Relationship Between Right Ventricular Function and Body Composition in Adolescents and Young Adults
Abstract
1. Introduction
2. Materials and Methods
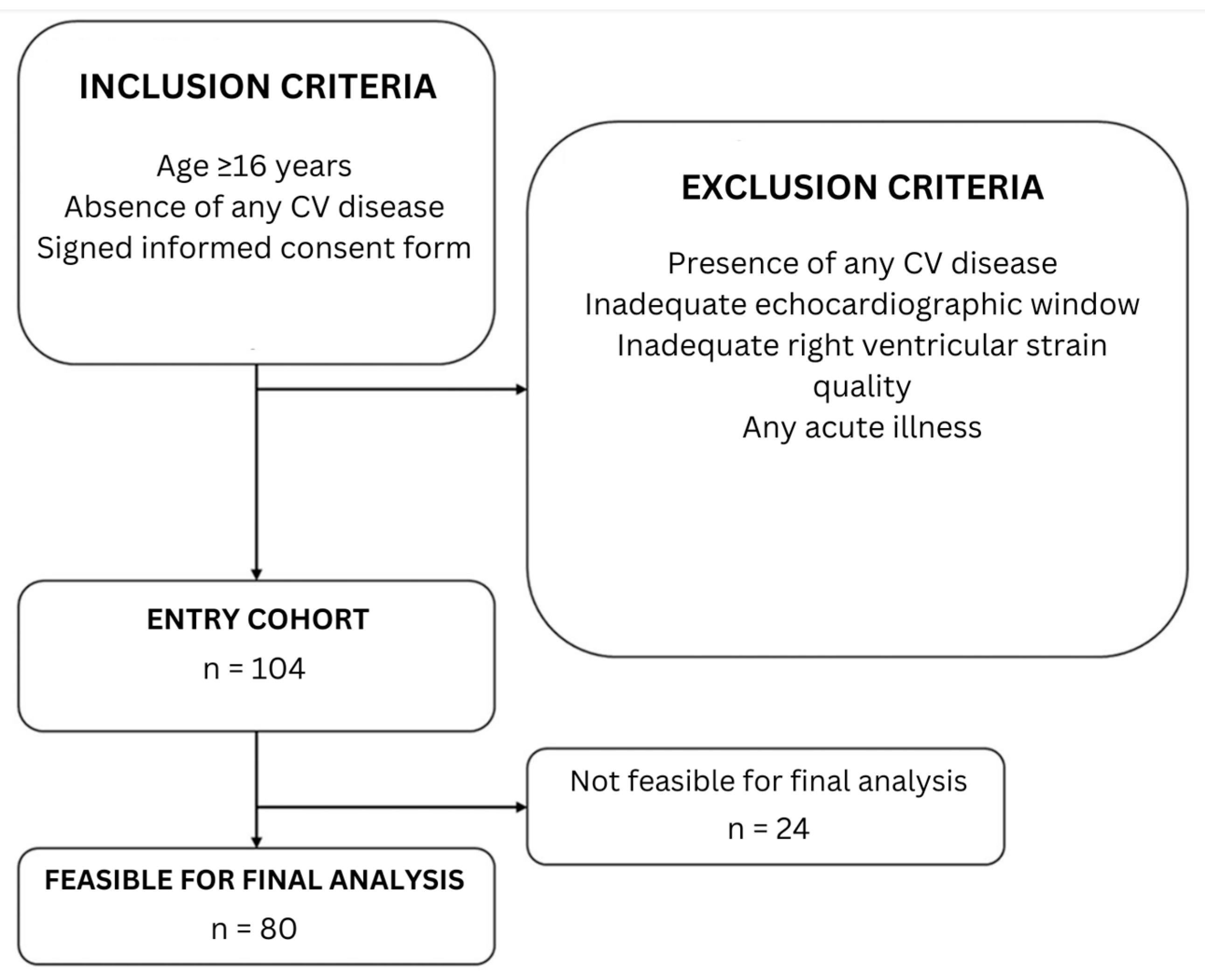
2.1. Study Population
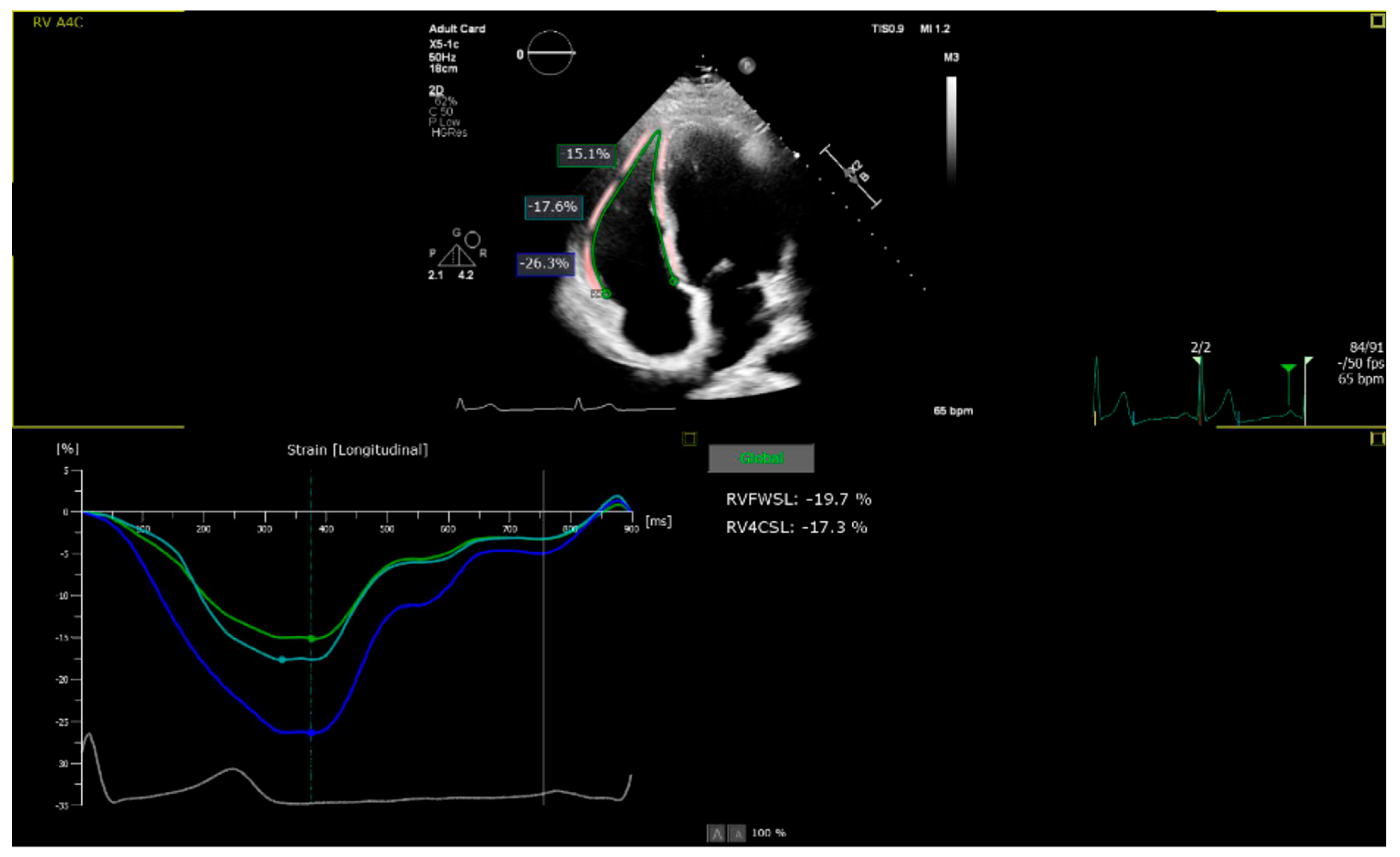
2.2. Echocardiographic Assessment
2.3. Laboratory Parameters
2.4. Body Composition Scale
2.5. Statistical Analysis
3. Results
3.1. Echocardiographic Findings
3.2. Correlations with Body Composition
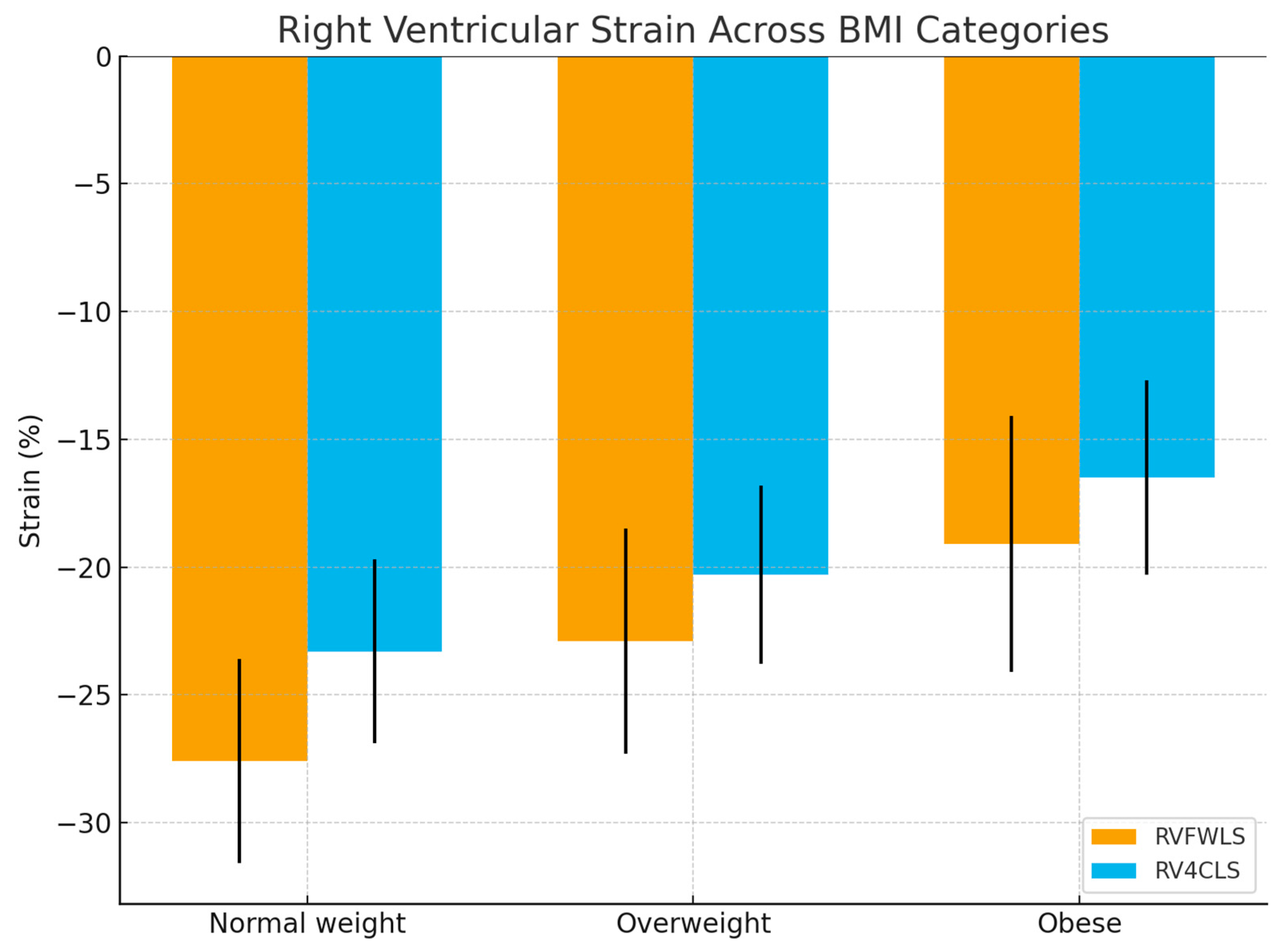
3.3. Trend Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D RV4CLS | Two-dimensional global longitudinal strain of the right ventricle |
| 2D RVFWLS | Two-dimensional longitudinal strain of the free wall of the right ventricle |
| 3D | Three-dimensional |
| BMI | Body mass index |
| CMR | Cardiac Magnetic Resonance |
| CVD | Cardiovascular diseases |
| ECW | Extracellular water |
| EF | Ejection fraction |
| FAC | Change in fractional area change |
| HF | Heart failure |
| ICW | Intracellular water |
| LV | Left ventricle |
| PASP | Systolic pressure in the pulmonary artery |
| RV | Right ventricle |
| RVBD, RVMD | Basal and mid-ventricular diameter of the RV |
| S’ | Maximum velocity of lateral tricuspid annular motion during systole |
| TAPSE | Amplitude of lateral tricuspid annular motion |
| TBW | Total Body Water |
| TR | Tricuspid regurgitation |
References
- Ren, J.; Wu, N.N.; Wang, S.; Sowers, J.R.; Zhang, Y. Obesity cardiomyopathy: Evidence, mechanisms, and therapeutic implications. Physiol. Rev. 2021, 101, 1745–1807. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Aryee, E.K.; Ozkan, B.; Ndumele, C.E. Heart Failure and Obesity: The Latest Pandemic. Prog. Cardiovasc. Dis. 2023, 78, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Schiattarella, G.G.; Lindberg, F.; Anker, M.S.; Bayes-Genis, A.; Bäck, M.; Braunschweig, F.; Bucciarelli-Ducci, C.; Butler, J.; Cannata, A.; et al. Heart failure and obesity: Translational approaches and therapeutic perspectives. A scientific statement of the Heart Failure Association of the ESC. Eur. J. Heart Fail. 2025, 27, 1273–1293. [Google Scholar] [CrossRef]
