Hepatic Focal Lesion Suspicious for Hepatocellular Carcinoma in a Patient with a History of Post-Traumatic Splenectomy: The Challenge of Differential Diagnosis with Intrahepatic Splenosis—Literature Review and Case Report
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
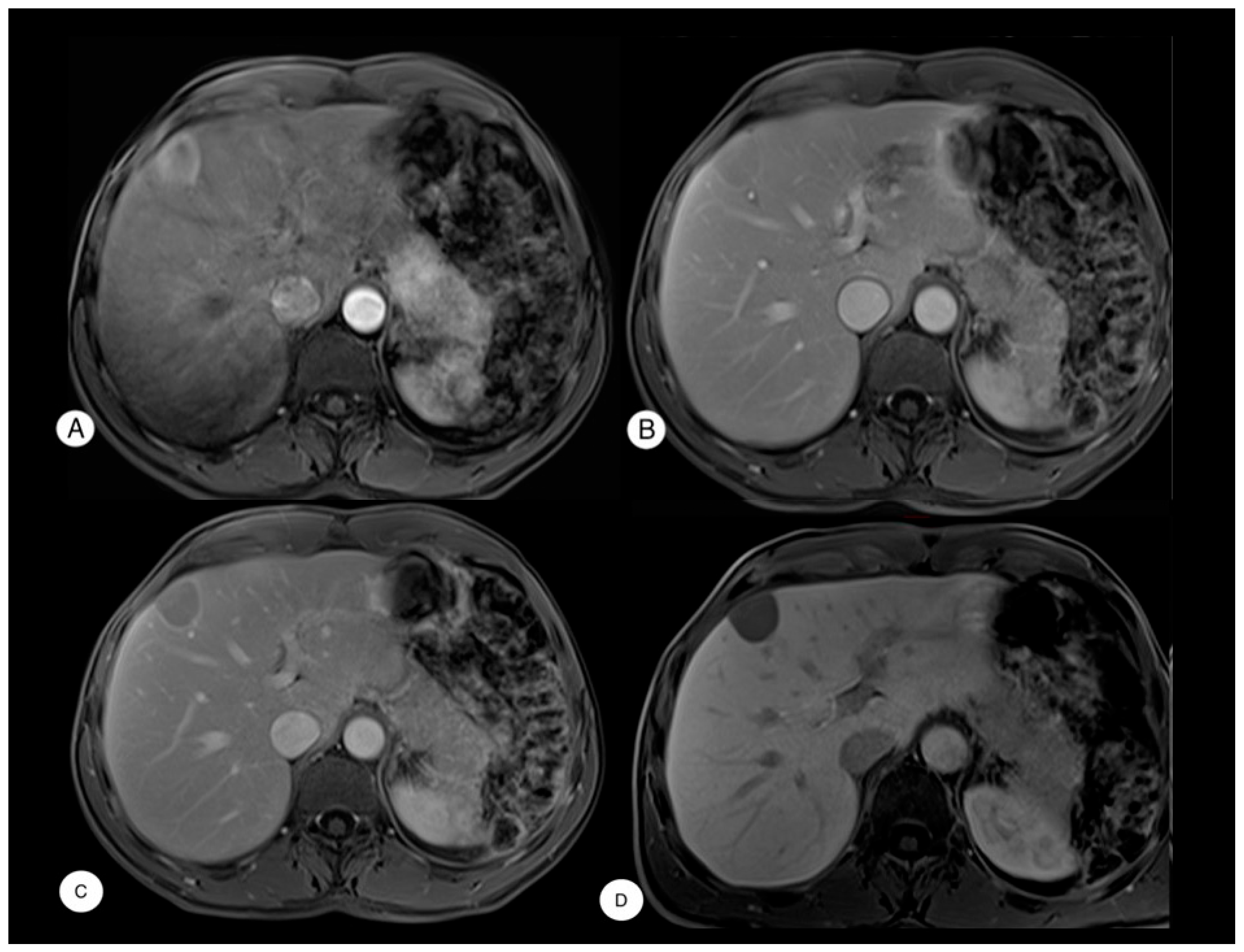
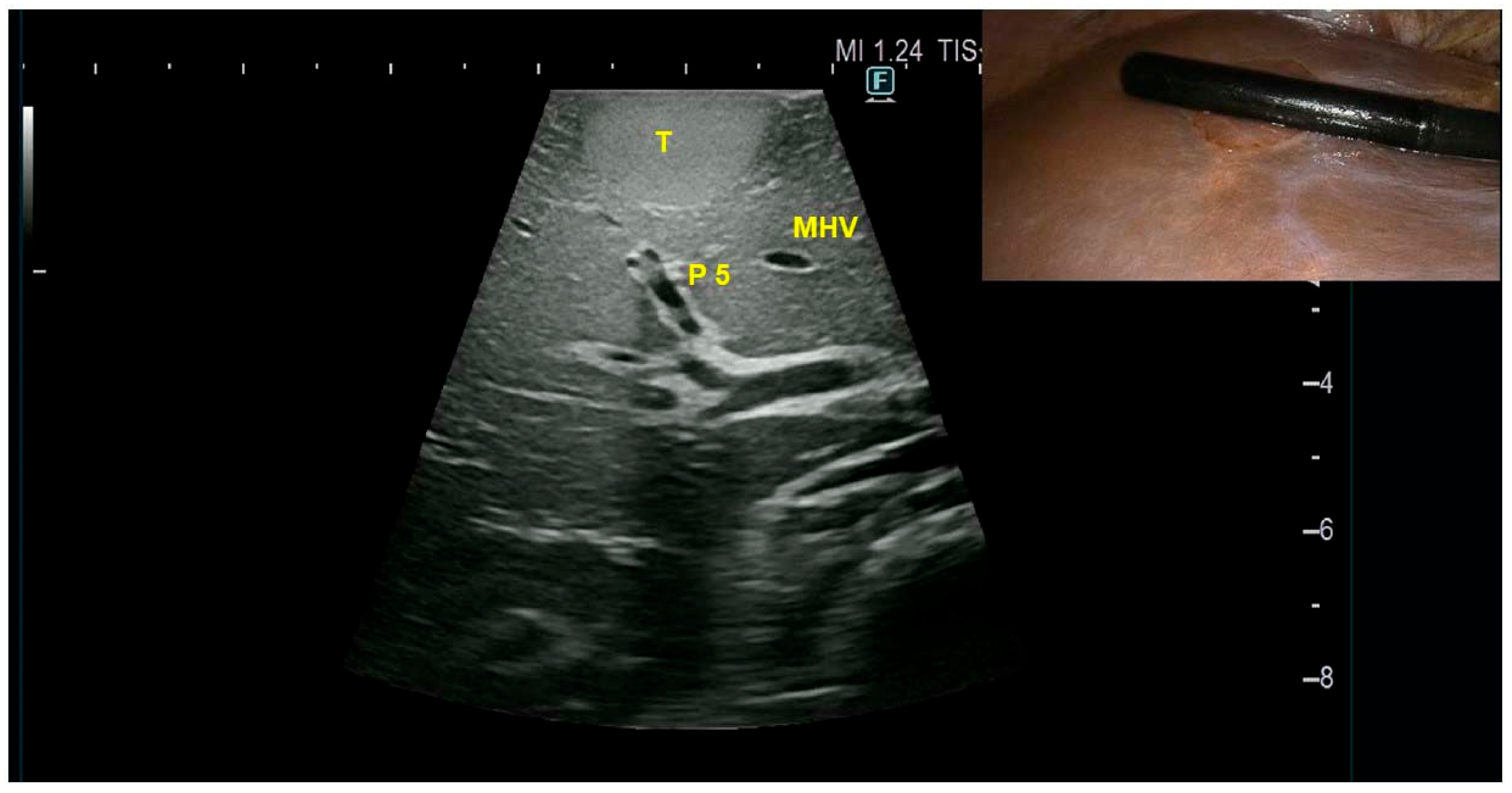
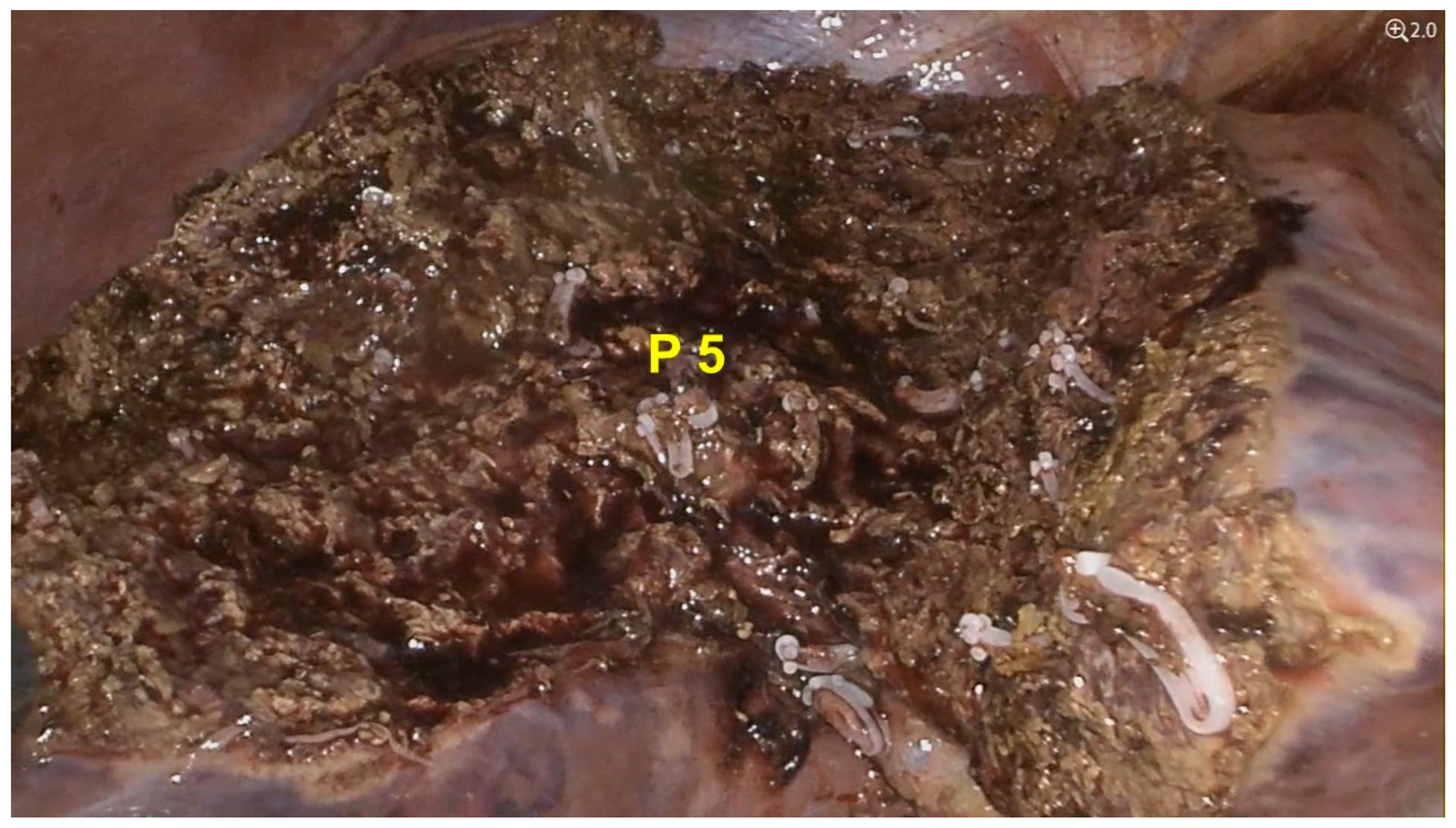
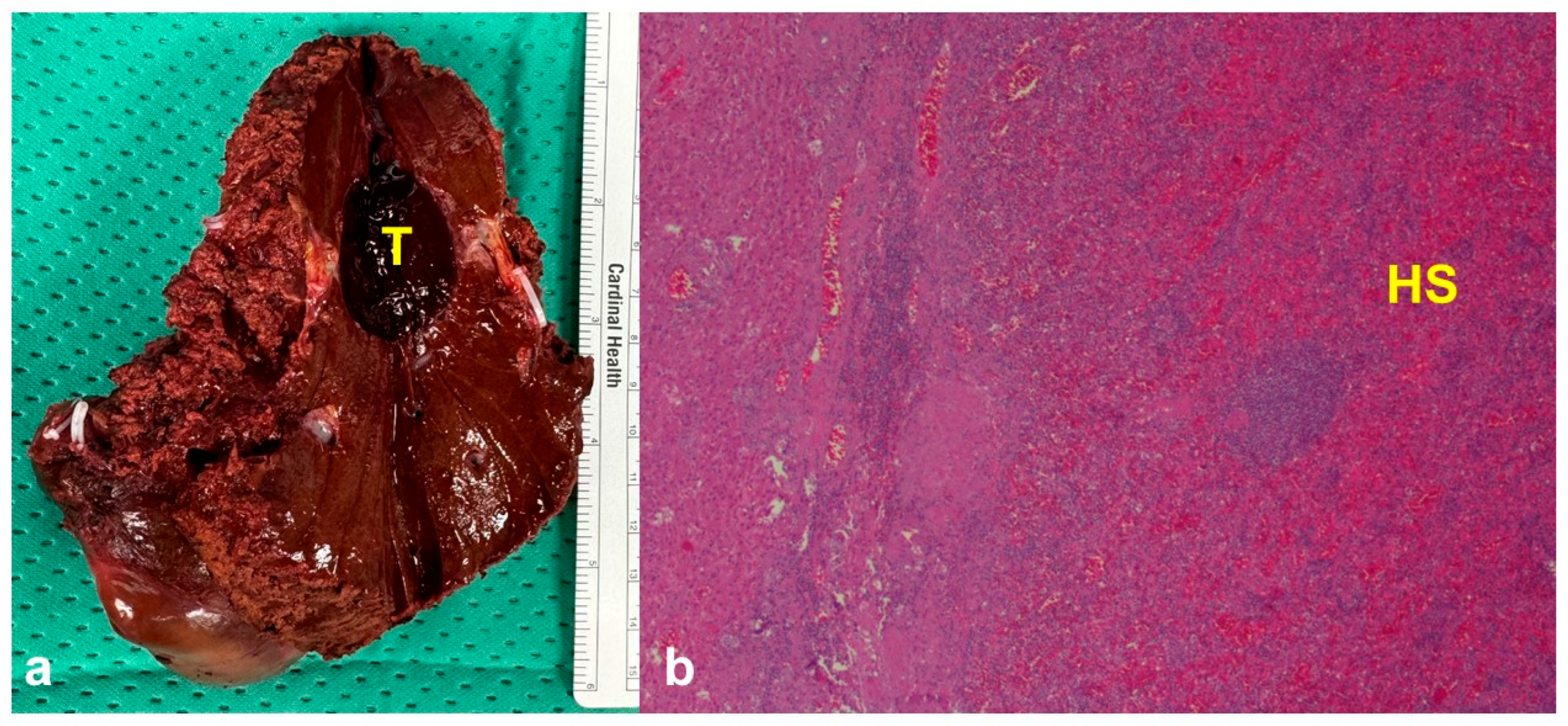
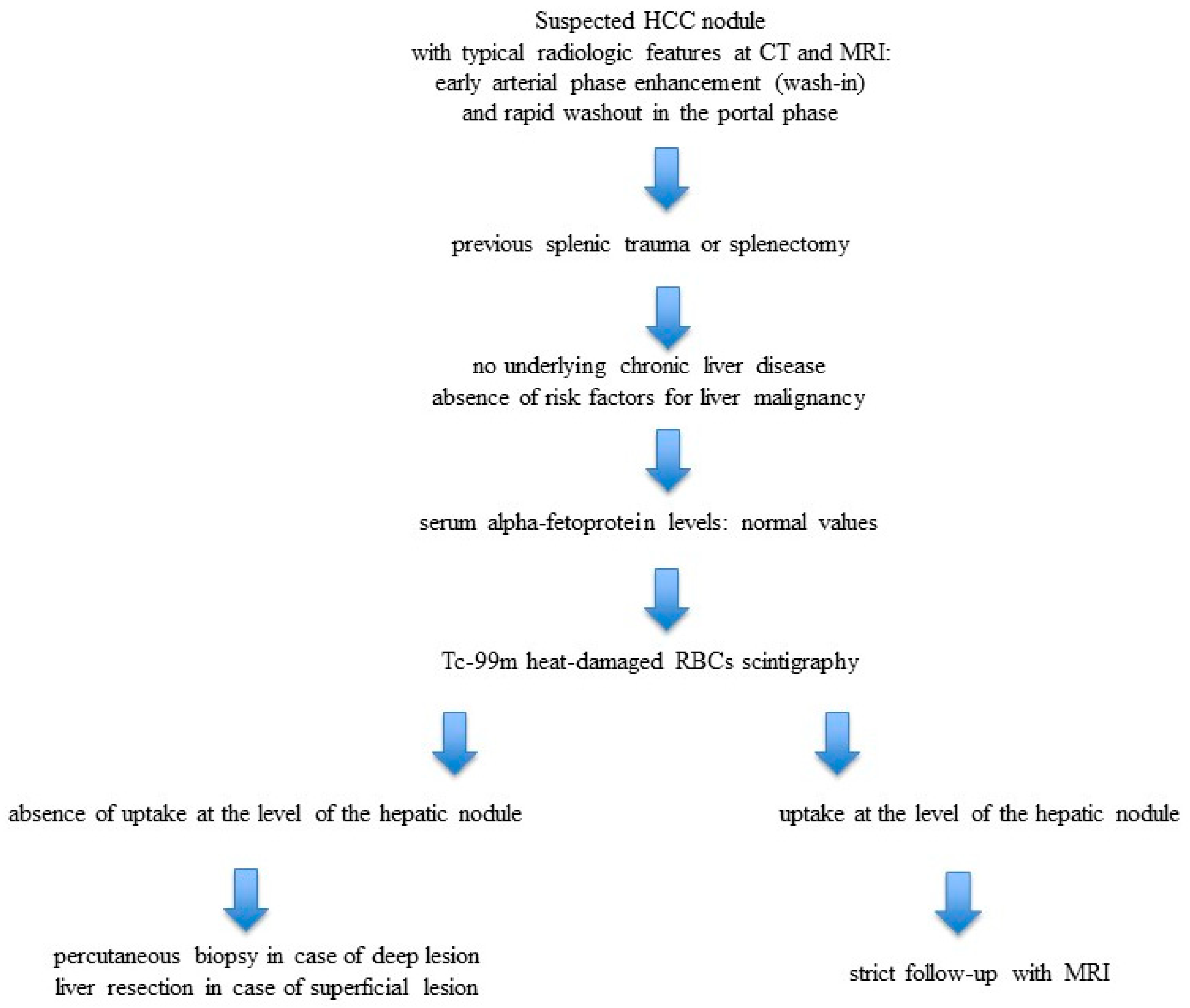
3. Results
Case Presentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| AFP | Alpha-fetoprotein |
| CEA | Carcinoembryonic antigen |
| CA19-9 | Carbohydrate antigen 19-9 |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HCC | Hepatocellular carcinoma |
| HS | Hepatic splenosis |
| IOUS | Intraoperative ultrasound |
| LI-RADS | Liver Imaging Reporting and Data System |
| MHV | Middle hepatic vein |
| MRI | Magnetic resonance imaging |
References
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J. Hepatol. 2025, 82, 315–374. [Google Scholar] [CrossRef] [PubMed]
- Teles, G.N.S.; Monteiro, P.E.Z.; Raphe, R. Intrahepatic splenosis mimicking hepatic neoplasia. Int. J. Surg. Case Rep. 2018, 44, 47–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, X.; Zeng, J.; Wang, Y.; Min, Y.; Shen, A.; Zhang, Y.; Deng, H.; Gong, N. Hepatic splenosis: Rare yet important—A case report and literature review. J. Int. Med. Res. 2019, 47, 1793–1801. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toh, W.S.; Chan, K.S.; Ding, C.S.L.; Tan, C.H.; Shelat, V.G. Intrahepatic splenosis: A world review. Clin. Exp. Hepatol. 2020, 6, 185–198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giuliante, F.; Ardito, F. Minimally invasive liver surgery in a hepato-biliary unit: Learning curve and indications. Updates Surg. 2015, 67, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Yoshimitsu, K.; Aibe, H.; Nobe, T.; Ezaki, T.; Tomoda, H.; Hayashi, I.; Koga, M. Intrahepatic splenosis mimicking a liver tumor. Abdom. Imaging 1993, 18, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Gruen, D.R.; Gollub, M.J. Intrahepatic splenosis mimicking hepatic adenoma. AJR Am. J. Roentgenol 1997, 168, 725–726. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.A.; Reid, I.N. Intrahepatic splenic tissue. J. Clin. Pathol. 1997, 50, 532–533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Angelica, M.; Fong, Y.; Blumgart, L.H. Isolated hepatic splenosis: First reported case. HPB Surg. 1998, 11, 39–42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Foroudi, F.; Ahern, V.; Peduto, A. Splenosis mimicking metastases from breast carcinoma. Clin. Oncol. 1999, 11, 190–192. [Google Scholar] [CrossRef] [PubMed]
- De Vuysere, S.; Van Steenbergen, W.; Aerts, R.; Van Hauwaert, H.; Van Beckevoort, D.; Van Hoe, L. Intrahepatic splenosis: Imaging features. Abdom. Imaging 2000, 25, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Pekkafali, Z.; Karsli, A.F.; Silit, E.; Başekim, C.C.; Narin, Y.; Mutlu, H.; Kizilkaya, E. Intrahepatic splenosis: A case report. Eur Radiol. 2002, 12 (Suppl. 3), S62–S65. [Google Scholar] [CrossRef] [PubMed]
