The Inflammatory–Dysplastic Spectrum in Oral Lichen Planus: A Study on Six Immunohistochemical Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Specimen Collection
2.2. Reagents and Antibodies
2.3. Tissue Processing and Staining Protocols
2.4. Evaluation of Immunohistochemical Staining
2.5. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics
3.2. Histological and Dysplasia Grades
3.3. Immunohistochemical Expression
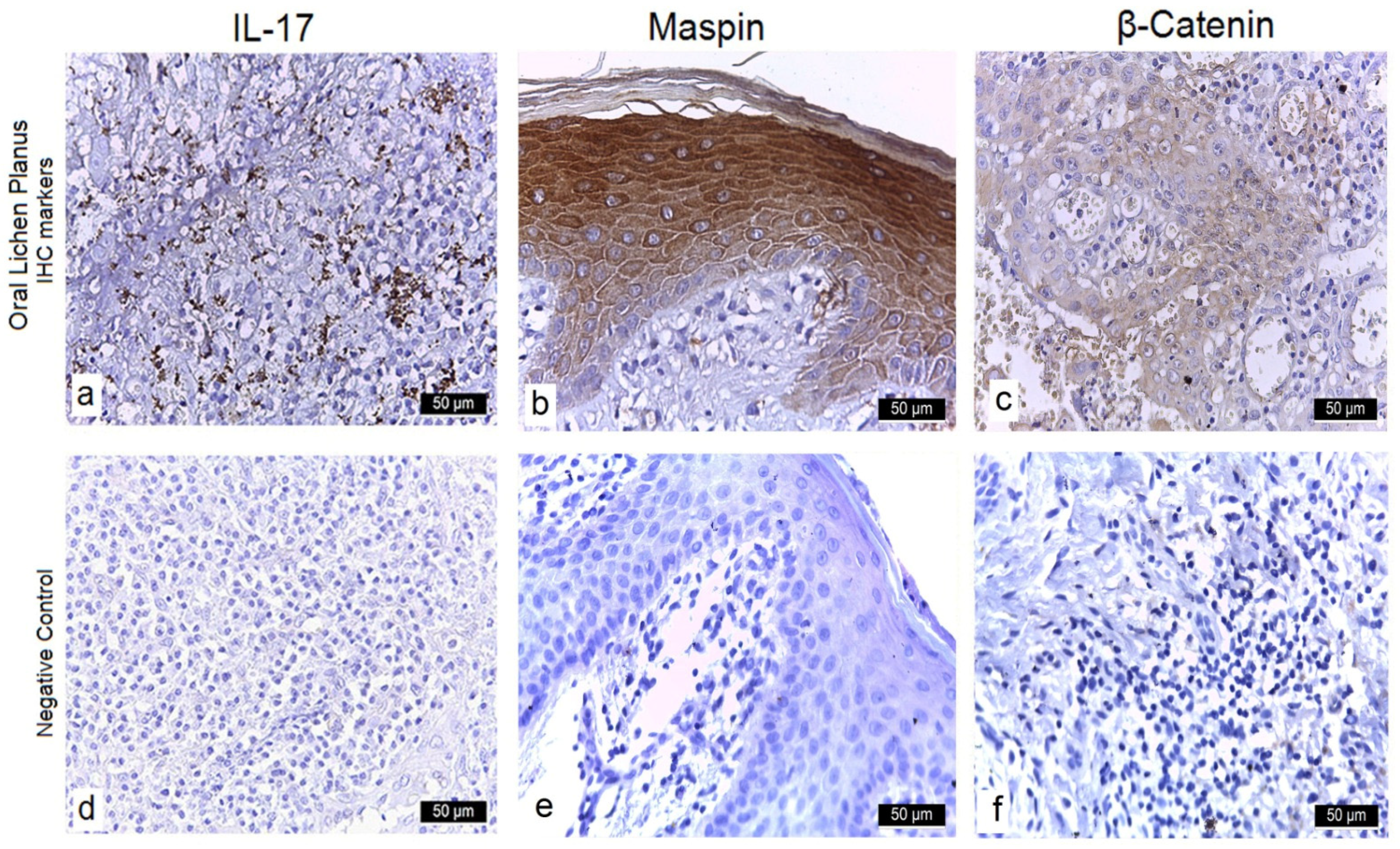
3.3.1. Interleukin-17 (IL-17)
3.3.2. Maspin
3.3.3. β-Catenin
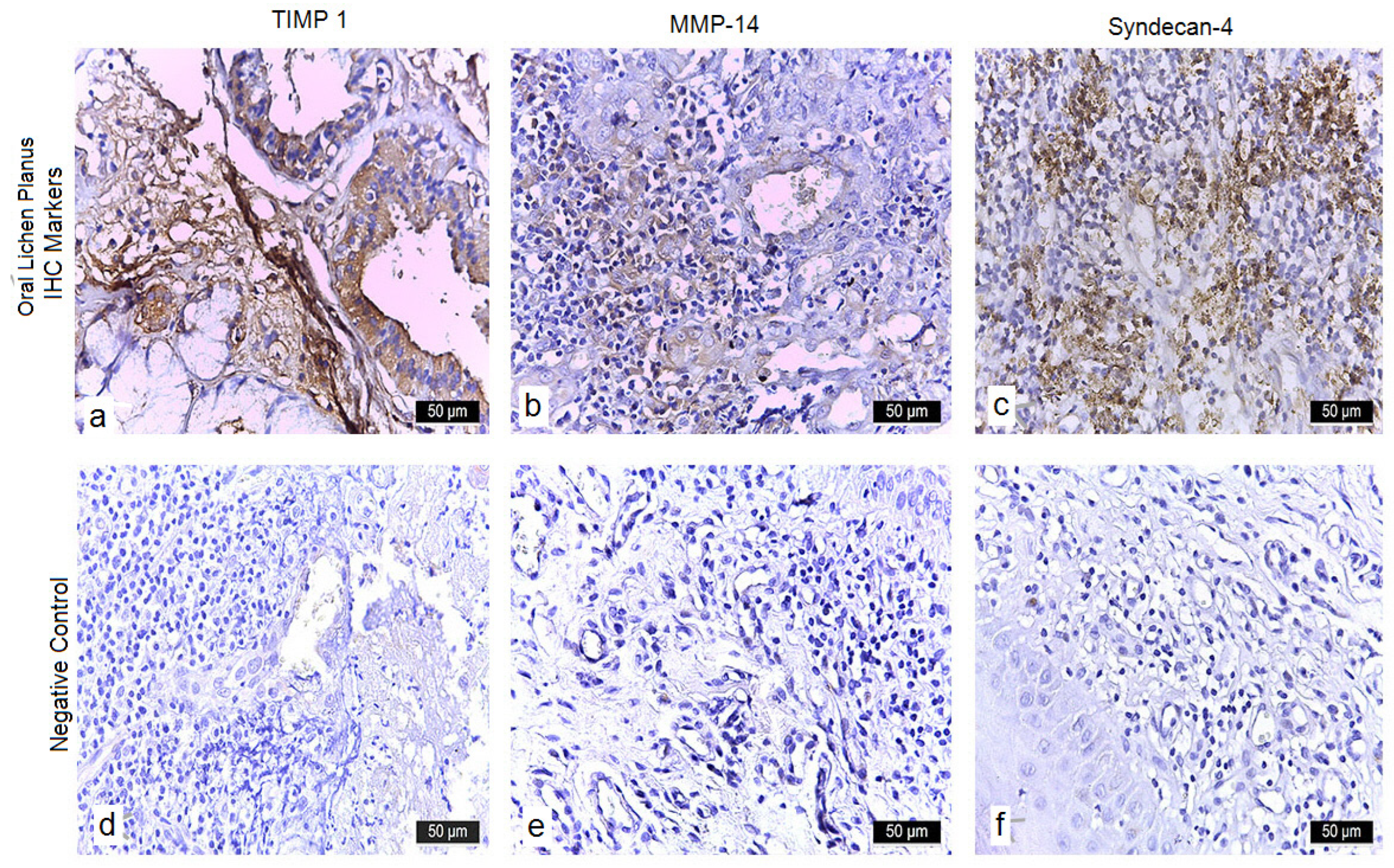
3.3.4. TIMP-1
3.3.5. MMP-14
3.3.6. Syndecan-4
3.4. Statistical Correlations
- β-Catenin and Dysplasia: Nuclear localization of β-catenin was observed predominantly in moderately dysplastic lesions (12.7% of cases), whereas it was uncommon in non-dysplastic cases (49.2% of the cohort) (χ2 test, p < 0.05).
