Significance of the Monitoring Right Ventricular Echocardiographic Parameters in Patients with Hypertrophic Cardiomyopathy Undergoing Alcohol Septal Ablation—A Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Alcohol Septal Ablation
2.3. Echocardiographic Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jurcut, R.; Barriales-Villa, R.; Biagini, E.; Garcia-Pavia, P.; Olivotto, I.; Protonotarios, A.; Arbustini, E.; Mogensen, J.; Elliott, P.; Arbelo, E.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar]
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Dearani, J.A.; Evanovich, L.; Ferrari, V.A.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1239–e1311. [Google Scholar] [CrossRef]
- Argirò, A.; Parikh, V.; Jurcut, R.; Finocchiaro, G.; Kaski, J.P.; Adler, E.; Olivotto, I. Hypertrophic cardiomyopathy. Nat. Rev. Dis. Primers. 2025, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Marian, A.J.; Braunwald, E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ. Res. 2017, 121, 749–770. [Google Scholar] [CrossRef]
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 372–389. [Google Scholar] [CrossRef]
- Braunwald, E. Hypertrophic Cardiomyopathy: A Brief Overview. Am. J. Cardiol. 2024, 212S, S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2025, 393, 1004–1015. [Google Scholar] [CrossRef]
- Sigwart, U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet 1995, 346, 211–214. [Google Scholar] [CrossRef]
- Veselka, J. Looking Back at 30 Years of Alcohol Septal Ablation and Looking Forward to the Future. Can. J. Cardiol. 2024, 40, 824–832. [Google Scholar] [CrossRef]
- Pastore, M.C.; De Carli, G.; Mandoli, G.E.; D’aScenzi, F.; Focardi, M.; Contorni, F.; Mondillo, S.; Cameli, M. The prognostic role of speckle tracking echocardiography in clinical practice: Evidence and reference values from the literature. Heart Fail. Rev. 2021, 26, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Muraru, D.; Parati, G.; Haugaa, K.; Voigt, J.U. How to do right ventricular strain. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, Y.L.; Debonnaire, P.; Bootsma, M.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Prevalence and Prognostic Implications of Right Ventricular Dysfunction in Patients With Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2019, 124, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Roşca, M.; Călin, A.; Beladan, C.C.; Enache, R.; Mateescu, A.D.; Gurzun, M.-M.; Varga, P.; Băicuş, C.; Coman, I.M.; Jurcuţ, R.; et al. Right ventricular remodeling, its correlates, and its clinical impact in hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2015, 28, 1329–1338. [Google Scholar] [CrossRef]
- Figliozzi, S.; Masci, P.-G.; Monti, L.; Stankowski, K.; Tondi, L.; Aivalioti, E.; Mavraganis, G.; Francone, M.; Condorelli, G.; Olivotto, I.; et al. Prognostic value of right ventricular involvement in hypertrophic cardiomyopathy: A systematic review and meta-analysis. Int. J. Cardiol. 2024, 413, 132390. [Google Scholar] [CrossRef]
