Skeletal Muscle 31P Magnetic Resonance Spectroscopy Study of Patients with Parkinson’s Disease: Energy Metabolism and Exercise Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. MRS Protocol
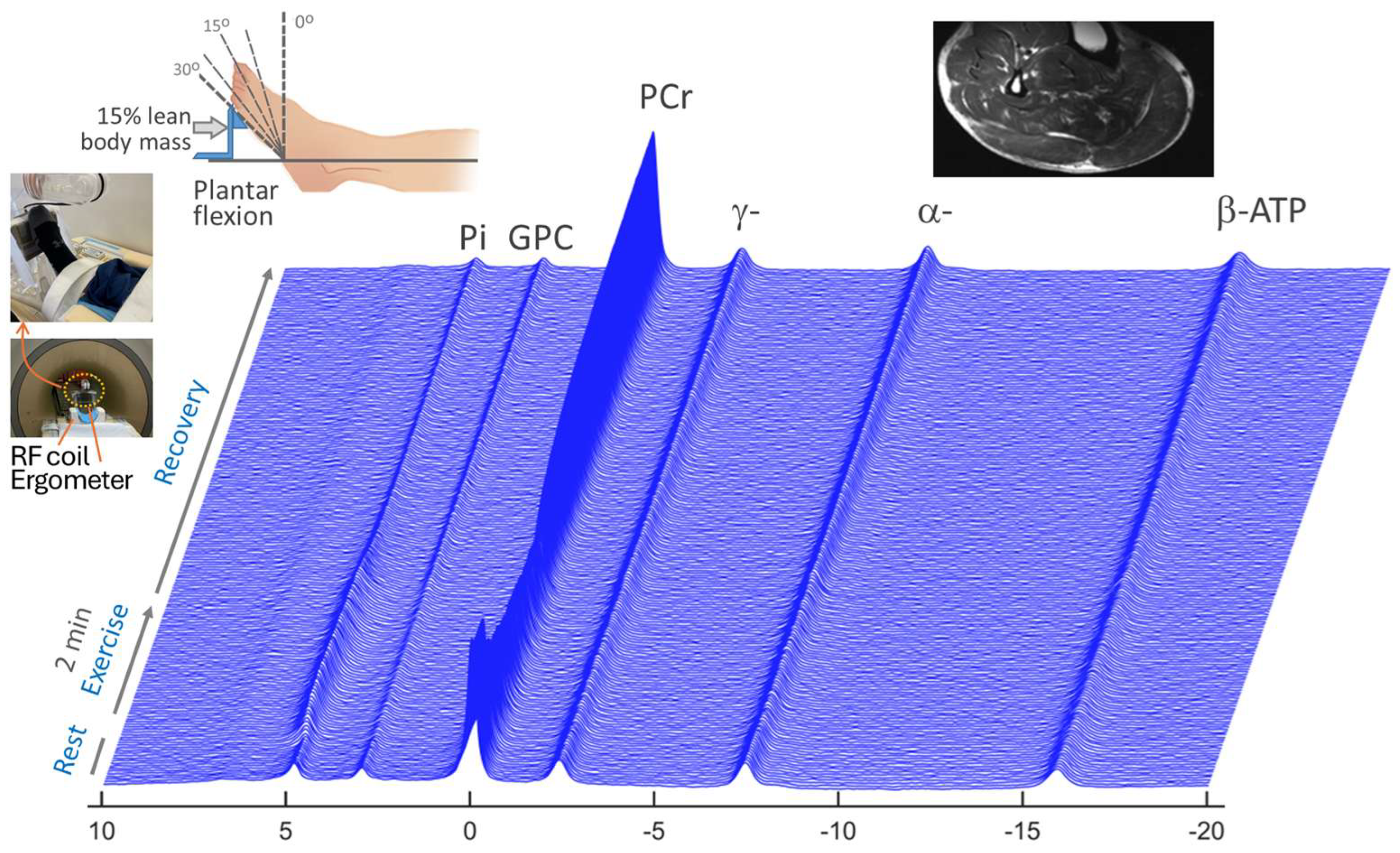
2.2.1. MRI Scanner, RF Coil, Exercise Device, and Subject Positioning
2.2.2. Dynamic 31P MRS with Plantar Flexion
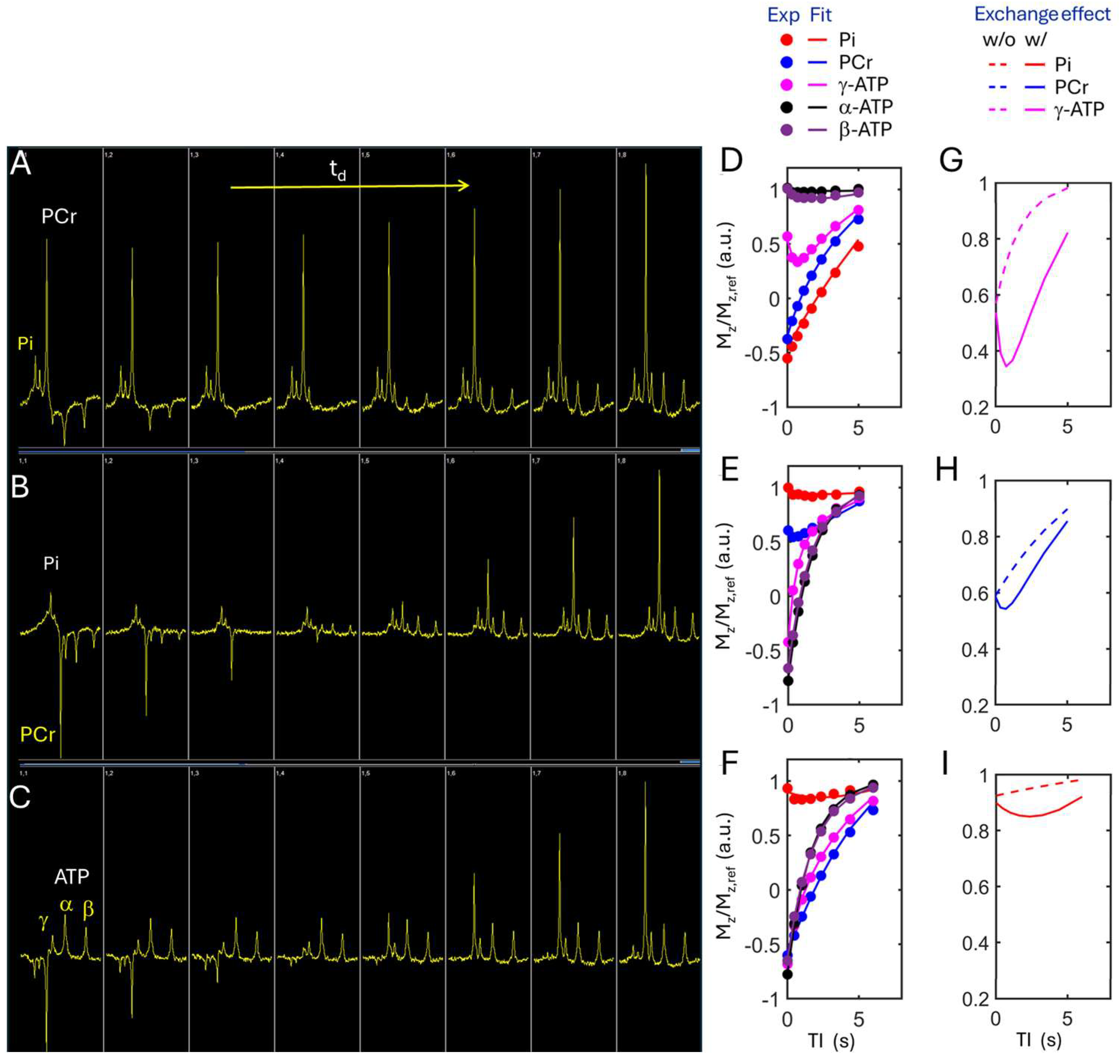
2.2.3. Kinetic 31P MRS at Resting State
2.3. 31P MRS Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Dynamic 31P MRS
3.2. Kinetic 31P MRS
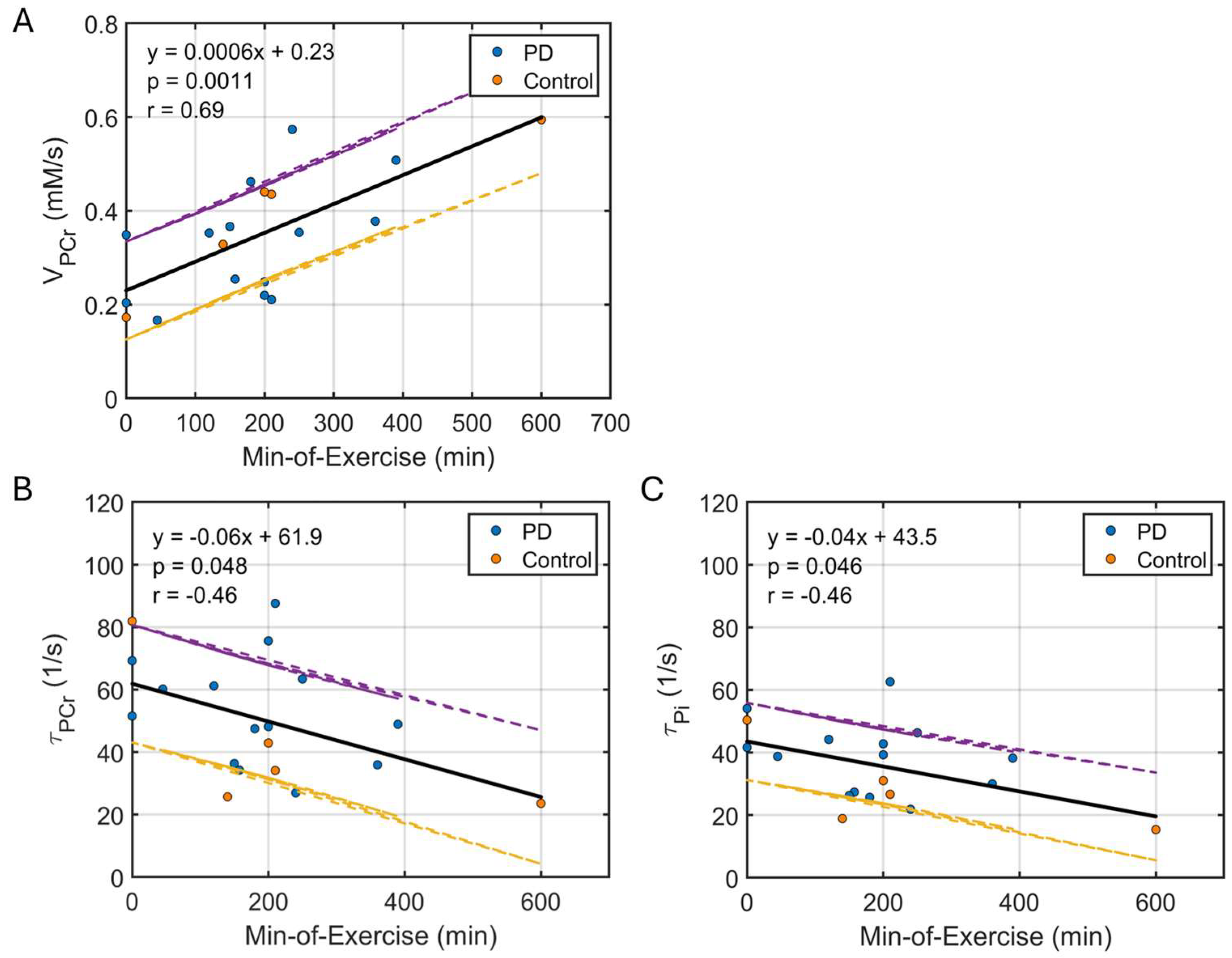
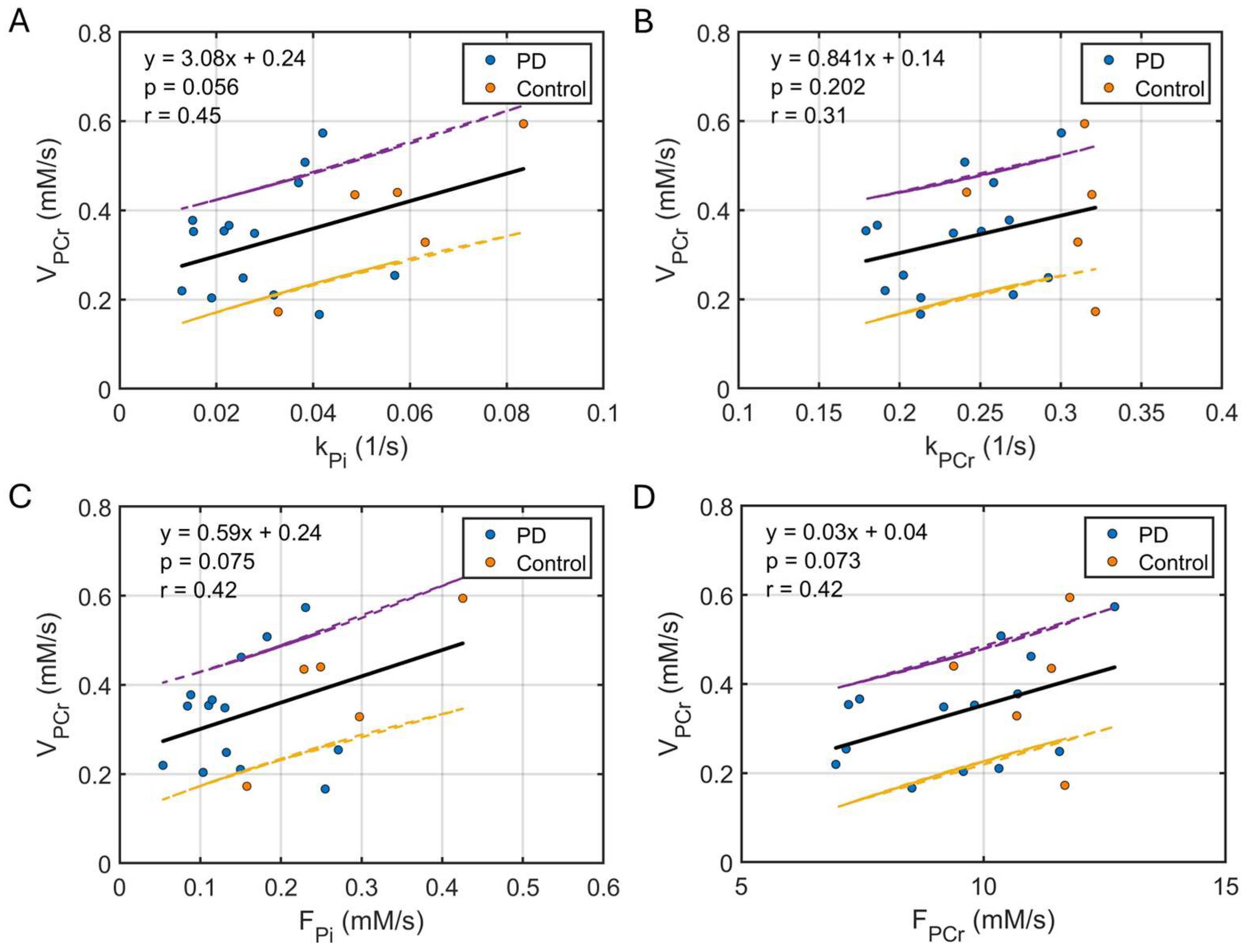
3.3. Correlation Between Kinetic and Dynamic Measurements
4. Discussion
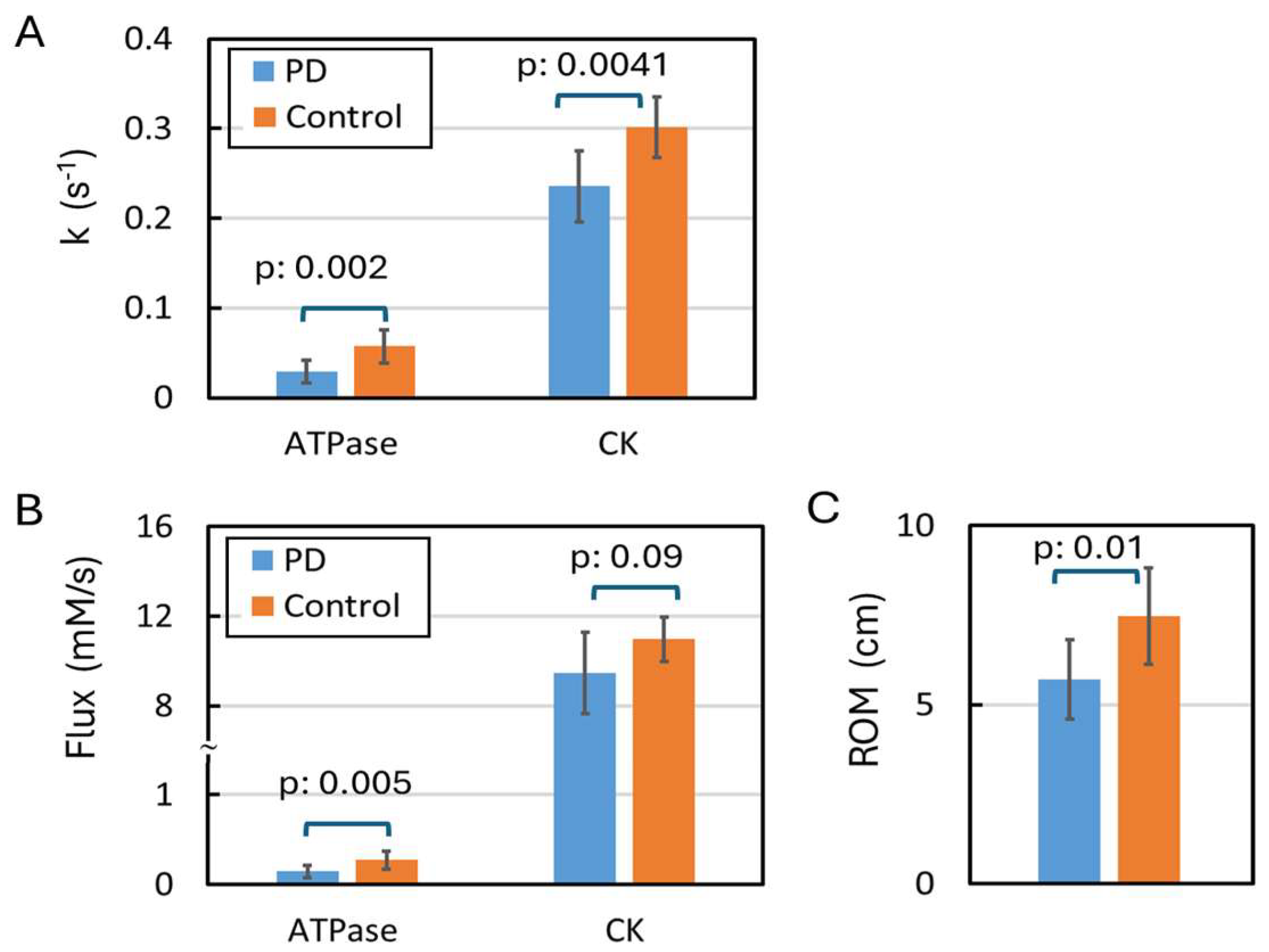
4.1. Declined ATP Kinetic Activities in Resting Muscle
4.2. Reduced Range of Motion (ROM)
4.3. Increased PCr Reserve
4.4. ATP Synthesis in Resting Versus Post-Exercise Muscle
4.5. Limitations
4.6. Other Remarks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| PwP | People with PD |
| QoL | Quality of Life |
| ATP | Adenosine triphosphate |
| ADP | Adenosine diphosphate |
| ROM | Range of motion |
| Cr | Creatine |
| PCr | Phosphocreatine |
| Pi | Inorganic phosphate |
| GPC | Glycerophosphocholine |
| MT | Magnetization Transfer |
