Cancer: Current Advances and Future Directions. Cancers 2024, 16, 2882. [Google Scholar] [CrossRef]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar]
- Dunne, E.G.; Fick, C.N.; Mastrogiacomo, B.; Tan, K.S.; Toumbacaris, N.; Vanstraelen, S.; Rocco, G.; Chaft, J.E.; Iyengar, P.; Gomez, D.; et al. Clinicopathologic and genomic features associated with brain metastasis after resection of lung adenocarcinoma. JTCVS Open 2024, 22, 458–469. [Google Scholar] [CrossRef]
- Wu, T.-M.; Liu, J.-B.; Liu, Y.; Shi, Y.; Li, W.; Wang, G.-R.; Ma, Y.-S.; Fu, D. Power and Promise of Next-Generation Sequencing in Liquid Biopsies and Cancer Control. Cancer Control. 2020, 27, 1073274820934805. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, H. Next-generation sequencing in liquid biopsy: Cancer screening and early detection. Hum. Genomics 2019, 13, 34. [Google Scholar] [PubMed]
- Kitagawa, S.; Seike, M. Liquid biopsy in lung cancer. Jap. J. Clin. Oncol. 2025, 55, 453–458. [Google Scholar] [CrossRef]
- de Jager, V.D.; Giacomini, P.; Fairley, J.A.; Toledo, R.A.; Patton, S.J.; Joosse, S.A.; Koch, C.; Deans, Z.C.; Agelaki, S.; Andersen, C.L.; et al. Reporting of molecular test results from cell-free DNA analyses: Expert consensus recommendations from the 2023 European Liquid Biopsy Society ctDNA Workshop. eBioMedicine 2025, 114, 105636. [Google Scholar] [CrossRef]
- Lin, C.; Liu, X.; Zheng, B.; Ke, R.; Tzeng, C.-M. Liquid Biopsy, ctDNA Diagnosis through NGS. Life 2021, 11, 890. [Google Scholar] [CrossRef]
- Bontoux, C.; Benzaquen, J.; Taly, V.; Baurès, A.; Allegra, M.; Lacoux, C.; Tanga, V.; Rignol, G.; Berthet, J.-P.; Marquette, C.-H.; et al. Preoperative liquid biopsy for EGFR-mutated detection at diagnostic in early-stage non-small cell lung cancer: Real-world experience of a single centre. J. Clin. Pathol. 2025, 78, 578–580. [Google Scholar]
- Aguilar, H.; López-Roldán, B.; Vilalta-Lacarra, A.; Alkorta-Aranburu, G.; Claramunt, R.; López-Guerrero, J.A.; Sandiego, S.; Gil-Bazo, I. Liquid biopsy for monitoring minimal residual disease in localized and locally-advanced non-small cell lung cancer after radical-intent treatment. J. Liq. Biopsy 2024, 4, 100145. [Google Scholar]
- Brunese, L.; Greco, B.; Setola, F.R.; Lassandro, F.; Guarracino, M.R.; De Rimini, M.; Piccolo, S.; De Rosa, N.; Muto, R.; Bianco, A.; et al. Non-small cell lung cancer evaluated with quantitative contrast-enhanced CT and PET-CT: Net enhancement and standardized uptake values are related to tumour size and histology. Med. Sci. Monit. 2013, 19, 95–101. [Google Scholar] [CrossRef]
- Hallermayr, A.; Keßler, T.; Fujera, M.; Liesfeld, B.; Bernstein, S.; von Ameln, S.; Schanze, D.; Steinke-Lange, V.; Pickl, J.M.A.; Neuhann, T.M.; et al. Impact of cfDNA Reference Materials on Clinical Performance of Liquid Biopsy NGS Assays. Cancers 2023, 15, 5024. [Google Scholar] [CrossRef] [PubMed]