- MMP-14 and Neovascularization: Positive MMP-14 expression showed a significant association with neovascularization in the lamina propria (χ2 test, p = 0.03), suggesting its role in early stromal remodeling.
- Syndecan-4 Loss and Dysplasia: Reduced Syndecan-4 expression was significantly correlated with the presence of epithelial dysplasia (50.8% of OLP cases) compared with non-dysplastic mucosa (49.2%) (χ2 test, p < 0.05), underscoring its role in maintaining epithelial polarity.
4. Discussion
4.1. Interpretation of Findings
4.2. Integration with Pathogenetic Mechanisms
4.3. Comparison with Existing Literature
4.4. Clinical Implications
4.5. Limitations
4.6. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OLP | Oral lichen planus |
| EMT | Epithelial–mesenchymal transition |
| IHC | Immunohistochemical |
| OPMD | Oral potentially malignant disorder |
| OSCC | Oral squamous cell carcinoma |
| H&E | Hematoxylin and eosin |
| PBS | Phosphate-buffered saline |
| DAB | 3,3′-Diaminobenzidine |
| Il-17 | Interleukin-17 |
| MMP-14 | Matrix metalloproteinase-14 |
References
- Carrozzo, M.; Thorpe, R. Oral lichen planus: A review. Minerva Stomatol. 2009, 58, 519–537. [Google Scholar] [PubMed]
- González-Moles, M.A.; Warnakulasuriya, S.; González-Ruiz, I.; González-Ruiz, L.; Ayén, Á.; Lenouvel, D.; Ruiz-Ávila, I.; Ramos-García, P. Clinicopathological and prognostic characteristics of oral squamous cell carcinomas arising in patients with oral lichen planus: A systematic review and a comprehensive meta-analysis. Oral. Oncol. 2020, 106, 104688. [Google Scholar] [CrossRef]
- Fitzpatrick, S.G.; Hirsch, S.A.; Gordon, S.C. The malignant transformation of oral lichen planus and oral lichenoid lesions: A systematic review. J. Am. Dent. Assoc. 2014, 145, 45–56. [Google Scholar] [CrossRef]
- Eisen, D. The clinical features, malignant potential, and systemic associations of oral lichen planus: A study of 723 patients. J. Am. Acad. Dermatol. 2002, 46, 207–214. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification. Oral. Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- Carbone, M.; Arduino, P.G.; Carrozzo, M.; Gandolfo, S.; Argiolas, M.R.; Bertolusso, G.; Conrotto, D.; Pentenero, M.; Broccoletti, R. Course of oral lichen planus: A retrospective study of 808 northern Italian patients. Oral. Dis. 2009, 15, 235–243. [Google Scholar] [CrossRef]
- Sugerman, P.B.; Savage, N.W.; Walsh, L.J.; Zhao, Z.Z.; Zhou, X.J.; Khan, A.; Seymour, G.J.; Bigby, M. The pathogenesis of oral lichen planus. Crit. Rev. Oral. Biol. Med. 2002, 13, 350–365. [Google Scholar] [CrossRef]
- Lavanya, N.; Jayanthi, P.; Rao, U.K.; Ranganathan, K. Oral lichen planus: An update on pathogenesis and treatment. J. Oral. Maxillofac. Pathol. 2011, 15, 127–132. [Google Scholar] [CrossRef]
- Zielińska, K.; Karczmarek-Borowska, B.; Kwaśniak, K.; Czarnik-Kwaśniak, J.; Ludwin, A.; Lewandowski, B.; Tabarkiewicz, J. Salivary IL-17A, IL-17F, and TNF-α are associated with disease advancement in patients with oral and oropharyngeal cancer. J. Immunol. Res. 2020, 2020, 3928504. [Google Scholar] [CrossRef]