| EBIT | Exchange-kinetics by Band Inversion Transfer |
| mEBIT | Modular EBIT |
| MRS | Magnetic Resonance Spectroscopy |
| ISIS | Image-Selected In vivo Spectroscopy |
| sLASER | Semi-Adiabatic Localization by Adiabatic Selective Refocusing |
| DRESS | Depth-Resolved Surface-Coil Spectroscopy |
References
- Rossi, A.; Berger, K.; Chen, H.; Leslie, D.; Mailman, R.B.; Huang, X. Projection of the prevalence of Parkinson’s disease in the coming decades: Revisited. Mov. Disord. 2018, 33, 156–159. [Google Scholar] [CrossRef]
- Pääsuke, M.; Ereline, J.; Gapeyeva, H.; Joost, K.; Mõttus, K.; Taba, P. Leg-extension strength and chair-rise performance in elderly women with Parkinson’s disease. J. Aging Phys. Act. 2004, 12, 511–524. [Google Scholar] [CrossRef]
- Robinson, K.; Dennison, A.; Roalf, D.; Noorigian, J.; Cianci, H.; Bunting-Perry, L.; Moberg, P.; Kleiner-Fisman, G.; Martine, R.; Duda, J.; et al. Falling risk factors in Parkinson’s disease. NeuroRehabilitation 2005, 20, 169–182. [Google Scholar] [CrossRef]
- Latt, M.D.; Lord, S.R.; Morris, J.G.; Fung, V.S. Clinical and physiological assessments for elucidating falls risk in Parkinson’s disease. Mov. Disord. 