- Montella, M.; Ciani, G.; Granata, V.; Fusco, R.; Grassi, F.; Ronchi, A.; Cozzolino, I.; Franco, R.; Marino, F.Z.; Urraro, F.; et al. Preliminary Experience of Liquid Biopsy in Lung Cancer Compared to Conventional Assessment: Light and Shadows. J. Pers. Med. 2022, 12, 1896. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Park, U.H.; Goh, C.J.; Park, D.; Lim, Y.G.; Lee, I.K.; Do, W.J.; Lee, K.J.; Kim, H.; Yun, S.Y.; et al. Enhancing Lung Cancer Classification through Integration of Liquid Biopsy Multi-Omics Data with Machine Learning Techniques. Cancers 2023, 15, 4556. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Pradelli, L.; Sicari, E.; Castellani, S.; Sivakumar, S.; Sokol, E.; Montesion, M.; Wieland, T.; Rambichler, J.; Minari, R.; et al. Liquid biopsy comprehensive genomic profiling of lung cancer in the Italian population: A real-world experience. Lung Cancer 2023, 185, 107359. [Google Scholar] [CrossRef]
- Sinkala, M.; Mulder, N.; Patrick Martin, D. Metabolic gene alterations impact the clinical aggressiveness and drug responses of 32 human cancers. Commun. Biol. 2019, 2, 414. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Locasale, J.W. Metabolomics: A Primer. Trends Biochem. Sci. 2017, 42, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2017, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Mandal, R.; Stanislaus, A.; Ramirez-Gaona, M. Cancer Metabolomics and the Human Metabolome Database. Metabolites 2016, 6, 10. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; DeBerardinis, R.J. Understanding the Intersections between Metabolism and Cancer Biology. Cell 2017, 168, 657–669. [Google Scholar] [CrossRef]
- Reid, M.A.; Dai, Z.; Locasale, J.W. The impact of cellular metabolism on chromatin dynamics and epigenetics. Nat. Cell Biol. 2017, 19, 1298–1306. [Google Scholar] [CrossRef]
- Gika, H.; Virgiliou, C.; Theodoridis, G.; Plumb, R.S.; Wilson, I.D. Untargeted LC/MS-based metabolic phenotyping (metabonomics/metabolomics): The state of the art. J. Chromatogr. B 2019, 1117, 136–147. [Google Scholar]
- Luengo, A.; Gui, D.Y.; Vander Heiden, M.G. Targeting Metabolism for Cancer Therapy. Cell Chem. Biol. 2017, 24, 1161–1180. [Google Scholar] [CrossRef]
- Luengo, A.; Li, Z.; Gui, D.Y.; Sullivan, L.B.; Zagorulya, M.; Do, B.T.; Ferreira, R.; Naamati, A.; Ali, A.; Lewis, C.A.; et al. Increased demand for NAD+ relative to ATP drives aerobic glycolysis. Mol. Cell 2021, 81, 691–707.e6. [Google Scholar] [CrossRef]
- Kreuzaler, P.; Panina, Y.; Segal, J.; Yuneva, M. Adapt and conquer: Metabolic flexibility in cancer growth, invasion and evasion. Mol. Metab. 2020, 33, 83–101. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Elstrom, R.L.; Bauer, D.E.; Buzzai, M.; Karnauskas, R.; Harris, M.H.; Plas, D.R.; Zhuang, H.; Cinalli, R.M.; Alavi, A.; Rudin, C.M.; et al. Akt Stimulates Aerobic Glycolysis in Cancer Cells. Cancer Res. 2004, 64, 3892–3899. [Google Scholar] [CrossRef] [PubMed]
- Baysal, B.E.; Ferrell, R.E.; Willett-Brozick, J.E.; Lawrence, E.C.; Myssiorek, D.; Bosch, A.; Mey, A.v.d.; Taschner, P.E.M.; Rubinstein, W.S.; Myers, E.N.; et al. Mutations in SDHD, a Mitochondrial Complex II Gene, in Hereditary Paraganglioma. Science 2000, 287, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, I.P.M.; Alam, N.A.; Rowan, A.J.; Barclay, E.; Jaeger, E.E.M.; Kelsell, D.; Leigh, I.; Gorman, P.; Lamlum, H.; Rahman, S.; et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 2002, 30, 406–410. [Google Scholar] [CrossRef]