- Wei, T.; Cong, X.; Wang, X.T.; Xu, X.J.; Min, S.N.; Ye, P.; Peng, X.; Wu, L.L.; Yu, G.Y. Interleukin-17A promotes tongue squamous cell carcinoma metastasis through activating miR-23b/versican pathway. Oncotarget 2017, 8, 6663–6680. [Google Scholar] [CrossRef]
- van der Meij, E.H.; van der Waal, I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J. Oral. Pathol. Med. 2003, 32, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Feldmeyer, L.; Suter, V.G.; Oeschger, C.; Cazzaniga, S.C.; Bornstein, M.M.; Simon, D.; Borradori, L.; Beltraminelli, H. Oral lichen planus and oral lichenoid lesions—An analysis of clinical and histopathological features. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23–IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef]
- Aggarwal, S.; Gurney, A.L. IL-17: Prototype member of an emerging cytokine family. J. Leukoc. Biol. 2002, 71, 1–8. [Google Scholar] [CrossRef]
- Miossec, P.; Kolls, J.K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Immunol. 2012, 12, 833–844. [Google Scholar] [CrossRef]
- Szatmári, T.; Ötvös, R.; Hjerpe, A.; Dobra, K. Syndecan-1 in cancer: Implications for cell signaling, differentiation, and prognostication. Dis. Markers. 2015, 2015, 796052. [Google Scholar] [CrossRef]
- Couchman, J.R. Syndecan-1 (CD138), carcinomas and EMT. Int. J. Mol. Sci. 2021, 22, 4227. [Google Scholar] [CrossRef]
- Couchman, J.R. Transmembrane signaling proteoglycans. Annu. Rev. Cell Dev. Biol. 2010, 26, 89–114. [Google Scholar] [CrossRef]
- Marioni, G.; Blandamura, S.; Giacomelli, L.; Calgaro, N.; Segato, P.; Leo, G.; Fischetto, D.; Staffieri, A.; de Filippis, C. Nuclear expression of maspin is associated with a lower recurrence rate and a longer disease-free interval after surgery for squamous cell carcinoma of the larynx. Histopathology 2005, 46, 576–582. [Google Scholar] [CrossRef]
- Condurache Hrițcu, O.M.; Ciobanu Apostol, D.G.; Toader, S.V.; Solcan, C.; Brănișteanu, D.E.; Toader, M.P.; Costan, V.V. Immunohistochemical assessment of maspin, β-catenin, and MMP-14 in oral potentially malignant lesions and oral squamous cell carcinoma: A retrospective observational study. Medicina 2025, 61, 1037. [Google Scholar] [CrossRef]
- Ramos-García, P.; González-Moles, M.Á. Prognostic and clinicopathological significance of the aberrant expression of β-catenin in oral squamous cell carcinoma: A systematic review and meta-analysis. Cancers 2022, 14, 479. [Google Scholar] [CrossRef]
- Balasundaram, P.; Singh, M.K.; Dinda, A.K.; Thakar, A.; Yadav, R. Study of β-catenin, E-cadherin and vimentin in oral squamous cell carcinoma with and without lymph node metastases. Diagn. Pathol. 2014, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Alamoud, K.A.; Kukuruzinska, M.A. Emerging insights into Wnt/β-catenin signaling in head and neck cancer. J. Dent. Res. 2018, 97, 665–673. [Google Scholar]
- Xie, J.; Huang, L.; Lu, Y.G.; Zheng, D.L. Roles of the Wnt signaling pathway in head and neck squamous cell carcinoma. Front. Mol. Biosci. 2021, 7, 590912. [Google Scholar] [CrossRef]
- Cathcart, J.; Pulkoski-Gross, A.; Cao, J. Targeting matrix metalloproteinases in cancer: Bringing new life to old ideas. Genes. Dis. 2015, 2, 26–34. [Google Scholar] [CrossRef]
- Kandhwal, M.; Behl, T.; Singh, S.; Sharma, N.; Arora, S.; Bhatia, S.; Al-Harrasi, A.; Sachdeva, M.; Bungau, S. Role of matrix metalloproteinase in wound healing. Am. J. Transl. Res. 2022, 14, 4391–4405. [Google Scholar]