- Gamulin, A.; Oberholzer, J.; Rubbia-Brandt, L.; Mentha, G. An unusual, presumably hepatic mass. Lancet 2002, 360, 2066. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Ryu, K.W.; Song, T.J.; Suh, S.O.; Kim, Y.C.; Koo, B.H.; Choi, S.Y. Hepatic splenosis diagnosed as hepatocellular carcinoma: Report of a case. Surg. Today 2002, 32, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Park, C.M.; Kim, C.H.; Choi, S.Y.; Park, S.W.; Kang, E.Y.; Seol, H.Y.; Cha, I.H. An interesting hepatic mass: Splenosis mimicking a hepatocellular carcinoma (2003:9b). Eur. Radiol. 2003, 13, 2713–2715. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, G.G.; Picciotto, F.P.; Marsilia, G.M.; Ascione, A. Hepatic splenosis misinterpreted as hepatocellular carcinoma in cirrhotic patients referred for liver transplantation: Report of two cases. Liver Transpl. 2004, 10, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Okazaki, H.; Takai, K.; Nishikawa, J.; Ohta, H.; Uekusa, T.; Yoshida, H.; Tanaka, K. Intrahepatic splenosis in a patient with chronic hepatitis C. J. Gastroenterol. 2004, 39, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Di Sarno, A.; Coppola, C.; Giorgio, A. Contrast-enhanced low-mechanical-index ultrasonography in hepatic splenosis. J. Ultrasound Med. 2006, 25, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.L.; Wang, L.Y.; Huang, C.I.; Hsieh, M.Y.; Lin, Z.Y.; Chuang, W.L.; Chang, W.T.; Wu, C.C.; Chen, C.Y. Abdominal splenosis mimicking hepatic tumor: A case report. Kaohsiung J. Med. Sci. 2008, 24, 602–606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, H.C.; Su, C.W.; Lu, C.L.; Chang, C.P.; Lin, H.C. Hepatic splenosis diagnosed by spleen scintigraphy. Am. J. Gastroenterol. 2008, 103, 1842–1844. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.H.; Ju, M.K.; Kim, J.Y.; Kang, C.M.; Kim, K.S.; Choi, J.S.; Han, K.H.; Park, M.S.; Park, Y.N.; Lee, W.J.; et al. Hepatic splenosis preoperatively diagnosed as hepatocellular carcinoma in a patient with chronic hepatitis B: A case report. J. Korean Med. Sci. 2008, 23, 336–341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grande, M.; Lapecorella, M.; Ianora, A.A.; Longo, S.; Rubini, G. Intrahepatic and widely distributed intraabdominal splenosis: Multidetector CT, US and scintigraphic findings. Intern. Emerg. Med. 2008, 3, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Imbriaco, M.; Camera, L.; Manciuria, A.; Salvatore, M. A case of multiple intra-abdominal splenosis with computed tomography and magnetic resonance imaging correlative findings. World J. Gastroenterol. 2008, 14, 1453–1455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakajima, T.; Fujiwara, A.; Yamaguchi, M.; Makiyama, A.; Wakae, T.; Fujita, K.; Yoshikawa, K.; Shiomi, T.; Ohishi, T.; Nakashima, T.; et al. Intrahepatic splenosis with severe iron deposition presenting with atypical magnetic resonance images. Intern. Med. 2008, 47, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xia, L.; Li, T.; Ju, M.; Liu, L.; Wu, Z.; Tang, Z. Intrahepatic splenosis mimicking hepatoma. BMJ Case Rep. 2009, 2009, bcr0620080230, Erratum in: BMJ Case Rep. 2009, 2009, bcr0620080230corr1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abu Hilal, M.; Harb, A.; Zeidan, B.; Steadman, B.; Primrose, J.N.; Pearce, N.W. Hepatic splenosis mimicking HCC in a patient with hepatitis C liver cirrhosis and mildly raised alpha feto protein; the important role of explorative laparoscopy. World J. Surg. Oncol. 2009, 7, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kashgari, A.A.; Al-Mana, H.M.; Al-Kadhi, Y.A. Intrahepatic splenosis mimicking hepatocellular carcinoma in a cirrhotic liver. Saudi Med. J. 2009, 30, 429–432. [Google Scholar] [PubMed]