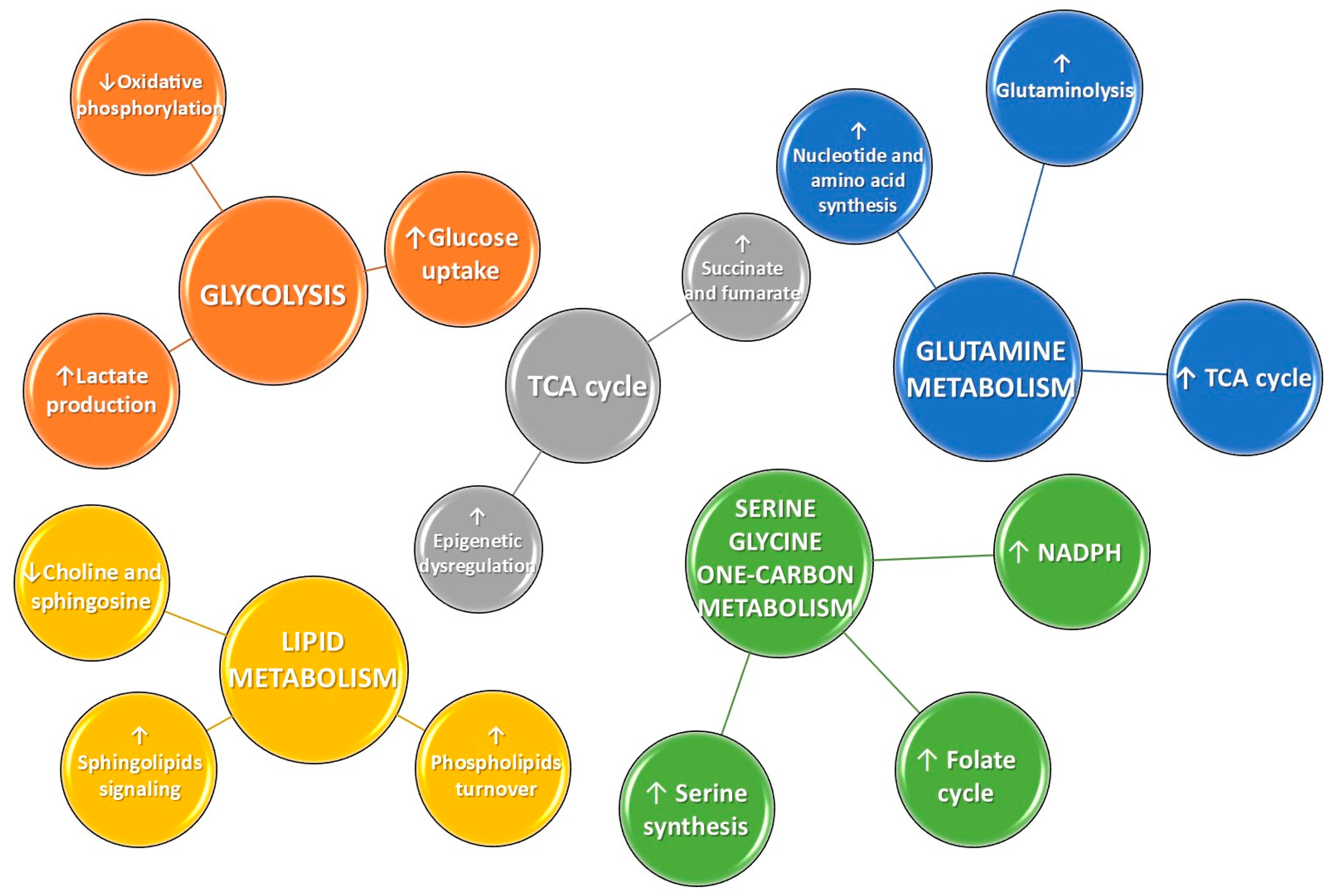
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- De Vitto, H.; Pérez-Valencia, J.; Radosevich, J.A. Glutamine at focus: Versatile roles in cancer. Tumor Biol. 2016, 37, 1541–1558. [Google Scholar] [CrossRef]
- Antonov, A.; Agostini, M.; Morello, M.; Minieri, M.; Melino, G.; Amelio, I. Bioinformatics analysis of the serine and glycine pathway in cancer cells. Oncotarget 2014, 5, 11004–11013. [Google Scholar] [CrossRef] [PubMed]
- Puchades-Carrasco, L.; Jantus-Lewintre, E.; Pérez-Rambla, C.; García-García, F.; Lucas, R.; Calabuig, S.; Blasco, A.; Dopazo, J.; Camps, C.; Pineda-Lucena, A. Serum metabolomic profiling facilitates the non-invasive identification of metabolic biomarkers associated with the onset and progression of non-small cell lung cancer. Oncotarget 2016, 7, 12904–12916. [Google Scholar] [CrossRef]
- Cantor, J.R.; Sabatini, D.M. Cancer Cell Metabolism: One Hallmark, Many Faces. Cancer Discov. 2012, 2, 881–898. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- Davidson, S.M.; Papagiannakopoulos, T.; Olenchock, B.A.; Heyman, J.E.; Keibler, M.A.; Luengo, A.; Bauer, M.R.; Jha, A.K.; O’Brien, J.P.; Pierce, K.A.; et al. Environment Impacts the Metabolic Dependencies of Ras-Driven Non-Small Cell Lung Cancer. Cell Metab. 2016, 23, 517–528. [Google Scholar] [CrossRef]
- de la Cruz-López, K.G.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front. Oncol. 2019, 9, 1143. [Google Scholar] [CrossRef]
- Wittig, R.; Coy, J.F. The role of glucose metabolism and glucose-associated signalling in cancer. Perspect. Medicin. Chem. 2008, 1, 64–82. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Peiris-Pagés, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Erratum: Cancer metabolism: A therapeutic perspective. Nat. Rev. Clin. Oncol. 2017, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Capello, M.; Fredolini, C.; Racanicchi, L.; Piemonti, L.; Liotta, L.A.; Novelli, F.; Petricoin, E.F. Proteomic Analysis Reveals Warburg Effect and Anomalous Metabolism of Glutamine in Pancreatic Cancer Cells. J. Proteome Res. 2012, 11, 554–563. [Google Scholar] [CrossRef]
- Ward, P.S.; Thompson, C.B. Signaling in control of cell growth and metabolism. Cold Spring Harb. Perspect. Biol. 2012, 4, a006783. [Google Scholar] [CrossRef] [PubMed]
- Berker, Y.; Vandergrift, L.A.; Wagner, I.; Su, L.; Kurth, J.; Schuler, A.; Dinges, S.S.; Habbel, P.; Nowak, J.; Mark, E.; et al. Magnetic Resonance Spectroscopy-based Metabolomic Biomarkers for Typing, Staging, and Survival Estimation of Early-Stage Human Lung Cancer. Sci. Rep. 2019, 9, 10319. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Wang, C.; Zhang, H.; Cai, Z. Non-targeted and targeted metabolomics approaches to diagnosing lung cancer and predicting patient prognosis. Oncotarget 2016, 7, 63437–63448. [Google Scholar] [CrossRef]