- Ozkan, B.; Ndumele, C.E. Exploring the Mechanistic Link Between Obesity and Heart Failure. Curr. Diabetes Rep. 2023, 23, 347–360. [Google Scholar] [CrossRef]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.T.; Deswal, A.; Fonarow, G.C.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef]
- Meems, L.M.G.; van Veldhuisen, D.J.; Sattar, N.; Lee, M.M.Y. Heart failure and obesity: Novel insights leading to new treatment paradigms. Nat Rev Cardiol. 2025, 1–13. [Google Scholar] [CrossRef]
- Lala, A.; Beavers, C.; Blumer, V.; Brewer, L.P.; De Oliveira-Gomes, D.; Dunbar, S.; Every, H.; Ferraro, R.; Ky, B.; Januzzi, J.; et al. The Continuum of Prevention and Heart Failure in Cardiovascular Medicine: A Joint Scientific Statement from the Heart Failure Society of America and The American Society for Preventive Cardiology. J. Card. Fail. 2025, 101069. [Google Scholar] [CrossRef]
- Tello, K.; Naeije, R.; de Man, F.; Guazzi, M. Pathophysiology of the right ventricle in health and disease: An update. Cardiovasc. Res. 2023, 119, 1891–1904. [Google Scholar] [CrossRef]
- Brown, T.M.; Pack, Q.R.; Aberegg, E.; Brewer, L.C.; Ford, Y.R.; Forman, D.E.; Gathright, E.C.; Khadanga, S.; Ozemek, C.; Thomas, R.J. Core Components of Cardiac Rehabilitation Programs: 2024 Update: A Scientific Statement from the American Heart Association and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2024, 150, e328–e347. [Google Scholar] [CrossRef]
- Goliopoulou, A.; Oikonomou, E.; Theofilis, P.; Tsigkou, V.; Makavos, G.; Kourampi, I.; Katsioupa, M.; Antoniou, V.-D.; Ikonomidis, I.; Lambadiari, V.; et al. Impairment in Right Ventricular-Pulmonary Arterial Coupling in Overweight and Obesity. J. Clin. Med. 2024, 13, 3389. [Google Scholar] [CrossRef]
- De Luca, M.; Ferrara, F.; Gargani, L.; Argiento, P.; Bandera, F.; Carbone, A.; Castaldo, R.; D’Agostino, A.; D’Alto, M.; D’Andrea, A.; et al. Exercise Doppler Echocardiography of the Right Heart and Pulmonary Circulation in Patients with Cardiovascular Risk Factors: Observations from the RIGHT Heart International NETwork (RIGHT-NET). Chest 2025. [Google Scholar] [CrossRef] [PubMed]