- Menth, M.; Herrmann, K.; Haug, A.; Raziorrouh, B.; Zach Oval, R.; Jung, C.M.; Otto, C. Intra-hepatic splenosis as an unexpected cause of a focal liver lesion in a patient with hepatitis C and liver cirrhosis: A case report. Cases J. 2009, 2, 8335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mescoli, C.; Castoro, C.; Sergio, A.; Ruol, A.; Farinati, F.; Rugge, M. Hepatic spleen nodules (HSN). Scand. J. Gastroenterol. 2010, 45, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Tsitouridis, I.; Michaelides, M.; Sotiriadis, C.; Arvaniti, M. CT and MRI of intraperitoneal splenosis. Diagn. Interv. Radiol. 2010, 16, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.C.; Cho, G.S.; Chung, G.A.; Kang, G.H.; Kim, Y.J.; Lee, M.S.; Kim, H.K.; Park, S.J. Intrahepatic splenosis mimicking liver metastasis in a patient with gastric cancer. J. Gastric Cancer. 2011, 11, 64–68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Ji, B.; Wang, G.; Wang, Y. Abdominal multiple splenosis mimicking liver and colon tumors: A case report and review of the literature. Int. J. Med. Sci. 2012, 9, 174–177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Snow-Lisy, D.; Klein, E.A. Hepatic splenosis diagnosed after inappropriate metastatic evaluation in patient with low-risk prostate cancer. Urology 2012, 79, e73–e74. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liang, Y.; Liang, X.; Yu, H.; Wang, Y.; Cai, X. Laparoscopic resection of isolated hepatic splenosis mimicking liver tumors: Case report with a literature review. Surg. Laparosc. Endosc. Percutaneous Tech. 2012, 22, e307–e311. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, R.; Peddu, P.; Karani, J. Hepatic splenosis presenting as arterialised liver lesion in a patient with NASH. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2853–2856. [Google Scholar] [PubMed]
- Krawczyk, M.; Schneider, G.; Farmakis, G.; Zimmer, V.; Lammert, F. Splenosis mimicking hepatic adenoma. J. Clin. Exp. Hepatol. 2013, 3, 351–352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Röther, M.; Dufour, J.F.; Schnüriger, B. An uncommon cause of a focal liver lesion. Post-traumatic splenosis. Gastroenterology 2013, 144, 510, 659. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.W.; Menon, T.; Rao, S. Post-traumatic intrahepatic splenosis mimicking a neuroendocrine tumour. BMJ Case Rep. 2013, 2013, bcr2012007885. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kandil, T.S.; El Sorogy, M.; Naiem, Y.; Elkashef, W.F. Post-splenectomy splenosis presenting as hepatocellular carcinoma in the left lateral section of the liver: A case report. Int. J. Surg. Case Rep. 2014, 5, 877–878. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sato, N.; Abe, T.; Suzuki, N.; Waragai, M.; Teranishi, Y.; Takano, Y.; Sato, A.; Azami, A.; Gotoh, M. Intrahepatic splenosis in a chronic hepatitis C patient with no history of splenic trauma mimicking hepatocellular carcinoma. Am. J. Case Rep. 2014, 15, 416–420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tinoco González, J.; Suárez Artacho, G.; Ramallo Solís, I.M.; Padillo Ruiz, F.J.; Angel, M. Intrahepatic splenosis as a differential diagnosis in focal liver lesions. Cir. Esp. 2014, 92, 690–691, (In English, Spanish). [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, B.; Chen, L.; Zhang, B.; Chen, X. Solitary perihepatic splenosis mimicking liver lesion: A case report and literature review. Medicine 2015, 94, e586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, C.; Liu, J.; Wang, F. Intrahepatic splenosis mimicking liver cancer: Report of a case and review of literature. Int. J. Clin. Exp. Pathol. 2015, 8, 1031–1035. [Google Scholar] [PubMed] [PubMed Central]