- Fahrmann, J.F.; Grapov, D.; Wanichthanarak, K.; DeFelice, B.C.; Salemi, M.R.; Rom, W.N.; Gandara, D.R.; Phinney, B.S.; Fiehn, O.; Pass, H.; et al. Integrated metabolomics and proteomics highlight altered nicotinamide and polyamine pathways in lung adenocarcinoma. Carcinogenesis 2017, 38, 271–280. [Google Scholar] [CrossRef]
- Zhang, W.C.; Shyh-Chang, N.; Yang, H.; Rai, A.; Umashankar, S.; Ma, S.; Soh, B.S.; Sun, L.L.; Tai, B.C.; Nga, M.E.; et al. Glycine Decarboxylase Activity Drives Non-Small Cell Lung Cancer Tumor-Initiating Cells and Tumorigenesis. Cell 2012, 148, 259–272. [Google Scholar] [CrossRef]
- Long, F.; Su, J.-H.; Liang, B.; Su, L.-L.; Jiang, S.-J. Identification of Gene Biomarkers for Distinguishing Small-Cell Lung Cancer from Non-Small-Cell Lung Cancer Using a Network-Based Approach. Biomed. Res. Int. 2015, 2015, 685303. [Google Scholar] [CrossRef]
- Fan, J.; Ye, J.; Kamphorst, J.J.; Shlomi, T.; Thompson, C.B.; Rabinowitz, J.D. Correction: Corrigendum: Quantitative flux analysis reveals folate-dependent NADPH production. Nature 2014, 513, 574. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, C.; Cao, Y.; Zhao, M.; Wang, K. The role of serine metabolism in lung cancer: From oncogenesis to tumor treatment. Front. Genet. 2023, 13, 1084609. [Google Scholar] [CrossRef]
- Huang, C.; Freter, C. Lipid Metabolism, Apoptosis and Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 924–949. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Z.; Li, A.; Li, H.; Wang, B.; Zhong, J.; Min, L.; Dai, L. Metabolomic profiling of human serum in lung cancer patients using liquid chromatography/hybrid quadrupole time-of-flight mass spectrometry and gas chromatography/mass spectrometry. J. Cancer Res. Clin. Oncol. 2015, 141, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Alberg, A.J.; Armeson, K.; Pierce, J.S.; Bielawski, J.; Bielawska, A.; Visvanathan, K.; Hill, E.G.; Ogretmen, B. Plasma Sphingolipids and Lung Cancer: A Population-Based, Nested Case–Control Study. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 1374–1382. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, H.; Ai, J.; Zhu, Y.; Li, Y.; Borgia, J.A.; Yang, J.S.; Zhang, J.; Jiang, B.; Gu, W.; et al. Global lipidomics identified plasma lipids as novel biomarkers for early detection of lung cancer. Oncotarget 2017, 8, 107899–107906. [Google Scholar] [CrossRef]
- Wood, D.E.; Kazerooni, E.A.; Baum, S.L.; Eapen, G.A.; Ettinger, D.S.; Hou, L.; Jackman, D.M.; Klippenstein, D.; Kumar, R.; Lackner, R.P.; et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 412–441. [Google Scholar] [CrossRef]
- Jonas, D.E.; Reuland, D.S.; Reddy, S.M.; Nagle, M.; Clark, S.D.; Weber, R.P.; Enyioha, C.; Malo, T.L.; Brenner, A.T.; Armstrong, C.; et al. Screening for Lung Cancer with Low-Dose Computed Tomography: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2021, 325, 971–987. [Google Scholar] [PubMed]
- US Preventive Services Task Force. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 962–970. [Google Scholar] [CrossRef]
- Bach, P.B.; Mirkin, J.N.; Oliver, T.K.; Azzoli, C.G.; Berry, D.A.; Brawley, O.W.; Byers, T.; Colditz, G.A.; Gould, M.K.; Jett, J.R.; et al. Benefits and Harms of CT Screening for Lung Cancer: A Systematic Review. JAMA 2012, 307, 2418–2429. [Google Scholar] [CrossRef]
