A Relapsed AML Case Featuring MYC and MECOM Rearrangements
Abstract
1. Introduction
2. Case Report
2.1. Case Presentation
2.2. Materials and Methods
2.2.1. Conventional Cytogenetics
2.2.2. Molecular Cytogenetics
2.3. Cytogenetic Results
2.3.1. Conventional Cytogenetics
2.3.2. Molecular Cytogenetics
3. Discussion
Review of the Literature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chakraborty, S.; Park, C.Y. Pathogenic Mechanisms in Acute Myeloid Leukemia. Curr. Treat. Options Oncol. 2022, 23, 1522–1534. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P. Diagnosis and Management of AML in Adults: 2022 Recommendations from an International Expert Panel on Behalf of the ELN. Blood J. Am. Soc. Hematol. 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Ozkan, E.; Lacerda, M.P. Genetics, Cytogenetic Testing and Conventional Karyotype; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Acute Myeloid Leukemia; Version 2; NCCN: Plymouth Meeting, PA, USA, 2025; Available online: https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf (accessed on 11 May 2025).
- Vosberg, S.; Greif, P.A. Clonal Evolution of Acute Myeloid Leukemia from Diagnosis to Relapse. Genes. Chromosomes Cancer 2019, 58, 839–849. [Google Scholar] [CrossRef]
- Rapaport, F.; Neelamraju, Y.; Baslan, T.; Hassane, D.; Gruszczynska, A.; Robert de Massy, M.; Farnoud, N.; Haddox, S.; Lee, T.; Medina-Martinez, J. Genomic and Evolutionary Portraits of Disease Relapse in Acute Myeloid Leukemia. Leukemia 2021, 35, 2688–2692. [Google Scholar] [CrossRef]
- Kim, N.; Hahn, S.; Choi, Y.J.; Cho, H.; Chung, H.; Jang, J.E.; Lyu, C.J.; Lee, S.-T.; Choi, J.R.; Cheong, J.-W. Comprehensive Insights into AML Relapse: Genetic Mutations, Clonal Evolution, and Clinical Outcomes. Cancer Cell Int. 2024, 24, 174. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tirado, C.A. A t (3; 8)(Q26. 2; Q24) Involving the EVI1 (MECOM) Gene. J. Assoc. Genet. Technol. 2018, 44, 92–99. [Google Scholar]
- Tang, G.; Hu, S.; Wang, S.A.; Xie, W.; Lin, P.; Xu, J.; Toruner, G.; Zhao, M.; Gu, J.; Doty, M. T(3;8)(Q26.2;Q24) Often Leads to MECOM/MYC Rearrangement and Is Commonly Associated with Therapy-Related Myeloid Neoplasms and/or Disease Progression. J. Mol. Diagn. 2019, 21, 343–351. [Google Scholar] [CrossRef]
- Smith, S.C.; Qdaisat, T.Z.S.; Althof, P.A.; Dave, B.J.; Sanmann, J.N. MECOM Rearrangement Involving the MYC Locus: Two Additional Patients with the Rare Translocation, t(3;8)(Q26.2;Q24), and Molecular Review. Leuk. Res. 2020, 95, 106387. [Google Scholar] [CrossRef]
- McGinnis, E.; Stubbins, R.J.; Li, D.; Hamadeh, Z.; Spence, T.J. Optical Genome Mapping As Standard-of-Care in Acute Leukemia: Diagnostic and Clinical Impacts 10 Months Post-Implementation. Blood 2024, 144, 1538. [Google Scholar] [CrossRef]
- Cheung, E.; Schumann, C.; Zhang, T.Y.; Fakhri, B.; Gotlib, J.; Liedtke, M.; Shomali, W.; Mannis, G. Cladribine and Low-Dose Cytarabine-Based Salvage Therapy for Relapsed/Refractory AML in a Predominantly Venetoclax-Exposed Cohort. Blood 2023, 142, 5927. [Google Scholar] [CrossRef]