- McCabe, C.; Oliveira, R.K.F.; Rahaghi, F.; Faria-Urbina, M.; Howard, L.; Axell, R.G.; Priest, A.N.; Waxman, A.B.; Systrom, D.M. Right ventriculo-arterial uncoupling and impaired contractile reserve in obese patients with unexplained exercise intolerance. Eur. J. Appl. Physiol. 2018, 118, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Melenovsky, V.; Kotrc, M.; Borlaug, B.A.; Marek, T.; Kovar, J.; Malek, I.; Kautzner, J. Relationships between right ventricular function, body composition, and prognosis in advanced heart failure. J. Am. Coll. Cardiol. 2013, 62, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Badano, L.P.; Muraru, D.; Parati, G.; Haugaa, K.; Voigt, J.U. How to do right ventricular strain. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 825–827. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Praz, F.; Borger, M.A.; Lanz, J.; Marin-Cuartas, M.; Abreu, A.; Adamo, M.; Marsan, N.A.; Barili, F.; Bonaros, N.; Cosyns, B.; et al. 2025 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2025, 67, ehaf194. [Google Scholar] [CrossRef]
- Mihos, C.G.; Liu, J.E.; Anderson, K.M.; Pernetz, M.A.; O’dRiscoll, J.M.; Aurigemma, G.P.; Ujueta, F.; Wessly, P.; American Heart Association Council on Peripheral Vascular Disease; Council on Cardiovascular and Stroke Nursing; et al. Speckle-Tracking Strain Echocardiography for the Assessment of Left Ventricular Structure and Function: A Scientific Statement from the American Heart Association. Circulation 2025, 152, e96–e109. [Google Scholar] [CrossRef]
- Yi, Y.; Baek, J.Y.; Lee, E.; Jung, H.W.; Jang, I.Y. A Comparative Study of High-Frequency Bioelectrical Impedance Analysis and Dual-Energy X-ray Absorptiometry for Estimating Body Composition. Life 2022, 12, 994. [Google Scholar] [CrossRef]
- Nakanishi, K.; Daimon, M.; Yoshida, Y.; Ishiwata, J.; Sawada, N.; Hirokawa, M.; Kaneko, H.; Nakao, T.; Mizuno, Y.; Morita, H.; et al. Relation of Body Mass Index to Adverse Right Ventricular Mechanics. Am. J. Cardiol. 2021, 144, 137–142. [Google Scholar] [CrossRef]