- Spicer, R.; Salek, R.M.; Moreno, P.; Cañueto, D.; Steinbeck, C. Navigating freely-available software tools for metabolomics analysis. Metabolomics 2017, 13, 106. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.D.; Souza, A.L.; Gerszten, R.E.; Clish, C.B. Targeted Metabolomics. Curr. Protoc. Mol. Biol. 2012, 98, 30.2.1–30.2.24. [Google Scholar] [CrossRef] [PubMed]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies—Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef]
- Bogner, W.; Otazo, R.; Henning, A. Accelerated MR spectroscopic imaging—A review of current and emerging techniques. NMR Biomed. 2021, 34, e4314. [Google Scholar] [CrossRef]
- Posse, S.; Otazo, R.; Dager, S.R.; Alger, J. MR spectroscopic imaging: Principles and recent advances. J. Magn. Reson. Imaging 2013, 37, 1301–1325. [Google Scholar] [CrossRef]
- Ščupáková, K.; Balluff, B.; Tressler, C.; Adelaja, T.; Heeren, R.M.A.; Glunde, K.; Ertaylan, G. Cellular resolution in clinical MALDI mass spectrometry imaging: The latest advancements and current challenges. Clin. Chem. Lab. Med. 2020, 58, 914–929. [Google Scholar] [CrossRef]
- Schulz, S.; Becker, M.; Groseclose, M.R.; Schadt, S.; Hopf, C. Advanced MALDI mass spectrometry imaging in pharmaceutical research and drug development. Curr. Opin. Biotechnol. 2019, 55, 51–59. [Google Scholar] [CrossRef]
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR Spectroscopy for Metabolomics Research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Crook, A.A.; Powers, R. Quantitative NMR-Based Biomedical Metabolomics: Current Status and Applications. Molecules 2020, 25, 5128. [Google Scholar] [CrossRef]
- Sengupta, A.; Weljie, A.M. NMR Spectroscopy–Based Metabolic Profiling of Biospecimens. Curr. Protoc. Protein Sci. 2019, 98, e98. [Google Scholar] [CrossRef] [PubMed]
- Siegal, G.; Selenko, P. Cells, drugs and NMR. J. Magn. Reson. 2019, 306, 202–212. [Google Scholar] [CrossRef]
- Kaebisch, E.; Fuss, T.L.; Vandergrift, L.A.; Toews, K.; Habbel, P.; Cheng, L.L. Applications of high-resolution magic angle spinning MRS in biomedical studies I-cell line and animal models. NMR Biomed. 2017, 30, e3700. [Google Scholar] [CrossRef]
- Hu, J.-M.; Sun, H.-T. Serum proton NMR metabolomics analysis of human lung cancer following microwave ablation. Radiat. Oncol. 2018, 13, 40. [Google Scholar] [CrossRef]
- Schult, T.A.; Lauer, M.J.; Berker, Y.; Cardoso, M.R.; Vandergrift, L.A.; Habbel, P.; Nowak, J.; Taupitz, M.; Aryee, M.; Mino-Kenudson, M.A.; et al. Screening human lung cancer with predictive models of serum magnetic resonance spectroscopy metabolomics. Proc. Natl. Acad. Sci. USA 2021, 118, e2110633118. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, S.; Wang, G.; Chen, F.; Bai, C. Staging research of human lung cancer tissues by high-resolution magic angle spinning proton nuclear magnetic resonance spectroscopy (HRMAS 1H NMR) and multivariate data analysis. Asia Pac. J. Clin. Oncol. 2017, 13, e232–e238. [Google Scholar] [CrossRef]