- Agha-Hosseini, F.; Moosavi, M.S.; Bahrami, H. A systematic review of interleukin-17 in oral lichen planus: From etiopathogenesis to treatment. Clin. Med. Res. 2023, 21, 201–215. [Google Scholar] [CrossRef]
- Abusleme, L.; Moutsopoulos, N.M. IL-17; overview and role in oral immunity and microbiome. Oral. Dis. 2017, 23, 854–865. [Google Scholar] [CrossRef]
- Li, C.; Dong, X.; Li, B. Tumor microenvironment in oral squamous cell carcinoma. Front. Immunol. 2024, 15, 1485174. [Google Scholar] [CrossRef]
- Hara, T.; Sato, A.; Yamamoto, C.; Kaji, T. Syndecan-1 downregulates syndecan-4 expression by suppressing the ERK1/2 and p38 MAPK signaling pathways in cultured vascular endothelial cells. Biochem. Biophys. Rep. 2021, 26, 101001. [Google Scholar] [CrossRef]
- Barbouri, D.; Afratis, N.; Gialeli, C.; Vynios, D.H.; Theocharis, A.D.; Karamanos, N.K. Syndecans as modulators and potential pharmacological targets in cancer progression. Front. Oncol. 2014, 4, 4. [Google Scholar] [CrossRef]
- Martinoli, C.; Gandini, S.; Luise, C.; Mazzarol, G.; Confalonieri, S.; Pelicci, P.G.; Testori, A.; Ferrucci, P.F. Maspin expression and melanoma progression: A matter of sub-cellular localization. Mod. Pathol. 2014, 27, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Boorghani, M.; Gholizadeh, N.; Taghavi Zenouz, A.; Vatankhah, M.; Mehdipour, M. Oral lichen planus: Clinical features, etiology, treatment and management; a review of literature. J. Dent. Res. Dent. Clin. Dent. Prospects 2010, 4, 3–9. [Google Scholar] [PubMed]
- Reyes, M.; Flores, T.; Betancur, D.; Peña-Oyarzún, D.; Torres, V.A. Wnt/β-catenin signaling in oral carcinogenesis. Int. J. Mol. Sci. 2020, 21, 4682. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Saini, J.; Bakshi, J.; Sharma, M.; Alnemare, A.K.; Bin Mahfoz, T.; Goyal, A.K. Tissue inhibitor of metalloproteinase-1 (TIMP-1) expression and its prognostic significance in oral cancer patients. J. Maxillofac. Oral. Surg. 2025, 24, 690–698. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Warnakulasuriya, S.; González-Ruiz, I.; Ayén, Á.; González-Ruiz, L.; Ruiz-Ávila, I.; Ramos-García, P. Dysplasia in oral lichen planus: Relevance, controversies and challenges. A position paper. Med. Oral. Patol. Oral. Cir. Bucal. 2021, 26, e541–e548. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Ramos-García, P. An evidence-based update on the potential for malignancy of oral lichen planus and related conditions: A systematic review and meta-analysis. Cancers 2024, 16, 608. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Keim-del Pino, C.; Ramos-García, P. Hallmarks of cancer expression in oral lichen planus: A scoping review of systematic reviews and meta-analyses. Int. J. Mol. Sci. 2022, 23, 13099. [Google Scholar] [CrossRef]
- Agarwal, N.; Carnelio, S.; Rodrigues, G. Immunohistochemical and clinical significance of matrix metalloproteinase-2 and its inhibitor in oral lichen planus. J. Oral. Maxillofac. Pathol. 2019, 23, 476. [Google Scholar] [CrossRef]
- Larsen, K.R.; Johansen, J.D.; Reibel, J.; Zachariae, C.; Pedersen, A.M.L. Serum cytokine profile and clinicopathological findings in oral lichen planus, oral lichenoid lesions and stomatitis. Clin. Exp. Dent. Res. 2017, 3, 220–226. [Google Scholar] [CrossRef]