- Lewis, A.J.M.; Abdesselam, I.; Rayner, J.J.; Byrne, J.; Borlaug, B.A.; Neubauer, S.; Rider, O.J. Adverse right ventricular remodelling, function, and stress responses in obesity: Insights from cardiovascular magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, J.F.; Aristizábal-Duque, C.H.; Sánchez, I.M.B.; Ortiz, M.R.; Almodóvar, A.R.; Ortega, M.D.; Martínez, F.E.; Saldaña, M.R.; del Pozo, F.J.F.; Álvarez-Ossorio, M.P.; et al. Relationship between overweight and obesity and cardiac dimensions and function in a paediatric population. Eur. J. Pediatr. 2022, 181, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yu, L.; Li, J.; Gao, X.; Wang, J.; Qu, G.; Shen, C.; Gan, L. Effect of obesity on cardiovascular morphofunctional phenotype: Study of Mendelian randomization. Medicine 2025, 104, e41858. [Google Scholar] [CrossRef]
- Toemen, L.; Santos, S.; Roest, A.A.; Jelic, G.; van der Lugt, A.; Felix, J.F.; Helbing, W.A.; Gaillard, R.; Jaddoe, V.W.V. Body Fat Distribution, Overweight, and Cardiac Structures in School-Age Children: A Population-Based Cardiac Magnetic Resonance Imaging Study. J. Am. Heart Assoc. 2020, 9, e014933. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, G.; Pacileo, G.; Del Giudice, E.M.; Natale, F.; Limongelli, G.; Verrengia, M.; Rea, A.; Fratta, F.; Castaldi, B.; D’Andrea, A.; et al. Abnormal myocardial deformation properties in obese, non-hypertensive children: An ambulatory blood pressure monitoring, standard echocardiographic, and strain rate imaging study. Eur. Heart J. 2006, 27, 2689–2695. [Google Scholar] [CrossRef]
- Xu, E.; Kachenoura, N.; della Valle, V.; Dubern, B.; Karsenty, A.; Tounian, P.; Dacher, J.; Layese, R.; Lamy, J.; le Pointe, H.D.; et al. Multichamber Dysfunction in Children and Adolescents with Severe Obesity: A Cardiac Magnetic Resonance Imaging Myocardial Strain Study. J. Magn. Reson. Imaging 2021, 54, 1393–1403. [Google Scholar] [CrossRef]