- Śpiewak, M.; Kłopotowski, M.; Mazurkiewicz, Ł.; Kowalik, E.; Petryka-Mazurkiewicz, J.; Miłosz-Wieczorek, B.; Klisiewicz, A.; Marczak, M. Predictors of right ventricular function and size in patients with hypertrophic cardiomyopathy. Sci. Rep. 2020, 10, 21054. [Google Scholar] [CrossRef]
- Seo, J.; Hong, Y.J.; Kim, Y.J.; Lkhagvasuren, P.; Cho, I.; Shim, C.Y.; Ha, J.-W.; Hong, G.-R. Prevalence, functional characteristics, and clinical significance of right ventricular involvement in patients with hypertrophic cardiomyopathy. Sci. Rep. 2020, 10, 21908. [Google Scholar] [CrossRef]
- Mahmod, M.; Raman, B.; Chan, K.; Sivalokanathan, S.; Smillie, R.W.; Samat, A.H.A.; Ariga, R.; Dass, S.; Ormondroyd, E.; Watkins, H.; et al. Right ventricular function declines prior to left ventricular ejection fraction in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2022, 24, 36. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Qiao, S.-B.; Hu, F.-H.; Yuan, J.-S.; Yang, W.-X.; Cui, J.-G.; Zhang, Y.; Zhou, Y.; Zhang, C.-L. Biventricular reverse remodeling after successful alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Am. J. Cardiol. 2015, 115, 493–498. [Google Scholar] [CrossRef]
- Mihos, C.G.; Escolar, E.; Fernandez, R. Right ventricular hypertrophy in apical hypertrophic cardiomyopathy. Echocardiography 2023, 40, 515–523. [Google Scholar] [CrossRef]
- Maron, M.S.; Hauser, T.H.; Dubrow, E.; Horst, T.A.; Kissinger, K.V.; Udelson, J.E.; Manning, W.J. Right ventricular involvement in hypertrophic cardiomyopathy. Am. J. Cardiol. 2007, 100, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Fan, C.; Tian, L.; Liu, Y.; Wang, H.; Zhao, S.; Duan, F.; Zhang, X.; Zhao, X.; Wang, F.; et al. The clinical features, outcomes and genetic characteristics of hypertrophic cardiomyopathy patients with severe right ventricular hypertrophy. PLoS ONE 2017, 12, e0174118. [Google Scholar] [CrossRef]
- Wen, S.; Pislaru, C.; Ommen, S.R.; Ackerman, M.J.; Pislaru, S.V.; Geske, J.B. Right Ventricular Enlargement and Dysfunction Are Associated With Increased All-Cause Mortality in Hypertrophic Cardiomyopathy. Mayo Clin. Proc. 2022, 97, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Monti, L.; Rossi, A.; Pontone, G.; Conte, E.; Nicoli, F.; di Odoardo, L.; Guglielmo, M.; Indolfi, E.; Bombace, S.; et al. The prognostic role of right ventricular dysfunction in patients with hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2023, 39, 1515–1523. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Wang, Y.; Zhang, M.; Zhu, W.; Cai, Q.; Jiang, W.; Sun, L.; Ding, X.; Ye, X.; et al. Impaired Right Ventricular Mechanics at Rest and During Exercise Are Associated With Exercise Capacity in Patients With Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2019, 8, e011269. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Knowles, J.W.; Pavlovic, A.; Perez, M.; Magavern, E.; Sinagra, G.; Haddad, F.; Ashley, E.A. Prevalence and clinical correlates of right ventricular dysfunction in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2014, 113, 361–367. [Google Scholar] [CrossRef]