2009, 24, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Shearin, S.M.; Medley, A.; Trudelle-Jackson, E.; Swank, C.; Querry, R. Plantarflexor strength, gait speed, and step length change in individuals with Parkinson’s disease. Int. J. Rehabil. Res. 2021, 44, 82–87. [Google Scholar] [CrossRef]
- Nallegowda, M.; Singh, U.; Handa, G.; Khanna, M.; Wadhwa, S.; Yadav, S.L.; Kumar, G.; Behari, M. Role of sensory input and muscle strength in maintenance of balance, gait, and posture in Parkinson’s disease: A pilot study. Am. J. Phys. Med. Rehabil. 2004, 83, 898–908. [Google Scholar] [CrossRef]
- Memme, J.M.; Slavin, M.; Moradi, N.; Hood, D.A. Mitochondrial Bioenergetics and Turnover during Chronic Muscle Disuse. Int. J. Mol. Sci. 2021, 22, 5179. [Google Scholar] [CrossRef]
- Khemraj, P.; Kuznyetsova, A.; Hood, D.A. Adaptations in mitochondrial quality control and interactions with innate immune signaling within skeletal muscle: A narrative review. J. Sport Health Sci. 2025, 1, 101049. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.H.; Katzenschlager, R.; Lim, S.Y.; Barton, B.; de Bie, R.M.A.; Seppi, K.; Coelho, M.; Sampaio, C. Movement Disorder Society Evidence-Based Medicine Committee. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov. Disord. 2018, 33, 1248–1266. [Google Scholar] [CrossRef] [PubMed]