- Sarlinova, M.; Baranovicova, E.; Skalicanova, M.; Dzian, A.; Petras, M.; Lehotsky, J.; Kalenska, D.; Racay, P.; Matakova, T.; Halasova, E. Metabolomic profiling of blood plasma of patients with lung cancer and malignant tumors with metastasis in the lungs showed similar features and promising statistical discrimination against controls. Neoplasma 2021, 68, 852–860. [Google Scholar] [CrossRef]
- Ahmed, N.; Kidane, B.; Wang, L.; Nugent, Z.; Moldovan, N.; McElrea, A.; Shariati-Ievari, S.; Qing, G.; Tan, L.; Buduhan, G.; et al. Metabolic Changes in Early-Stage Non–Small Cell Lung Cancer Patients after Surgical Resection. Cancers 2021, 13, 3012. [Google Scholar] [CrossRef] [PubMed]
- Seow, W.J.; Shu, X.-O.; Nicholson, J.K.; Holmes, E.; Walker, D.I.; Hu, W.; Cai, Q.; Gao, Y.-T.; Xiang, Y.-B.; Moore, S.C.; et al. Association of Untargeted Urinary Metabolomics and Lung Cancer Risk Among Never-Smoking Women in China. JAMA Netw. Open. 2019, 2, e1911970. [Google Scholar] [CrossRef]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-omics approaches to disease. Genome Biol. 2017, 18, 83. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488. [Google Scholar] [CrossRef]
- Shur, J.D.; Doran, S.J.; Kumar, S.; ap Dafydd, D.; Downey, K.; O’Connor, J.P.B.; Papanikolaou, N.; Messiou, C.; Koh, D.-M.; Orton, M.R. Radiomics in Oncology: A Practical Guide. Radiographics 2021, 41, 1717–1732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-P.; Zhang, X.-Y.; Cheng, Y.-T.; Li, B.; Teng, X.-Z.; Zhang, J.; Lam, S.; Zhou, T.; Ma, Z.-R.; Sheng, J.-B.; et al. Artificial intelligence-driven radiomics study in cancer: The role of feature engineering and modeling. Mil. Med. Res. 2023, 10, 22. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.; Jin, Q. Radiomics and Its Feature Selection: A Review. Symmetry 2023, 15, 1834. [Google Scholar] [CrossRef]
- Nardone, V.; Reginelli, A.; Grassi, R.; Boldrini, L.; Vacca, G.; D’Ippolito, E.; Annunziata, S.; Farchione, A.; Belfiore, M.P.; Desideri, I.; et al. Delta radiomics: A systematic review. Radiol. Med. 2021, 126, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, M.P.; Sansone, M.; Monti, R.; Marrone, S.; Fusco, R.; Nardone, V.; Grassi, R.; Reginelli, A. Robustness of Radiomics in Pre-Surgical Computer Tomography of Non-Small-Cell Lung Cancer. J. Pers. Med. 2022, 83, 132023. [Google Scholar] [CrossRef]
- Reginelli, A.; Nardone, V.; Giacobbe, G.; Belfiore, M.P.; Grassi, R.; Schettino, F.; Del Canto, M.; Grassi, R.; Cappabianca, S. Radiomics as a New Frontier of Imaging for Cancer Prognosis: A Narrative Review. Diagnostics 2021, 11, 1796. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Copley, S.J.; Viola, P.; Lu, H.; Aboagye, E.O. Radiomics and artificial intelligence for precision medicine in lung cancer treatment. Semin. Cancer Biol. 2023, 93, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhao, S.; Li, Y.; Heibi, Y.; Wu, H.; Jiang, Y. Development and validation of a machine learning-based nomogram for predicting prognosis in lung cancer patients with malignant pleural effusion. Sci. Rep. 2025, 15, 9714. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Zhou, G.; Tian, X.; Guo, F.; Zhang, L.; Zhang, Z. Identifying Lipid Metabolism-Related Therapeutic Targets and Diagnostic Markers for Lung Adenocarcinoma by Mendelian Randomization and Machine Learning Analysis. Thorac. Cancer 2025, 16, e70020. [Google Scholar]