- Madan, N.; Aly, D.; Kathol, M.; Buddhavarapu, A.; Rieth, T.; Sherman, A.; Forsha, D. Relationship Between Obesity and Global Longitudinal Strain in the Pediatric Single Ventricle Fontan Population Across Ventricular Morphologies. J. Am. Heart Assoc. 2024, 13, e028616. [Google Scholar] [CrossRef]
- Houston, B.A.; Brittain, E.L.; Tedford, R.J. Right Ventricular Failure. N. Engl. J. Med. 2023, 388, 1111–1125. [Google Scholar] [CrossRef]
- Batalli-Këpuska, A.; Bajraktari, G.; Zejnullahu, M.; Azemi, M.; Shala, M.; Batalli, A.; Ibrahimi, P.; Jashari, F.; Henein, M.Y. Abnormal systolic and diastolic myocardial function in obese asymptomatic adolescents. Int. J. Cardiol. 2013, 168, 2347–2351. [Google Scholar] [CrossRef]
- Siurana, J.M.; Ventura, P.S.; Yeste, D.; Riaza-Martin, L.; Arciniegas, L.; Clemente, M.; Torres, M.; Amigó, N.; Giralt, G.; Roses-Noguer, F.; et al. Myocardial Geometry and Dysfunction in Morbidly Obese Adolescents (BMI 35–40 kg/m2). Am. J. Cardiol. 2021, 157, 128–134. [Google Scholar] [CrossRef]
- Cortez, R.M.; Okoshi, M.P.; Okoshi, K. A Review of the Roles and Limitations of Noninvasive Imaging Methods for Investigating Cardiovascular Disease in Individuals with Obesity. Med. Sci. Monit. 2022, 28, e937362. [Google Scholar] [CrossRef]
- Edward, J.; Banchs, J.; Parker, H.; Cornwell, W. Right ventricular function across the spectrum of health and disease. Heart 2023, 109, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Aaron, C.; Tandri, H.; Barr, R.; Johnson, C.; Bagiella, E.; Chahal, H.; Jain, A.; Kizer, J.; Lima, J.; Bluemke, D.; et al. Obesity and right ventricular structure and function: The MESA-Right Ventricle Study. Chest 2012, 141, 388–395. [Google Scholar]
- Ma, J.I.; Zern, E.K.; Parekh, J.K.; Owunna, N.; Jiang, N.; Wang, D.; Rambarat, P.K.; Pomerantsev, E.; Picard, M.H.; Ho, J.E. Obesity Modifies Clinical Outcomes of Right Ventricular Dysfunction. Circ. Heart Fail. 2023, 16, e010524. [Google Scholar] [CrossRef] [PubMed]
- Sokmen, A.; Sokmen, G.; Acar, G.; Akcay, A.; Koroglu, S.; Koleoglu, M.; Yalcintas, S.; Aydin, M.N. The impact of isolated obesity on right ventricular function in young adults. Arq. Bras. Cardiol. 2013, 101, 160–168. [Google Scholar] [CrossRef]