- David, F.J.; Rafferty, M.R.; Robichaud, J.A.; Prodoehl, J.; Kohrt, W.M.; Vaillancourt, D.E.; Corcos, D.M. Progressive resistance exercise and Parkinson’s disease: A review of potential mechanisms. Park. Dis. 2012, 2012, 124527. [Google Scholar] [CrossRef]
- Skinner, J.W.; Needle, A.R. Exploring the role of ankle muscle function in gait impairments and fall risk in Parkinson’s disease. Hum. Mov. Sci. 2025, 99, 103316. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; Burnfield, J.M. Gait Analysis: Normal and Pathological Function. J. Sports Sci. Med. 2010, 9, 353. [Google Scholar] [PubMed Central]
- Shearin, S.; Medley, A.; Trudelle-Jackson, E.; Swank, C.; Querry, R. Differences in predictors for gait speed and gait endurance in Parkinson’s disease. Gait Posture 2021, 87, 49–53. [Google Scholar] [CrossRef]
- Dietz, V.; Leenders, K.L.; Colombo, G. Leg muscle activation during gait in Parkinson’s disease: Influence of body unloading. Electroencephalogr. Clin. Neurophysiol./Electromyogr. Mot. Control 1997, 105, 400–405. [Google Scholar] [CrossRef]
- Sofuwa, O.; Nieuwboer, A.; Desloovere, K.; Willems, A.M.; Chavret, F.; Jonkers, I. Quantitative gait analysis in Parkinson’s disease: Comparison with a healthy control group. Arch. Phys. Med. Rehabil. 2005, 86, 1007–1013. [Google Scholar] [CrossRef]