- Xie, Y.; Li, X.; Yang, S.; Jia, F.; Han, Y.; Huang, M.; Chen, L.; Zou, W.; Deng, C.; Liang, Z. Radiomics models using machine learning algorithms to differentiate the primary focus of brain metastasis. Transl. Cancer Res. 2025, 14, 731–742. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, H.; Lin, J.; Wang, J. Review of the application of dual-energy CT combined with radiomics in the diagnosis and analysis of lung cancer. J. Appl. Clin. Med. Phys. 2025, 26, e70020. [Google Scholar] [CrossRef]
- Wang, X.-y.; Wu, S.-h.; Ren, J.; Zeng, Y.; Guo, L.-l. Predicting Gene Comutation of EGFR and TP53 by Radiomics and Deep Learning in Patients With Lung Adenocarcinomas. J. Thorac. Imaging 2025, 40, e0817. [Google Scholar] [CrossRef]
- Kirienko, M.; Sollini, M.; Corbetta, M.; Voulaz, E.; Gozzi, N.; Interlenghi, M.; Gallivanone, F.; Castiglioni, I.; Asselta, R.; Duga, S.; et al. Radiomics and gene expression profile to characterise the disease and predict outcome in patients with lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3643–3655. [Google Scholar] [CrossRef]
- Zhao, M.; Xue, G.; He, B.; Deng, J.; Wang, T.; Zhong, Y.; Li, S.; Wang, Y.; He, Y.; Chen, T.; et al. Integrated multiomics signatures to optimize the accurate diagnosis of lung cancer. Nat. Commun. 2025, 16, 84. [Google Scholar] [CrossRef]
- Ryan, J.; Kavanagh, J.; Coleman, N. Oncogene-driven lung cancer in the era of radiogenomics: Current evidence and future developments. Discov. Oncol. 2025, 16, 1585. [Google Scholar] [CrossRef] [PubMed]
- Boubnovski Martell, M.; Linton-Reid, K.; Chen, M.; Aboagye, E.O. Radiomics for lung cancer diagnosis, management, and future prospects. Clin. Radiol. 2025, 86, 106926. [Google Scholar] [CrossRef] [PubMed]

| Metabolite | Alteration in Cancer | Role/Significance |
|---|---|---|
| Glucose | Decreased serum levels due to high cellular uptake and consumption |
|
| Lactate | Increased intracellular levels due to enhanced glucose metabolism |
|
| Glutamine | Decreased intracellular levels due to enhanced glutaminolysis |
|
| Glutamate | Increased intracellular levels due to enhanced glutaminolysis |
|
| Cysteine | Increased intracellular level due to high cellular uptake and biosynthesis |
|
| Histidine | Decreased intracellular levels due to enhanced utilization |
|
| Threonine | Decreased intracellular levels due to enhanced utilization |
|
| Serine | Decreased intracellular levels due to enhanced utilization |
|
| Choline | Increased intracellular levels due to enhanced uptake |
|
| Sphingosine | Decreased intracellular levels due to enhanced utilization |
|
| Sphingosine-1-phosphate (S1P) | Increased intracellular levels due to enhanced sphingosine conversion |
|
| LDL/VLDL | Increased intracellular levels due to enhanced cellular uptake |
|
| Patient Groups | Sample Types | Results Compared to Healthy Controls | Reference |
|---|---|---|---|