- Houston, B.A.; Brittain, E.L.; Tedford, R.J. Right Ventricular Failure. N. Engl. J. Med. 2023, 388, 1111–1125. [Google Scholar] [CrossRef]
- Tello, K.; Naeije, R.; de Man, F.; Guazzi, M. Pathophysiology of the right ventricle in health and disease: An update. Cardiovasc. Res. 2023, 119, 1891–1904. [Google Scholar] [CrossRef] [PubMed]
- Calore, C.; Mangia, M.; Basso, C.; Corrado, D.; Thiene, G. Hypertrophic Cardiomyopathy: New Clinical and Therapeutic Perspectives of an "Old" Genetic Myocardial Disease. Genes 2025, 16, 74. [Google Scholar] [CrossRef]
- Ommen, S.R.; Nishimura, R.A.; Schaff, H.V.; Dearani, J.A. Hypertrophic Cardiomyopathy: State of the Art. Mayo Clin. Proc. 2025, 100, 557–566. [Google Scholar] [CrossRef] [PubMed]

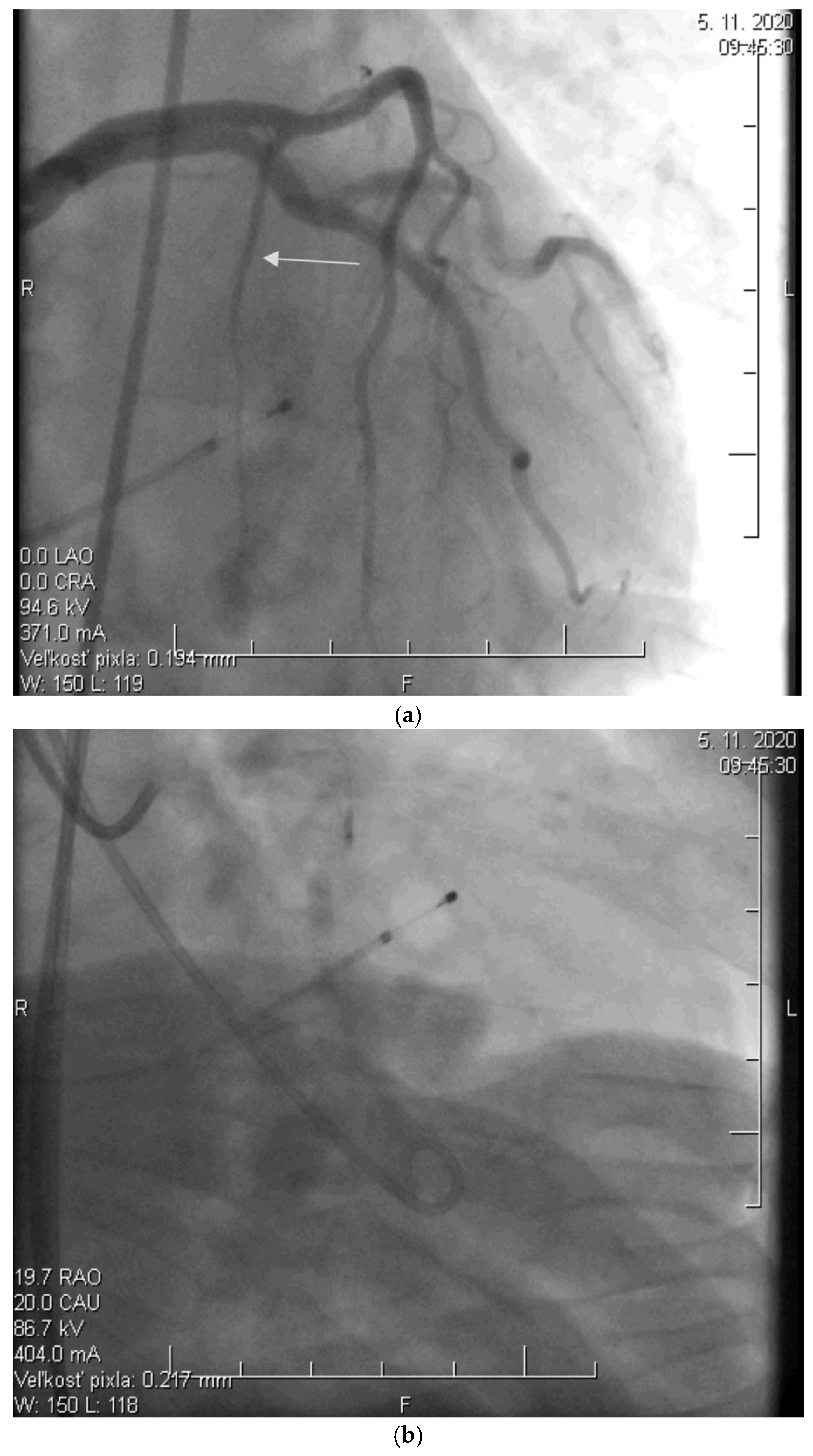

| Before ASA | 0–3 M | 0–1 Y | 0–3 Y | 0–5 Y | |||||
|---|---|---|---|---|---|---|---|---|---|
| p | p | p | p | ||||||
| RVWT, mm | 7.1 ± 1.9 | 7.0 ± 1.9 | <0.001 | 6.7 ± 1.8 | <0.001 | 6.6 ± 1.6 | <0.001 | 6.5 ± 1.6 | <0.001 |
| RVOTPROX, mm | 29.2 ± 2.1 | 29.7 ± 2.0 | ns | 29.8 ± 1.8 | ns | 29.8 ± 2.3 | ns | 30.1 ± 2.2 | ns |
| RVD, mm | 39.0 ± 4.3 | 39.2 ± 4.2 | <0.05 | 39.2 ± 4.3 | <0.05 | 39.7 ± 4.0 | ns | 39.9 ± 3.8 | ns |
| RVEDV, mL | 143.7 ± 49.2 | 143.9 ± 49.0 | ns | 144.1 ± 48.5 | ns | 145.2 ± 47.9 | ns | 145.7 ± 48.6 | ns |
| TAPSE, mm | 23.3 ± 4.7 | 23.0 ± 4.4 | ns | 22.7 ± 4.3 | ns | 22.5 ± 3.9 | ns | 22.2 ± 4.0 | ns |
| S’, mm | 10.9 ± 1.4 | 10.7 ± 1.3 | ns | 10.9 ± 1.2 | ns | 10.5 ± 1.3 | ns | 10.4 ± 1.4 | ns |
| LVOTG, mmHg | 83.0 ± 32.8 | 19.9 ±12.7 | <0.001 | 21.2 ± 13.8 | <0.001 | 20.4 ± 15.5 | <0.001 | 34.2 ± 30.9 | <0.001 |
| NYHA | 2.8 ± 0.4 | 2.6 ± 0.3 | <0.001 | 2.6 ± 0.5 | <0.001 | 2.3 ± 0.6 | <0.001 | 2.0 ± 0.5 | <0.01 |
| LVD, mm | 40.3 ± 4.8 | 40.7 ± 4.9 | <0.05 | 41.1 ± 4.7 | <0.05 | 43.6 ± 4.9 | <0.05 | 44.2 ± 5.3 | <0.01 |
| IVSD, mm | 19.4 ± 3.5 | 19.1 ± 2.7 | <0.001 | 16.4 ± 3.3 | <0.001 | 14.8 ± 2.9 | <0.001 | 14.6 ± 3.6 | <0.001 |
| PWD, mm | 14.6 ± 3.3 | 14.2 ± 2.0 | <0.01 | 13.7 ± 2.1 | <0.01 | 13.2 ± 2.4 | <0.01 | 13.0 ± 1.8 | <0.001 |
| LAD, mm | 43.2 ± 6.9 | 43.0 ± 5.4 | ns | 42.7 ± 4.9 | ns | 42.4 ± 6.1 | ns | 43.8 ± 5.9 | ns |
| LVEF, % | 64.9 ± 5.5 | 60.6 ± 6.1 | <0.01 | 61.8 ± 7.2 | <0.01 | 64.1 ± 7.4 | ns | 62.3 ± 7.0 | ns |
| 0–3 M | 0–1 Y | 0–3 Y | 0–5 Y | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| RVWT, mm | 0.62 | <0.001 | 0.63 | <0.001 | 0.52 | ns | 0.67 | ns |