| Overweight or Obesity N = 55 | Normal Weight N = 25 | r | p | |
|---|---|---|---|---|
| Demographics | ||||
| Female, n (%) | 23 (42) | 10 (40) | 0.081 | |
| Age, years | 19.5 ± 2.8 | 20.4 ± 2.96 | 0.816 | |
| Weight (kg) | 77.1 ± 3.9 | 71.8 ± 2.7 | 0.005 | |
| Height (cm) | 173.2 ± 4.7 | 175.4 ± 4.5 | 0.005 | |
| BMI (kg/m2) | 31.4 ± 4.0 | 21.5 ± 2.0 | p < 0.0001 | |
| Caucasian ethnicity (%) | 55 (100) | 25 (100) | ||
| Clinical characteristics | ||||
| SBP, mmHg | 122 ± 21 | 121 ± 19 | 0.008 | |
| DBP, mmHg | 75 ± 12 | 77 ± 13 | 0.007 | |
| 24 h SBP, mmHg | 109 ± 11 | 111 ± 13 | 0.005 | |
| 24 h DBP, mmHg | 66 ± 9 | 67 ± 9 | 0.076 | |
| Heart rate, bpm | 64 ± 10 | 65 ± 11 | 0.214 | |
| 24 h heart rate, bpm | 65 ± 10 | 67 ± 11 | 0.459 | |
| Smoker, n (%) | 9 (16) | 4 (16) | 0.573 | |
| Laboratory parameters | ||||
| AST, μkat/L | 0.47 ± 0.21 | 0.41 ± 0.13 | −0.18 | 0.249 |
| ALT, μkat/L | 0.46 ± 0.31 | 0.31 ± 0.16 | −0.16 | 0.061 |
| GGT, μkat/L | 0.39 ± 0.24 | 0.26 ± 0.07 | −0.05 | 0.819 |
| CHOL, mmol/L | 4.33 ± 0.64 | 4.24 ± 0.74 | −0.19 | 0.312 |
| TAG, mmol/L | 1.15 ± 0.54 | 0.79 ± 0.40 | −0.09 | 0.624 |
| LDL, mmol/L | 2.84 ± 0.72 | 2.54 ± 0.62 | −0.22 | 0.238 |
| HDL, mmol/L | 1.26 ± 0.42 | 1.57 ± 0.41 | −0.04 | 0.796 |
| Albumin, g/L | 45.62 ± 2.23 | 45.65 ± 2.55 | 0.09 | 0.085 |
| Uric acid, mmol/L | 325.89 ± 80.26 | 269.83 ± 82.70 | 0.08 | 0.552 |
| Total serum protein, kg | 72.46 ± 2.97 | 73.85 ± 3.73 | 0.10 | 0.074 |
| C-peptide, nmol/L | 1242.61 ± 619.82 | 712.1 ± 385.2 | 0.13 | 0.306 |
| Insulin, mmol/L | 13.37 ± 6.57 | 10.01 ± 6.90 | 0.05 | 0.495 |
| Body composition | ||||
| Total water, L | 45.53 ± 8.64 | 38.21 ± 7.45 | 0.24 | 0.083 |
| Total minerals, kg | 4.03 ± 0.83 | 3.47 ± 0.60 | 0.29 | 0.057 |
| Fat mass, kg | 25.46 ± 13.39 | 9.83 ± 5.36 | −0.41 | 0.035 |
| Lean body mass, kg | 62.84 ± 11.53 | 52.03 ± 10.11 | 0.59 | 0.009 |
| Echocardiography | ||||
| RVBD (mm) | 35.4 ± 3.6 | 31.9 ± 3.9 | 0.67 | p < 0.05 |
| RVMD (mm) | 27.8 ± 3.9 | 25.7 ± 3.9 | 0.12 | p < 0.04 |