- Kirkwood, R.N.; Trede, R.G.; Moreira Bde, S.; Kirkwood, S.A.; Pereira, L.S. Decreased gastrocnemius temporal muscle activation during gait in elderly women with history of recurrent falls. Gait Posture 2011, 34, 60–64. [Google Scholar] [CrossRef]
- Matinolli, M.; Korpelainen, J.T.; Sotaniemi, K.A.; Myllylä, V.V.; Korpelainen, R. Recurrent falls and mortality in Parkinson’s disease: A prospective two-year follow-up study. Acta Neurol Scand. 2011, 123, 193–200. [Google Scholar] [CrossRef]
- Ren, J.; Sherry, A.D.; Malloy, C.R. Modular 31 P wideband inversion transfer for integrative analysis of adenosine triphosphate metabolism, T1 relaxation and molecular dynamics in skeletal muscle at 7T. Magn. Reson. Med. 2019, 81, 3440–3452. [Google Scholar] [CrossRef]
- Moon, H.E.; Paek, S.H. Mitochondrial Dysfunction in Parkinson’s Disease. Exp. Neurobiol. 2015, 24, 103–116. [Google Scholar] [CrossRef]
- Van Laar, V.S.; Berman, S.B. Mitochondrial dynamics in Parkinson’s disease. Exp. Neurol. 2009, 218, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Mischley, L.K.; Shankland, E.; Liu, S.Z.; Bhayana, S.; Fox, D.J.; Marcinek, D.J. ATP and NAD+ Deficiency in Parkinson’s Disease. Nutrients 2023, 15, 943. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, H.; Song, C.; Tian, L.; Wang, P.; Li, T.; Cheng, C.; AlNusaif, M.; Li, S.; Liang, Z.; et al. LRRK2G2019S Gene Mutation Causes Skeletal Muscle Impairment in Animal Model of Parkinson’s Disease. J. Cachexia Sarcopenia Muscle 2024, 15, 2595–2607. [Google Scholar] [CrossRef]
- Krumpolec, P.; Vallova, S.; Slobodova, L.; Tirpakova, V.; Vajda, M.; Schon, M.; Klepochova, R.; Janakova, Z.; Straka, I.; Sutovsky, S.; et al. Aerobic-Strength Exercise Improves Metabolism and Clinical State in Parkinson’s Disease Patients. Front. Neurol. 2017, 8, 698. [Google Scholar] [CrossRef]
- Iansek, R.; Danoudis, M. Freezing of Gait in Parkinson’s Disease: Its Pathophysiology and Pragmatic Approaches to Management. Mov. Disord. Clin. Pract. 2016, 4, 290–297. [Google Scholar] [CrossRef]
- Laukka, J.J.; Kain, K.M.; Rathnam, A.S.; Sohi, J.; Khatib, D.; Kamholz, J.; Stanley, J.A. Altered high-energy phosphate and membrane metabolism in Pelizaeus-Merzbacher disease using phosphorus magnetic resonance spectroscopy. Brain Commun. 2022, 4, fcac202. [Google Scholar] [CrossRef]
- Leukodystrophy. Available online: https://www.ninds.nih.gov/health-information/disorders/leukodystrophy# (accessed on 27 June 2025).