- Li, T.; Yang, X.Y.; Tang, Z.Y. Intrahepatic and intraperitoneal splenosis mimicking hepatocellular carcinoma with abdominal wall metastasis in a patient with hepatitis C cirrhotic liver. Surgery 2015, 157, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Tamm, A.; Decker, M.; Hoskinson, M.; Abele, J.; Patel, V. Heat-damaged RBC scan: A case of intrahepatic splenosis. Clin. Nucl. Med. 2015, 40, 453–454. [Google Scholar] [CrossRef] [PubMed]
- Grambow, E.; Weinrich, M.; Zimpfer, A.; Kloker, K.; Klar, E. Ectopic Spleen Tissue—An Underestimated Differential Diagnosis of a Hypervascularised Liver Tumour. Viszeralmedizin 2015, 31, 445–447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toktaş, O.; Yavuz, A.; İliklerden, Ü.; Yılmaz, D.; Bayram, İ. Intrahepatic splenosis after splenectomy performed for idiopathic thrombocytopenic purpura. Ulus. Cerrahi Derg. 2015, 31, 247–249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fung, A.; Chok, K.; Lo, A.; Lo, C.M. Hepatobiliary and Pancreatic: Hepatic splenosis: A rare differential of a liver mass in an HBV endemic area. J. Gastroenterol. Hepatol. 2016, 31, 1238. [Google Scholar] [CrossRef] [PubMed]
- He, Z.L.; Xu, X.; Ke, Q.H.; Zheng, S.S. Successful diagnosis of intrahepatic splenosis mimicking hepatic tumor. Kaohsiung J. Med. Sci. 2016, 32, 224–225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jereb, S.; Trotovsek, B.; Skrbinc, B. Hepatic splenosis mimicking liver metastases in a patient with history of childhood immature teratoma. Radiol. Oncol. 2016, 50, 212–217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Riggi, M.A.; Fusco, F.; Fantini, C.; D’Agostino, A.; Cioffi, L.; Russo, G.; Belli, G. Laparoscopic surgical treatment of hepatic splenosis. A case report. Ann. Ital. Chir. 2016, 87, S2239253X16024889. [Google Scholar] [PubMed]
- Wang, M.Y.; Li, B.; Chen, D.; Liu, A.L.; Qamar, S.; Sun, M.Y. Spleen implanting in the fatty liver mimicking hepatocarcinoma in a patient with hepatitis B&C: A case report and literature review. Medicine 2017, 96, e7217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keck, C.; Shetty, A.; Mischen, B.; Willner, I. Hepatic Splenosis Masquerading as Hepatocellular Carcinoma in a Chronic Hepatitis C Patient. Am. J. Gastroenterol. 2017, 112, 1493. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; Li, X.F.; Yan, Z.L.; Wang, Y.; Ma, J.Y.; Shi, L.H.; Zhang, X.F. Intrahepatic splenosis mimics hepatocellular carcinoma in a patient with chronic hepatitis B: A case report and literature review. Medicine 2017, 96, e8680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Somsap, K.; Chamadol, N.; Titapun, A.; Pairojkul, C.; Sangkhamanon, S. MR imaging findings of a patient with isolated intrahepatic splenosis mistaken for hepatocellular carcinoma. BJR Case Rep. 2016, 3, 20150242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aramoana, J.; Wylie, N.; Koea, J.B. Hepatic splenosis. ANZ J. Surg. 2018, 88, E359–E360. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; Ginolfi, F.; Moscati, S.; Giordano, B.; Ferrara, N.; Panico, C.; Imbriaco, M. Multiple intra-hepatic and abdominal splenosis: An easy call if you know about it. Acta Radiol. Open. 2018, 7, 2058460118772324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guzman Rojas, P.; Parikh, J.; Mahne, A.; Vishnubhotla, P.; Oharriz, J.J. Where is My Spleen?