| FAC (%) | 44.7 ± 5.0 | 46.4 ± 5.6 | 0.15 | ns |
| 2D RVFWLS (%) | 20.8 ± 5.2 | 27.6 ± 4.0 | 0.32 | p < 0.001 |
| 2D RV4CLS (%) | 18.2 ± 3.8 | 23.3 ± 3.6 | 0.29 | p < 0.001 |
| LVEF, % | 56.70 ± 3.90 | 58.40 ± 4.25 | −0.24 | p <0.01 |
| S’(cm/s) | 13.1 ± 1.6 | 14.1 ± 1.64 | −0.09 | p < 0.01 |
| TAPSE, mm | 22.93 ± 2.5 | 23.1 ± 2.5 | −0.16 | ns |
| PASP, mmHg | 26.57 ± 5.7 | 22.7 ± 4.7 | 0.21 | p < 0.01 |
| Echocardiographic Parameters | Body Composition Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TP | TW | MIN | FM | LBM | ||||||
| r | p | r | p | r | p | r | p | r | p | |
| S’ (cm/s) | −0.10 | <0.01 | −0.09 | ns | −0.06 | ns | −0.02 | <0.01 | −0.11 | <0.01 |
| TAPSE, mm | −0.17 | ns | −0.15 | ns | −0.01 | ns | −0.02 | ns | −0.11 | ns |
| PASP, mmHg | 0.19 | <0.01 | 0.12 | ns | 0.09 | ns | 0.17 | <0.01 | 0.23 | <0.01 |
| RVBD (mm) | 0.05 | <0.01 | −0.04 | ns | 0.12 | ns | 0.10 | <0.001 | 0.05 | <0.01 |
| RVMD (mm) | 0.12 | <0.05 | 0.09 | ns | 0.13 | ns | 0.32 | <0.04 | 0.14 | <0.04 |
| FAC (%) | 0.24 | ns | 0.23 | ns | 0.15 | ns | 0.30 | ns | 0.25 | ns |
| 2D RVFWLS (%) | −0.25 | <0.01 | 0.28 | ns | 0.32 | ns | −0.22 | <0.001 | 0.29 | <0.01 |
| 2D RV4CLS (%) | −0.24 | <0.01 | 0.26 | ns | 0.29 | ns | −0.29 | <0.001 | 0.29 | <0.001 |
| Echocardiographic Parameters | Body Composition Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TP | TW | MIN | FM | LBM | ||||||
| r | p | r | p | r | p | r | p | r | p | |
| S’ (cm/s) | −0.12 | <0.01 | −0.08 | ns | −0.05 | ns | −0.03 | <0.01 | −0.09 | <0.01 |
| TAPSE, mm | −0.14 | ns | −0.13 | ns | −0.02 | ns | −0.01 | ns | −0.12 | ns |
| PASP, mmHg | 0.21 | <0.01 | 0.11 | ns | 0.07 | ns | 0.18 | <0.01 | 0.22 | <0.01 |
| RVBD (mm) | 0.06 | <0.01 | −0.03 | ns | 0.10 | ns | 0.11 | <0.05 | 0.06 | <0.01 |
| RVMD (mm) | 0.14 | <0.05 | 0.10 | ns | 0.09 | ns | 0.33 | <0.05 | 0.15 | <0.04 |
| FAC (%) | 0.21 | ns | 0.20 | ns | 0.18 | ns | 0.28 | ns | 0.23 | ns |
| 2D RVFWLS (%) | −0.28 | <0.01 | 0.27 | ns | 0.30 | ns | −0.17 | <0.001 | 0.29 | <0.01 |