- Jiang, L.; Ma, Z.; Song, L.; Zhu, C.; Li, J.; Su, Z.; Liu, H. Expression of interleukin-17 in oral tongue squamous cell carcinoma and its effect on biological behavior. Sci. Rep. 2025, 15, 3195. [Google Scholar] [CrossRef] [PubMed]
- Ladjevac, N.; Milovanovic, M.; Jevtovic, A.; Arsenijevic, D.; Stojanovic, B.; Dimitrijevic Stojanovic, M.; Stojanovic, B.; Arsenijevic, N.; Arsenijevic, A.; Milovanovic, J. The role of IL-17 in the pathogenesis of oral squamous cell carcinoma. Int. J. Mol. Sci. 2023, 24, 9874. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saleh, H.A.; Nabil, G.; Badawy, S.A.M.M. Comparative immunohistochemical expression of β-catenin and CD163 between dysplastic/non-dysplastic oral lichen planus and lichenoid lesions (ex vivo study). BMC Oral. Health 2024, 24, 1122. [Google Scholar] [CrossRef] [PubMed]
- Nosratzehi, T.; Alijani, E.; Moodi, M. Salivary MMP-1, MMP-2, MMP-3 and MMP-13 levels in patients with oral lichen planus and squamous cell carcinoma. Asian Pac. J. Cancer Prev. 2017, 18, 1947–1951. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, W.; Zhang, Y.; Wang, X.; Liu, H.; Xu, S.; Zhao, Z.; Chen, D. Changes in the expression of Col IV, gelatinase and TIMP-1 in oral leukoplakia. Int. J. Clin. Exp. Pathol. 2017, 10, 8535–8543. [Google Scholar] [PubMed Central]
- Almutairi, S.; Kalloush, H.M.; Manoon, N.A.; Bardaweel, S.K. Matrix metalloproteinases inhibitors in cancer treatment: An updated review (2013–2023). Molecules 2023, 28, 5567. [Google Scholar] [CrossRef]
- Condurache Hrițcu, O.M.; Botez, A.E.; Olinici, D.T.; Onofrei, P.; Stoica, L.; Grecu, V.B.; Toader, P.M.; Gheucă-Solovăstru, L.; Cotrutz, E.C. Molecular markers associated with potentially malignant oral lesions. Exp. Ther. Med. 2021, 22, 834. [Google Scholar] [CrossRef]
| Characteristic | OLP Group (n = 63) | Control Group (n = 20) |
|---|---|---|
| Timeframe | January 2015–January 2023 | January 2015–January 2023 |
| Source | Archived biopsy specimens from the Department of Anatomical Pathology at “Sfântul Spiridon” Emergency Clinical Hospital | Normal oral mucosa obtained during minor surgical procedures (e.g., third molar extraction) |
| Inclusion Criteria | Age > 18 years; no other chronic illness; histopathologically confirmed OLP (modified WHO criteria) | Age > 18 years; no other chronic illness; clinically and histologically normal mucosa (no inflammation or dysplasia) |
| Exclusion Criteria | Current smokers or alcohol abuse; prior head and neck radiotherapy; immunosuppressive therapy; systemic inflammatory diseases; lack of histopathological confirmation; | Current smokers or alcohol abuse; prior head and neck radiotherapy; immunosuppressive therapy; systemic inflammatory diseases; lack of histopathological confirmation; |
| Marker | Clone | Host | Dilution | Supplier | Catalog No. |
|---|---|---|---|---|---|
| IL-17 | Polyclonal | Rabbit | 1:100 | Biorbyt, Cambridge, UK | ORB13500 |
| β-Catenin | SAB4500543 | Rabbit | 1:50 | Sigma-Aldrich, St. Louis, MO, USA | SAB4500543-100UG |
| Maspin | Clone C-8 | Mouse | 1:100 | Santa Cruz Biotechnology, Dallas, TX, USA | SC-271694 |