—A Case of Splenosis Diagnosed Years Later after Splenectomy. Cureus 2018, 10, e2618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Budak, E.; Oral, A.; Yazici, B.; Guler, E.; Omur, O. Splenosis of the Liver Capsule. Clin. Nucl. Med. 2018, 43, e460–e462. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.; Bergson, J.; Yaipen, O. Intrahepatic Splenosis: Incidental Liver Lesion after Splenectomy. J. Comput. Assist. Tomogr. 2018, 42, 730–731. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Z.; Chen, J.; Song, P.; Du, Y.; Wang, L.; Wan, D.; Zheng, S. Management of intrahepatic splenosis:a case report and review of the literature. World J. Surg. Oncol. 2018, 16, 119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smolen, B.; Khoury, J.; Baruch, Y.; Saadi, T. Non-invasive evaluation of a liver mass in a patient post splenectomy. Scott. Med. J. 2019, 64, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Guedes, T.P.; Fernandes, B.; Pedroto, I. Hepatobiliary and Pancreatic: Symptomatic hepatic splenosis. J. Gastroenterol. Hepatol. 2019, 34, 2061. [Google Scholar] [CrossRef] [PubMed]
- Ananthan, K.; Yusuf, G.T.; Kumar, M. Intrahepatic and intra-abdominal splenosis: A case report and review of literature. World J. Hepatol. 2019, 11, 773–779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kosydar, S.R.; Sanchirico, P.J.; Pfeiffer, D.C. A case of thoracoabdominal splenosis. Radiol. Case Rep. 2019, 15, 7–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martín-Marcuartu, J.J.; Fernández-Rodríguez, P.; Tirado-Hospital, J.L.; Jiménez-Hoyuela, J.M. Labeled heat-denatured red blood cell scintigraphy in hepatic splenosis in a cirrhotic patient. Cir. Esp. 2020, 98, 158, (In English, Spanish). [Google Scholar] [CrossRef] [PubMed]
- Sansone, V.; Falsetti, L.; Tovoli, F.; Golfieri, R.; Cescon, M.; Piscaglia, F. An Uncommon Focal Liver Lesion: Intrahepatic Splenosis. J. Gastrointest. Liver Dis. 2020, 29, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Kawada, S.; Ichikawa, T.; Ueda, H.; Ito, K.; Inoue, K.; Mori, K. A case of intrahepatic splenosis: Usefulness of splenic scintigraphy. Abdom. Radiol. 2020, 45, 2274–2278. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Yang, L.; Huang, J.; Deng, L.; Nie, L.; Lu, Q. Contrast-enhanced ultrasonographic imaging of hepatic splenosis: A case report. Medicine 2021, 100, e24243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Richardson, L.; Gardner, K.; Eberhardt, S.; Thompson, W. A case of hepatic splenosis in the setting of iron overload; multimodal and literature review. Radiol. Case Rep. 2021, 16, 2499–2504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Djekidel, M.; Michalski, M. Hybrid Imaging with SPECT-CT and SPECT-MR in Hepatic Splenosis. J. Nucl. Med. Technol. 2021; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Connor, S.; Hamilton, S.; Hore, T.; Sakowska, M. Rare case of intrahepatic splenosis masquerading as malignancy. J. Med. Imaging Radiat. Oncol. 2022, 66, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Pessarelli, T.; Crespi, S.; Antonelli, B.; Maggioni, M.; Iavarone, M. Hepatic splenosis mimicking hepatocellular carcinoma in metabolic associated fatty liver disease. Dig. Liver Dis. 2022, 54, 1725–1726. [Google Scholar] [CrossRef] [PubMed]