- Delia, M.; Pastore, D.; Carluccio, P.; Pasciolla, C.; Ricco, A.; Rossi, A.R.; Casieri, P.; Mestice, A.; Albano, F.; Specchia, G. FLAG-Ida Regimen as Bridge Therapy to Allotransplantation in Refractory/Relapsed Acute Myeloid Leukemia Patients. Clin. Lymphoma Myeloma Leuk. 2017, 17, 767–773. [Google Scholar] [CrossRef]
- Issa, G.C.; Zarka, J.; Sasaki, K.; Qiao, W.; Pak, D.; Ning, J.; Short, N.J.; Haddad, F.; Tang, Z.; Patel, K.P. Predictors of Outcomes in Adults with Acute Myeloid Leukemia and KMT2A Rearrangements. Blood Cancer J. 2021, 11, 162. [Google Scholar] [CrossRef]
- Iacobucci, I.; Mullighan, C.G. KMT2A-Rearranged Leukemia: The Shapeshifter. Blood J. Am. Soc. Hematol. 2022, 140, 1833–1835. [Google Scholar] [CrossRef]
- Boddu, P.; Pemmaraju, N.; Tang, G. MYC Rearrangements in Myeloid Neoplasms. Blood 2017, 130, 5077. [Google Scholar]
- Tang, R.; Cheng, A.; Guirales, F.; Yeh, W.; Tirado, C.A. C-MYC Amplification in AML. J. Assoc. Genet. Technol. 2021, 47, 202–212. [Google Scholar]
- Call, S.G.; Duren, R.P.; Panigrahi, A.K.; Nguyen, L.; Freire, P.R.; Grimm, S.L.; Coarfa, C.; Conneely, O.M. Targeting Oncogenic Super Enhancers in MYC-Dependent AML Using a Small Molecule Activator of NR4A Nuclear Receptors. Sci. Rep. 2020, 10, 2851. [Google Scholar] [CrossRef]
- Cho, S.W.; Xu, J.; Sun, R.; Mumbach, M.R.; Carter, A.C.; Chen, Y.G.; Yost, K.E.; Kim, J.; He, J.; Nevins, S.A. Promoter of LncRNA Gene PVT1 Is a Tumor-Suppressor DNA Boundary Element. Cell 2018, 173, 1398–1412. [Google Scholar] [CrossRef]
- Tolomeo, D.; Agostini, A.; Visci, G.; Traversa, D.; Storlazzi, C.T. PVT1: A Long Non-Coding RNA Recurrently Involved in Neoplasia-Associated Fusion Transcripts. Gene 2021, 779, 145497. [Google Scholar] [CrossRef]
- Lugthart, S.; Gröschel, S.; Beverloo, H.B.; Kayser, S.; Valk, P.J.M.; van Zelderen-Bhola, S.L.; Jan Ossenkoppele, G.; Vellenga, E.; van den Berg-de Ruiter, E.; Schanz, U. Clinical, Molecular, and Prognostic Significance of WHO Type Inv(3)(Q21q26.2)/t(3;3)(Q21;Q26.2) and Various Other 3q Abnormalities in Acute Myeloid Leukemia. J. Clin. Oncol. 2010, 28, 3890–3898. [Google Scholar] [CrossRef]
- Ottema, S.; Mulet-Lazaro, R.; Beverloo, H.B.; Erpelinck, C.; van Herk, S.; van der Helm, R.; Havermans, M.; Grob, T.; Valk, P.J.M.; Bindels, E. Atypical 3q26/MECOM Rearrangements Genocopy Inv(3)/t(3;3) in Acute Myeloid Leukemia. Blood J. Am. Soc. Hematol. 2020, 136, 224–234. [Google Scholar] [CrossRef]
- Lennon, P.A.; Abruzzo, L.V.; Medeiros, L.J.; Cromwell, C.; Zhang, X.; Yin, C.C.; Kornblau, S.M.; Konopieva, M.; Lin, P. Aberrant EVI1 Expression in Acute Myeloid Leukemias Associated with the t(3;8)(Q26;Q24). Cancer Genet. Cytogenet. 2007, 177, 37–42. [Google Scholar] [CrossRef]
- Pei Lin T(3;8)(Q26;Q24) PVT1/MECOM. Available online: http://atlasgeneticsoncology.org/haematological/1463/t(3;8)(q26;q24)-pvt1-mecom (accessed on 12 May 2025).