| NSCLC; HC | Serum | (+) Lactate, leucine/isoleucine, N-acetyl-cysteine, glutamate, creatine, acetate, glicerol (−) HDL, LDL, VLDL, choline, glucose, glutamine, threonine, histidine, adipic acid | [71] |
| 93 NSCLC; 29 HC | Serum, tissue | (+) Lactate, glutamate (−) Glycerophosphocholine | [80] |
| 25 LC; 25 HC | Serum | (+) β-hydroxybutyrate, acetoacetate, lactate, glutamine, glutamate, asparagine, aspartate, histidine, tyrosine, isoleucine, leucine, cysteine (−) Glucose, LDL, VLDL, unsaturated lipids, glycerophosphocholine, phosphocholine, choline, trimethylamine N-oxide, betaine, methionine, tryptophan | [81] |
| 39 NSCLC; 43 HC | Serum | (+) Lactate, proline, tyrosine, phenylalanine, alanine, tryptophan, glutamate, glycoprotein (−) Glucose, taurine, glutamine, glycine, threonine, phosphocreatine | [108] |
| 79 NSCLC; 79 HC | Serum | (+) ADP, AMP, lactate, fructose-6-phosphate, diphospho-glycerate, 3-phosphoglycerate, tryptophan, succinate (−) ATP, GTP, NADP, 1,7-Dimethyl-xanthine, carnosine, carnitine, taurine, tyrosine | [109] |
| 32 LC | Tissue | (+) Lipids, aspartate, glycerophosphocholine, phosphocholine | [110] |
| 132 PLC; 47 SLC; 77 HC | Plasma | (+) Glucose, acetate, citrate, creatinine, 3-hydroxybutyrate, proline (−) Lactate, pyruvate, succinate, tyrosine, alanine, tryptophan, threonine, lipoproteins | [111] |
| 29 NSCLC urine; 32 NSCLC serum (sampling before and after surgery) | Urine, serum | (+) N6-methyladenosine (−) Leucyl proline, isopentenyladenine, fumaric acid, ADMA | [112] |
| 275 LC; 278 HC | Urine | (−) 5-methyl-2-furoic acid | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierri, M.; Ciani, G.; Brunese, M.C.; Lauro, G.; Terracciano, S.; Iorizzi, M.; Nardone, V.; Chini, M.G.; Bifulco, G.; Cappabianca, S.; et al. Advancing Non-Small-Cell Lung Cancer Management Through Multi-Omics Integration: Insights from Genomics, Metabolomics, and Radiomics. Diagnostics 2025, 15, 2586. https://doi.org/10.3390/diagnostics15202586
Pierri M, Ciani G, Brunese MC, Lauro G, Terracciano S, Iorizzi M, Nardone V, Chini MG, Bifulco G, Cappabianca S, et al. Advancing Non-Small-Cell Lung Cancer Management Through Multi-Omics Integration: Insights from Genomics, Metabolomics, and Radiomics. Diagnostics. 2025; 15(20):2586. https://doi.org/10.3390/diagnostics15202586
Chicago/Turabian StylePierri, Martina, Giovanni Ciani, Maria Chiara Brunese, Gianluigi Lauro, Stefania Terracciano, Maria Iorizzi, Valerio Nardone, Maria Giovanna Chini, Giuseppe Bifulco, Salvatore Cappabianca, and et al. 2025. "Advancing Non-Small-Cell Lung Cancer Management Through Multi-Omics Integration: Insights from Genomics, Metabolomics, and Radiomics" Diagnostics 15, no. 20: 2586. https://doi.org/10.3390/diagnostics15202586
APA StylePierri, M., Ciani, G., Brunese, M. C., Lauro, G., Terracciano, S., Iorizzi, M., Nardone, V., Chini, M. G., Bifulco, G., Cappabianca, S., & Reginelli, A. (2025). Advancing Non-Small-Cell Lung Cancer Management Through Multi-Omics Integration: Insights from Genomics, Metabolomics, and Radiomics. Diagnostics, 15(20), 2586. https://doi.org/10.3390/diagnostics15202586