- Peethambaran, M.S.P.; Matthew, C.; Rajendran, R.R. Accessory Spleen Mimicking an Intrahepatic Neoplasm: A Rare Case Report. Cureus. 2023, 15, e39185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Umeda, I.; Tomihara, H.; Maeda, S.; Kitagawa, A.; Miyamoto, K.; Kawabata, R.; Nishikawa, K.; Noura, S.; Yasuhara, Y.; Miyamoto, A. A rare intrahepatic splenosis mimicking hepatocellular carcinoma: A case report. Mol. Clin. Oncol. 2023, 19, 91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosi, M.; Adotti, V.; Citone, M.; Marra, F. Intrahepatic splenosis mimicking hepatocellular carcinoma. United Eur. Gastroenterol. J. 2024, 12, 412–414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Z.Y.; Zheng, R.Y.; Zhang, C. A case of multiple hepatic splenoses? Asian J. Surg. 2025, 48, 1323–1324. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.; Kawashita, Y.; Tateishi, M.; Ueda, T.; Yamaguchi, J.; Washida, Y.; Hachitanda, Y. Masquerading Spleen: A Perplexing Case of Intrahepatic Splenosis Mimicking Hepatocellular Carcinoma. Cureus 2024, 16, e70145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Escribano Cruz, S.; Garrido Gómez, E.; García Latorre, R.; García González, M. Hepatic splenosis, description of an unusual behavior on contrast-enhanced ultrasonography (CEUS). Rev. Esp. Enferm. Dig. 2024, 116, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Marrana, F.; Tavares, C.; Furtado, A.; Moreira Marques, T.; Faria, G. From a Suspicious Liver Mass to Splenosis: A Case Report. Cureus. 2025, 17, e79141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Graziani, T.; Baldari, G.; Sammartano, A.; Scarlattei, M.; Migliari, S.; Pescarenico, M.G.; Cidda, C.; Bola, S.; Ruffini, L. SPECT/CT with 99mTc labelled heat-denatured erythrocyte to detect thoracic and abdominal splenosis. Acta Biomed. 2020, 91, e2020098. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holzgreve, A.; Völter, F.; Delker, A.; Kunz, W.G.; Fabritius, M.P.; Brendel, M.; Albert, N.L.; Bartenstein, P.; Unterrainer, M.; Unterrainer, L.M. Detection of Splenic Tissue Using 99mTc-Labelled Denatured Red Blood Cells Scintigraphy-A Quantitative Single Center Analysis. Diagnostics 2022, 12, 486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Author (Year) | Age/Sex | Previous Splenectomy | Alpha-Fetoprotein (ng/mL) | Primary Diagnosis | 99mTc Scintigraphy | Treatment | Final Diagnosis |
|---|---|---|---|---|---|---|---|
| Yoshimitsu K (1993) [6] | 51/F | Yes | Normal | HCC | No | Surgery | HS |
| Gruen DR (1997) [7] | 38/F | Yes | N/A | Adenoma vs. FNH | No | Surgery | HS |
| Davidson LA (1997) [8] | 54/M | Yes | N/A | N/A | No | Necropsy | HS |
| D’ Angelica M (1998) [9] | 38/F | Yes | N/A | Adenoma vs. FNH | No | Surgery | HS |
| Foroudi F (1999) [10] | 59/M | Yes | N/A | Metastasis | Yes | - | HS |
| De Vuysere S (2000) [11] | 50/M | Yes | Normal | HS | No | Biopsy | HS |
| Pekkafali Z (2002) [12] | 21/M | Yes | N/A | HS | Yes | - | HS |
| Gamulin A (2002) [13] | 49/M | Yes | N/A | Lymphoma | No | Surgery | HS |
| Lee JB (2002) [14] | 43/M | Yes | Normal | HCC | No | Surgery | HS |
| Kim KA (2003) [15] | 43/M | Yes | Normal | HCC | No | Surgery | HS |
| Di Costanzo GG (2004) [16] | 58/M | Yes | 412 | HCC | Yes | Biopsy | HS |
| Di Costanzo GG (2004) [16] | 48/F | Yes | 327 | HCC | No | Biopsy | HS |
| Kondo M (2004) [17] | 55/M | Yes | N/A | HCC | No | Biopsy | HS |
| Ferraioli G (2006) [18] | 40/M | Yes | Normal | HS | No | Biopsy | HS |