- Blood Proteins for the Treatment of Parkinson’s Disease. Available online: https://www.michaeljfox.org/grant/blood-proteins-treatment-parkinsons-disease (accessed on 27 June 2025).
- Lim, E.L.; Hollingsworth, K.G.; Thelwall, P.E.; Taylor, R. Measuring the acute effect of insulin infusion on ATP turnover rate in human skeletal muscle using phosphorus—31 magnetic resonance saturation transfer spectroscopy. NMR Biomed. 2010, 23, 952–957. [Google Scholar]
- Ren, J.; Sherry, A.D.; Malloy, C.R. A simple approach to evaluate the kinetic rate constant for ATP synthesis in resting human skeletal muscle at 7 T. NMR Biomed. 2016, 29, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Sherry, A.D.; Malloy, C.R. Amplification of the effects of magnetization exchange by 31P band inversion for measuring adenosine triphosphate synthesis rates in human skeletal muscle. Magn. Reson. Med. 2015, 74, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Sherry, A.D.; Malloy, C.R. Efficient 31P band inversion transfer approach for measuring creatine kinase activity, ATP synthesis, and molecular dynamics in the human brain at 7 T. Magn. Reson. Med. 2017, 78, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.G.; Gerstenblith, G.; Bottomley, P.A. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc. Natl. Acad. Sci. USA 2005, 102, 808–813. [Google Scholar] [CrossRef]
- Giacona, J.M.; Afridi, A.; Bezan Petric, U.; Johnson, T.; Pastor, J.; Ren, J.; Sandon, L.; Malloy, C.; Pandey, A.; Shah, A.; et al. Association between dietary phosphate intake and skeletal muscle energetics in adults without cardiovascular disease. J. Appl. Physiol. 2024, 136, 1007–1014. [Google Scholar] [CrossRef]
- Valkovič, L.; Ukropcová, B.; Chmelík, M.; Baláž, M.; Bogner, W.; Schmid, A.I.; Frollo, I.; Zemková, E.; Klimeš, I.; Ukropec, J.; et al. Interrelation of 31P-MRS metabolism measurements in resting and exercised quadriceps muscle of overweight-to-obese sedentary individuals. NMR Biomed. 2013, 26, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Valkovič, L.; Chmelík, M.; Meyerspeer, M.; Gagoski, B.; Rodgers, C.T.; Krššák, M.; Andronesi, O.C.; Trattnig, S.; Bogner, W. Dynamic 31 P-MRSI using spiral spectroscopic imaging can map mitochondrial capacity in muscles of the human calf during plantar flexion exercise at 7 T. NMR Biomed. 2016, 29, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Kelly, N.A.; Ford, M.P.; Standaert, D.G.; Watts, R.L.; Bickel, C.S.; Moellering, D.R.; Tuggle, S.C.; Williams, J.Y.; Lieb, L.; Windham, S.T.; et al. Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with Parkinson’s disease. J. Appl. Physiol. 2014, 116, 582–592. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef]
- Jin, H.; Kanthasamy, A.; Ghosh, A.; Anantharam, V.; Kalyanaraman, B.; Kanthasamy, A.G. Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: Preclinical and clinical outcomes. Biochim. Biophys. Acta. 2014, 1842, 1282–1294. [Google Scholar] [CrossRef] [PubMed]
- Onaolapo, O.J.; Odeniyi, A.O.; Onaolapo, A.Y. Parkinson’s Disease: Is there a Role for Dietary and Herbal Supplements? CNS Neurol. Disord. Drug Targets 2021, 20, 343–365. [Google Scholar] [CrossRef]