| MMP-14 | SAB4501901 | Rabbit | 1:100 | Sigma-Aldrich, St. Louis, MO, USA | SAB4501901-100UG |
| Syndecan-4 | SAB4502721 | Rabbit | 1:100 | Sigma-Aldrich, St. Louis, MO, USA | SAB4502721-100UG |
| TIMP-1 | HPA053417 | Rabbit | 1:100 | Atlas Antibodies, Bromma, Sweden | HPA053417-100UL |
| Affected Site | Number of Cases (n = 63) | Percentage (%) |
|---|---|---|
| Buccal mucosa | 56 | 89.2 |
| Lateral border of the tongue | 37 | 58.6 |
| Gingiva | 11 | 16.7 |
| Labial mucosa (lips) | 11 | 16.7 |
| Histopathological Feature | OLP Group (n = 63) | Control Group (n = 20) |
|---|---|---|
| Basal cell degeneration | Present in 63 (100%) | Absent |
| Saw-tooth rete ridges | Present in 63 (100%) | Absent |
| Band-like lymphocytic infiltrate | Present in 63 (100%) | Absent |
| Epithelial dysplasia—none | 31 (49.2%) | 20 (100%) |
| Epithelial dysplasia—mild | 24 (38.1%) | 0 (0%) |
| Epithelial dysplasia—moderate | 8 (12.7%) | 0 (0%) |
| Marker | Localization | OLP Group | Control Group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (0) | (+) | (++) | (+++) | (0) | (+) | (++) | (+++) | ||
| IL-17 | Cytoplasmic/Membranous | 0 (0.0%) | 11 (17.5%) | 0 (0.0%) | 52 (82.5%) | 20 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Maspin | Cytoplasmic/Nuclear | 0 (0.0%) | 0 (0.0%) | 5 (7.9%) | 58 (92.1%) | 19 (95.0%) | 1(5.0%) | 0 (0.0%) | 0(0.0%) |
| β-Catenin | Cytoplasmic/Nuclear | 0 (0.0%) | 0 (0.0%) | 7 (11.1%) | 56 (88.9%) | 20 (100.0%) | 0(0.0%) | 0 (0.0%) | 0 (0.0%) |
| TIMP-1 | Membranous/Cytoplasmic | 0 (0.0%) | 0 (0.0%) | 14 (22.2%) | 49 (77.8%) | 0 (0.0%) | 20 (100.0%) | 0 (0.0%) | 0 (0.0%) |
| MMP-14 | Cytoplasmic/Membranous | 0 (0.0%) | 63 (100%) | 0 (0.0%) | 0 (0.0%) | 20 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Syndecan-4 | Membranous | 0 (0.0%) | 0 (0.0%) | 12 (19.0%) | 51 (81.0%) | 0 (0.0%) | 20 (100.0%) | 0 (0.0%) | 0 (0.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Condurache Hrițcu, O.M.; Costan, V.-V.; Toader, Ș.V.; Ciobanu Apostol, D.G.; Solcan, C.; Brănișteanu, D.E.; Toader, M.P. The Inflammatory–Dysplastic Spectrum in Oral Lichen Planus: A Study on Six Immunohistochemical Markers. Diagnostics 2025, 15, 2443. https://doi.org/10.3390/diagnostics15192443
Condurache Hrițcu OM, Costan V-V, Toader ȘV, Ciobanu Apostol DG, Solcan C, Brănișteanu DE, Toader MP. The Inflammatory–Dysplastic Spectrum in Oral Lichen Planus: A Study on Six Immunohistochemical Markers. Diagnostics. 2025; 15(19):2443. https://doi.org/10.3390/diagnostics15192443
Chicago/Turabian StyleCondurache Hrițcu, Oana Mihaela, Victor-Vlad Costan, Ștefan Vasile Toader, Delia Gabriela Ciobanu Apostol, Carmen Solcan, Daciana Elena Brănișteanu, and Mihaela Paula Toader. 2025. "The Inflammatory–Dysplastic Spectrum in Oral Lichen Planus: A Study on Six Immunohistochemical Markers" Diagnostics 15, no. 19: 2443. https://doi.org/10.3390/diagnostics15192443
APA StyleCondurache Hrițcu, O. M., Costan, V.-V., Toader, Ș. V., Ciobanu Apostol, D. G., Solcan, C., Brănișteanu, D. E., & Toader, M. P. (2025). The Inflammatory–Dysplastic Spectrum in Oral Lichen Planus: A Study on Six Immunohistochemical Markers. Diagnostics, 15(19), 2443. https://doi.org/10.3390/diagnostics15192443