| Yeh ML (2008) [19] | 64/M | Yes | Normal | HCC | No | Surgery | HS |
| Lu HC (2008) [20] | 59/M | Yes | Normal | HS | Yes | - | HS |
| Choi GH (2008) [21] | 32/M | Yes | 17.3 | HCC | No | Surgery | HS |
| Grande M (2008) [22] | 41/M | Yes | Normal | HS | Yes | - | HS |
| Imbriaco M (2008) [23] | 39/M | Yes | N/A | Hepatic Tumor | No | Surgery | HS |
| Nakajima T (2008) [24] | 41/M | Yes | N/A | HS | No | Biopsy | HS |
| Yu H (2009) [25] | 54/M | Yes | Normal | Hepatoma | No | Surgery | HS |
| Abu Hilal M (2009) [26] | 60/M | Yes | Mild rise | HCC | No | Surgery | HS |
| Kashgari AA (2009) [27] | 52/M | Yes | Normal | HCC | No | Biopsy | HS |
| Menth M (2009) [28] | 43/M | Yes | 6.4 | HCC | Yes | - | HS |
| Mescoli C (2010) [29] | 68/F | No | N/A | FNH | No | Biopsy | HS |
| Mescoli C (2010) [29] | 54/M | Yes | N/A | Metastasis | No | Surgery | HS |
| Tsitouridis I (2010) [30] | 63/M | Yes | N/A | HS | No | Surgery | HS |
| Tsitouridis I (2010) [30] | 64/M | Yes | N/A | Peritoneal implant | No | Biopsy | HS |
| Kang KC (2011) [31] | 54/M | Yes | N/A | Metastasis | No | Surgery | HS |
| Liu Y (2012) [32] | 49/F | Yes | N/A | Metastasis | No | Surgery | HS |
| Li H (2012) [33] | 61/M | Yes | N/A | HS | No | Biopsy | HS |
| Liu K (2012) [34] | 38/M | Yes | Normal | Hepatic Tumor | No | Surgery | HS |
| Inchingolo R (2013) [35] | 53/M | Yes | Normal | HCC vs. Adenoma | No | Surgery | HS |
| Krawczyk M (2013) [36] | 39/F | Yes | N/A | HS | Yes | - | HS |
| Röther M (2013) [37] | 62/M | Yes | Normal | HCC | No | Surgery | HS |
| Leong CW (2013) [38] | 56/M | Yes | N/A | Carcinoid NET | No | Surgery | HS |
| Kandil TS (2014) [39] | 45/F | Yes | Normal | HCC | No | Surgery | HS |
| Sato N (2014) [40] | 58/M | No | Elevated | HCC | No | Surgery | HS |
| Tinoco Gonzales J, (2014) [41] | 60/M | Yes | N/A | HCC | No | Surgery | HS |
| Wu C (2015) [42] | 33/M | Yes | Normal | HCC | No | Surgery | HS |
| Liu C (2015) [43] | 33/M | Yes | Normal | HCC | No | Biopsy | HS |
| Li T (2015) [44] | 67/F | Yes | Elevated | HCC | No | Surgery | HS |
| Tamm A (2015) [45] | 43/M | Yes | N/A | Suspicious nodule | Yes | - | HS |
| Grambow E (2015) [46] | 53/M | Yes | Normal | HCC | No | Surgery | HS |
| Toktas O (2015) [47] | 40/F | Yes | N/A | Suspicious nodule | No | Surgery | HS |
| Fung A (2016) [48] | 55/M | Yes | Normal | Hepatic Tumor | No | Surgery | HS |
| He ZL (2016) [49] | 51/M | Yes | N/A | Metastasis | No | Biopsy | HS |
| Jereb S (2016) [50] | 22/M | Yes | Normal | Metastasis | No | Biopsy | HS |
| De Riggi MA (2016) [51] | 31/M | Yes | N/A | Hepatic Tumor | No | Surgery | HS |
| Wang MY (2017) [52] | 42/M | Yes | 1.531 | HCC | No | Surgery | HS |
| Keck C (2017) [53] | 66/M | Yes | Normal | HS | No | Biopsy | HS |
| Wang WC (2017) [54] | 54/M | Yes | Normal | HCC | No | Surgery | HS |
| Somsap K (2017) [55] | 51/M | Yes | Normal | HCC | No | Surgery | HS |
| Teles GNS (2018) [2] | 73/M | Yes | Normal | Hepatic Tumor | No | Surgery | HS |
| Aramoana J (2018) [56] | 58/M | Yes | N/A | HS | No | Surgery | HS |
| Vergara D (2018) [57] | 69/M | Yes | Normal | HS | No | Biopsy | HS |
| Guzman Rojas P (2018) [58] | 43/M | Yes | N/A | Adenoma | No | Biopsy | HS |
| Budak E (2018) [59] | 46/M | Yes | N/A | HCC vs. HS | Yes | - | HS |
| Varghese J (2018) [60] | 50/M | Yes | N/A | Suspicious nodule | No | CT splenic-like enhancement | HS |
| Xuan Z (2018) [61] | 54/M | Yes | Normal | HCC | No | Surgery | HS |