| RVOTPROX, mm | −0.71 | ns | −0.72 | ns | −0.80 | ns | −0.81 | ns |
| RVD, mm | −0.48 | ns | −0.27 | ns | −0.52 | ns | −0.12 | ns |
| RVEDV, mL | −0.61 | ns | −0.78 | ns | −0.71 | ns | −0.28 | ns |
| TAPSE, mm | −0.63 | ns | −0.77 | ns | −0.59 | ns | −0.56 | ns |
| S’, mm | −0.32 | ns | −0.51 | ns | −0.22 | ns | −0.28 | ns |
| 0–3 M | 0–1 Y | 0–3 Y | 0–5 Y | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| RVWT, mm | 0.53 | <0.01 | 0.54 | <0.01 | 0.61 | ns | 0.69 | ns |
| RVOTPROX, mm | 0.65 | ns | 0.68 | ns | 0.68 | ns | 0.78 | ns |
| RVD, mm | 0.52 | ns | 0.24 | ns | 0.57 | ns | 0.46 | ns |
| RVEDV, mL | 0.64 | ns | 0.76 | ns | 0.64 | ns | 0.52 | ns |
| TAPSE, mm | 0.68 | ns | 0.79 | ns | 0.51 | ns | 0.66 | ns |
| S’, mm | 0.40 | ns | 0.46 | ns | 0.39 | ns | 0.42 | ns |
| 0–3 M | 0–1 Y | 0–3 Y | 0–5 Y | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| RVWT, mm | 0.43 | <0.01 | 0.59 | <0.01 | 0.68 | ns | 0.64 | ns |
| RVOTPROX, mm | 0.58 | ns | 0.72 | ns | 0.64 | ns | 0.72 | ns |
| RVD, mm | 0.63 | ns | 0.27 | ns | 0.61 | ns | 0.51 | ns |
| RVEDV, mL | 0.59 | ns | 0.82 | ns | 0.62 | ns | 0.58 | ns |
| TAPSE, mm | 0.63 | ns | 0.77 | ns | 0.59 | ns | 0.72 | ns |
| S’, mm | 0.44 | ns | 0.53 | ns | 0.48 | ns | 0.51 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poruban, T.; Schusterova, I.; Pella, D.; Fedacko, J.; Sieradzka Uchnar, K.A.; Sepesiova, B.; Gurbalova, S. Significance of the Monitoring Right Ventricular Echocardiographic Parameters in Patients with Hypertrophic Cardiomyopathy Undergoing Alcohol Septal Ablation—A Single-Center Experience. Diagnostics 2025, 15, 2509. https://doi.org/10.3390/diagnostics15192509
Poruban T, Schusterova I, Pella D, Fedacko J, Sieradzka Uchnar KA, Sepesiova B, Gurbalova S. Significance of the Monitoring Right Ventricular Echocardiographic Parameters in Patients with Hypertrophic Cardiomyopathy Undergoing Alcohol Septal Ablation—A Single-Center Experience. Diagnostics. 2025; 15(19):2509. https://doi.org/10.3390/diagnostics15192509
Chicago/Turabian StylePoruban, Tibor, Ingrid Schusterova, Dominik Pella, Jan Fedacko, Karolina Angela Sieradzka Uchnar, Barbora Sepesiova, and Silvia Gurbalova. 2025. "Significance of the Monitoring Right Ventricular Echocardiographic Parameters in Patients with Hypertrophic Cardiomyopathy Undergoing Alcohol Septal Ablation—A Single-Center Experience" Diagnostics 15, no. 19: 2509. https://doi.org/10.3390/diagnostics15192509
APA StylePoruban, T., Schusterova, I., Pella, D., Fedacko, J., Sieradzka Uchnar, K. A., Sepesiova, B., & Gurbalova, S. (2025). Significance of the Monitoring Right Ventricular Echocardiographic Parameters in Patients with Hypertrophic Cardiomyopathy Undergoing Alcohol Septal Ablation—A Single-Center Experience. Diagnostics, 15(19), 2509. https://doi.org/10.3390/diagnostics15192509