| 2D RV4CLS (%) | −0.22 | <0.05 | 0.21 | ns | 0.28 | ns | −0.27 | <0.001 | 0.31 | <0.001 |
| Echocardiographic Parameters | Body Composition Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TP | TW | MIN | FM | LBM | ||||||
| r | p | r | p | r | p | r | p | r | p | |
| S’ (cm/s) | −0.11 | <0.01 | −0.07 | ns | −0.08 | ns | −0.04 | <0.01 | −0.10 | <0.001 |
| TAPSE, mm | −0.16 | ns | −0.12 | ns | −0.03 | ns | −0.01 | ns | −0.09 | ns |
| PASP, mmHg | 0.18 | <0.05 | 0.13 | ns | 0.10 | ns | 0.16 | <0.01 | 0.21 | <0.01 |
| RVBD (mm) | 0.07 | <0.01 | −0.02 | ns | 0.09 | ns | 0.12 | <0.001 | 0.07 | <0.01 |
| RVMD (mm) | 0.14 | <0.05 | 0.08 | ns | 0.12 | ns | 0.30 | <0.04 | 0.13 | <0.04 |
| FAC (%) | 0.23 | ns | 0.22 | ns | 0.14 | ns | 0.27 | ns | 0.26 | ns |
| 2D RVFWLS (%) | −0.27 | <0.01 | 0.29 | ns | 0.30 | ns | −0.23 | <0.01 | 0.28 | <0.01 |
| 2D RV4CLS (%) | −0.20 | <0.001 | 0.23 | ns | 0.25 | ns | −0.30 | <0.001 | 0.28 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sieradzka Uchnar, K.A.; Toth, S.; Schusterova, I.; Pella, D.; Gurbalova, S.; Poruban, T. Relationship Between Right Ventricular Function and Body Composition in Adolescents and Young Adults. Diagnostics 2025, 15, 2487. https://doi.org/10.3390/diagnostics15192487
Sieradzka Uchnar KA, Toth S, Schusterova I, Pella D, Gurbalova S, Poruban T. Relationship Between Right Ventricular Function and Body Composition in Adolescents and Young Adults. Diagnostics. 2025; 15(19):2487. https://doi.org/10.3390/diagnostics15192487
Chicago/Turabian StyleSieradzka Uchnar, Karolina Angela, Stefan Toth, Ingrid Schusterova, Dominik Pella, Silvia Gurbalova, and Tibor Poruban. 2025. "Relationship Between Right Ventricular Function and Body Composition in Adolescents and Young Adults" Diagnostics 15, no. 19: 2487. https://doi.org/10.3390/diagnostics15192487
APA StyleSieradzka Uchnar, K. A., Toth, S., Schusterova, I., Pella, D., Gurbalova, S., & Poruban, T. (2025). Relationship Between Right Ventricular Function and Body Composition in Adolescents and Young Adults. Diagnostics, 15(19), 2487. https://doi.org/10.3390/diagnostics15192487