| Smolen B (2019) [62] | 35/M | Yes | Normal | Adenoma vs. FNH | Yes | - | HS |
| Guedes TP (2019) [63] | 68/M | Yes | Normal | HCC vs. adenoma | No | Surgery | HS |
| Ananthan K (2019) [64] | 57/M | No | Normal | HS | Yes | - | HS |
| Kosydar SR (2019) [65] | 38/M | Yes | N/A | HS | No | - | HS |
| Luo X (2019) [3] | 41/M | Yes | Normal | Metastasis | No | Surgery | HS |
| Martín-Marcuartu JJ, (2020) [66] | 66/M | Yes | N/A | HS | Yes | Liver transplantation for other reason | HS |
| Sansone V (2020) [67] | 46/M | Yes | N/A | Adenoma vs. Hemangioma | No | Surgery | HS |
| Kawada S (2020) [68] | 39/M | Yes | N/A | Suspicious nodule | Yes | Surgery | HS |
| Zhong X (2021) [69] | 55/M | Yes | N/A | Suspicious nodule | No | Surgery | HS |
| Richardson L (2021) [70] | 68/M | Yes | N/A | HS | No | - | HS |
| Djekidel M (2021) [71] | 29/F | Yes | N/A | Hepatic Tumor | Yes | - | HS |
| Anderson M (2022) [72] | 54/M | Yes | N/A | Hepatic Tumor | Yes | - | HS |
| Pessarelli T (2022) [73] | 56/M | Yes | N/A | HCC | No | Surgery | HS |
| M S P (2023) [74] | 57/M | Yes | N/A | Suspicious nodule | No | Surgery | HS |
| Umeda I (2023) [75] | 46/M | Yes | Normal | HCC | No | Surgery | HS |
| Rosi M (2024) [76] | 50/M | Yes | N/A | HCC | No | Surgery | HS |
| Hu ZY (2024) [77] | 53/M | N/A | N/A | Hepatic Tumor | No | Surgery | HS |
| Ikeda N (2024) [78] | 56/F | Yes | Normal | HCC | No | Surgery | HS |
| Escribano Cruz S (2024) [79] | 47/M | Yes | N/A | Adenoma | No | Biopsy | HS |
| Marrana F (2025) [80] | 51/M | Yes | N/A | Adenoma | No | Surgery | HS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanzafame, A.; Perrone, G.; Campisi, A.; Razionale, F.; Panettieri, E.; Genco, E.; Giustiniani, M.C.; Coppola, A.; Giuliante, F.; Ardito, F. Hepatic Focal Lesion Suspicious for Hepatocellular Carcinoma in a Patient with a History of Post-Traumatic Splenectomy: The Challenge of Differential Diagnosis with Intrahepatic Splenosis—Literature Review and Case Report. Diagnostics 2025, 15, 2442. https://doi.org/10.3390/diagnostics15192442
Lanzafame A, Perrone G, Campisi A, Razionale F, Panettieri E, Genco E, Giustiniani MC, Coppola A, Giuliante F, Ardito F. Hepatic Focal Lesion Suspicious for Hepatocellular Carcinoma in a Patient with a History of Post-Traumatic Splenectomy: The Challenge of Differential Diagnosis with Intrahepatic Splenosis—Literature Review and Case Report. Diagnostics. 2025; 15(19):2442. https://doi.org/10.3390/diagnostics15192442
Chicago/Turabian StyleLanzafame, Andrea, Giulio Perrone, Andrea Campisi, Francesco Razionale, Elena Panettieri, Enza Genco, Maria Cristina Giustiniani, Alessandro Coppola, Felice Giuliante, and Francesco Ardito. 2025. "Hepatic Focal Lesion Suspicious for Hepatocellular Carcinoma in a Patient with a History of Post-Traumatic Splenectomy: The Challenge of Differential Diagnosis with Intrahepatic Splenosis—Literature Review and Case Report" Diagnostics 15, no. 19: 2442. https://doi.org/10.3390/diagnostics15192442
APA StyleLanzafame, A., Perrone, G., Campisi, A., Razionale, F., Panettieri, E., Genco, E., Giustiniani, M. C., Coppola, A., Giuliante, F., & Ardito, F. (2025). Hepatic Focal Lesion Suspicious for Hepatocellular Carcinoma in a Patient with a History of Post-Traumatic Splenectomy: The Challenge of Differential Diagnosis with Intrahepatic Splenosis—Literature Review and Case Report. Diagnostics, 15(19), 2442. https://doi.org/10.3390/diagnostics15192442