- Plascencia-Villa, G.; Perry, G. Exploring Molecular Targets for Mitochondrial Therapies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 12486. [Google Scholar] [CrossRef]
- Borsche, M.; Pereira, S.L.; Klein, C.; Grünewald, A. Mitochondria and Parkinson’s Disease: Clinical, Molecular, and Translational Aspects. J. Park. Dis. 2021, 11, 45–60. [Google Scholar] [CrossRef]

| PD | Control | p-Val | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | PD 1 | Ctrl 1 | PD-vs-Ctrl 2 | |
| kPi, s−1 | 0.03 | 0.03 | 0.06 | 0.05 | 0.87 | 0.39 | 0.0016 * |
| (0.02) | (0.01) | (0.02) | (0.02) | ||||
| kPCr, s−1 | 0.23 | 0.24 | 0.32 | 0.29 | 0.37 | 0.25 | 0.0041 * |
| (0.05) | (0.04) | (0.04) | (0.04) | ||||
| FPi, mM/s | 0.15 | 0.15 | 0.31 | 0.24 | 0.93 | 0.39 | 0.0054 * |
| (0.09) | (0.07) | (0.13) | (0.12) | ||||
| FPCr, mM/s | 8.92 | 10.01 | 11.37 | 10.61 | 0.16 | 0.39 | 0.094 |
| (2.08) | (1.87) | (1.36) | (1.31) | ||||
| Pi, mM | 5.09 | 5.11 | 4.85 | 4.26 | 0.94 | 0.42 | 0.078 |
| (0.55) | (0.97) | (0.80) | (1.32) | ||||
| PCr, mM | 38.9 | 41.1 | 35.9 | 37.2 | 0.05 | 0.30 | 0.012 * |
| (2.2) | (3.2) | (2.2) | (1.5) | ||||
| τPCr, s−1 | 51.5 | 55.2 | 40.1 | 43.2 | 0.60 | 0.84 | 0.25 |
| (18.7) | (14.8) | (22.0) | (25.5) | ||||
| τPi, s−1 | 38.6 | 38.4 | 23.2 | 33.7 | 0.98 | 0.31 | 0.13 |
| (11.8) | (13.4) | (7.6) | (20.2) | ||||
| VPCr, mM/s | 0.31 | 0.35 | 0.37 | 0.42 | 0.40 | 0.61 | 0.37 |
| (0.13) | (0.13) | (0.12) | (0.20) | ||||
| VPCr/FPi, a.u. | 2.73 | 3.07 | 1.39 | 2.22 | 0.62 | 0.25 | 0.09 |
| (1.48) | (2.02) | (0.83) | (1.24) | ||||
| PCrex/PCrrest | 0.62 | 0.66 | 0.64 | 0.60 | 0.23 | 0.50 | 0.65 |
| (0.14) | (0.13) | (0.09) | (0.09) | ||||
| Piex/Pirest | 2.99 | 2.82 | 2.69 | 3.22 | 0.08 | 0.30 | 0.39 |
| (0.92) | (0.79) | (0.88) | (0.60) | ||||
| pHrest | 7.02 | 7.02 | 7.04 | 7.05 | 0.67 | 0.67 | 0.13 |
| (0.04) | (0.04) | (0.03) | (0.03) | ||||
| pHex | 6.98 | 7.01 | 7.00 | 7.04 | 0.32 | 0.21 | 0.16 |
| (0.09) | 0.06 | (0.05) | (0.04) | ||||
| ROM 3, cm | 5.90 | 6.12 | 7.10 | 8.31 | 0.20 | 0.20 | 0.01 * |
| (1.52) | (1.36) | (1.43) | (1.07) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Patel, N.; Johnson, T.; Querry, R.; Shearin, S. Skeletal Muscle 31P Magnetic Resonance Spectroscopy Study of Patients with Parkinson’s Disease: Energy Metabolism and Exercise Performance. Diagnostics 2025, 15, 2573. https://doi.org/10.3390/diagnostics15202573
Ren J, Patel N, Johnson T, Querry R, Shearin S. Skeletal Muscle 31P Magnetic Resonance Spectroscopy Study of Patients with Parkinson’s Disease: Energy Metabolism and Exercise Performance. Diagnostics. 2025; 15(20):2573. https://doi.org/10.3390/diagnostics15202573
Chicago/Turabian StyleRen, Jimin, Neha Patel, Talon Johnson, Ross Querry, and Staci Shearin. 2025. "Skeletal Muscle 31P Magnetic Resonance Spectroscopy Study of Patients with Parkinson’s Disease: Energy Metabolism and Exercise Performance" Diagnostics 15, no. 20: 2573. https://doi.org/10.3390/diagnostics15202573
APA StyleRen, J., Patel, N., Johnson, T., Querry, R., & Shearin, S. (2025). Skeletal Muscle 31P Magnetic Resonance Spectroscopy Study of Patients with Parkinson’s Disease: Energy Metabolism and Exercise Performance. Diagnostics, 15(20), 2573. https://doi.org/10.3390/diagnostics15202573