- Papadopoulou, V.; Schoumans, J.; Scarpelli, I.; Blum, S. Description of an Institutional Cohort of Myeloid Neoplasms Carrying ETV6-Locus Deletions or ETV6 Rearrangements. Acta Haematol. 2023, 146, 401–407. [Google Scholar] [CrossRef]
- Yip, B.H.; Steeples, V.; Repapi, E.; Armstrong, R.N.; Llorian, M.; Roy, S.; Shaw, J.; Dolatshad, H.; Taylor, S.; Verma, A. The U2AF1 S34F Mutation Induces Lineage-Specific Splicing Alterations in Myelodysplastic Syndromes. J. Clin. Investig. 2017, 127, 2206–2221. [Google Scholar] [CrossRef]
- Badar, T.; Vanegas, Y.A.M.; Nanaa, A.; Foran, J.M.; Al-Kali, A.; Mangaonkar, A.; Murthy, H.; Alkhateeb, H.B.; Viswanatha, D.; He, R. U2AF1 Pathogenic Variants in Myeloid Neoplasms and Precursor States: Distribution of Co-Mutations and Prognostic Heterogeneity. Blood Cancer J. 2023, 13, 149. [Google Scholar] [CrossRef]
- Hu, Z.; Medeiros, L.J.; Wang, W.; Chen, Z.; Tang, G.; Hodjat, P.; Yang, S.; Fang, L.; Li, Y.; Verstovsek, S. 3q26. 2/EVI1 Rearrangement Is Associated with Poor Prognosis in Classical Philadelphia Chromosome-Negative Myeloproliferative Neoplasms. Mod. Pathol. 2017, 30, 940–951. [Google Scholar] [CrossRef][Green Version]
- Tang, Z.; Tang, G.; Hu, S.; Patel, K.P.; Yin, C.C.; Wang, W.; Lin, P.; Toruner, G.A.; Ok, C.Y.; Gu, J. Deciphering the Complexities of MECOM Rearrangement-Driven Chromosomal Aberrations. Cancer Genet. 2019, 233, 21–31. [Google Scholar] [CrossRef]
- Liu, K.; Tirado, C.A. MECOM: A Very Interesting Gene Involved Also in Lymphoid Malignancies. J. Assoc. Genet. Technol. 2019, 45, 109–114. [Google Scholar]
- Ohanian, M.; Rozovski, U.; Kanagal-Shamanna, R.; Abruzzo, L.V.; Loghavi, S.; Kadia, T.; Futreal, A.; Bhalla, K.; Zuo, Z.; Huh, Y.O. MYC Protein Expression Is an Important Prognostic Factor in Acute Myeloid Leukemia. Leuk Lymphoma 2019, 60, 37–48. [Google Scholar] [CrossRef]
- Mertens, F.; Johansson, B.; Billström, R.; Engquist, L.; Mitelman, F. A Case of Myelodysplastic Syndrome with High Platelet Counts and at (3; 8)(Q26; Q24). Cancer Genet. Cytogenet. 1987, 27, 1–4. [Google Scholar] [CrossRef]
- Baldazzi, C.; Luatti, S.; Zuffa, E.; Papayannidis, C.; Ottaviani, E.; Marzocchi, G.; Ameli, G.; Bardi, M.A.; Bonaldi, L.; Paolini, R. Complex Chromosomal Rearrangements Leading to MECOM Overexpression Are Recurrent in Myeloid Malignancies with Various 3q Abnormalities. Genes Chromosomes Cancer 2016, 55, 375–388. [Google Scholar] [CrossRef]
- Kendrick, T.S.; Buic, D.; Fuller, K.A.; Erber, W.N. Abnormalities in Chromosomes 5 and 7 in Myelodysplastic Syndrome and Acute Myeloid Leukemia. Ann. Lab. Med. 2025, 45, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.; Baughn, L.B.; Akkari, Y.; Chartrand, S.; LaBarge, B.; Claxton, D.; Lennon, P.A.; Cujar, C.; Kolhe, R.; Kroeger, K. Optical Genome Mapping in Acute Myeloid Leukemia: A Multicenter Evaluation. Blood Adv. 2023, 7, 1297–1307. [Google Scholar] [CrossRef]
- Levy, B.; Kanagal-Shamanna, R.; Sahajpal, N.S.; Neveling, K.; Rack, K.; Dewaele, B.; Olde Weghuis, D.; Stevens-Kroef, M.; Puiggros, A.; Mallo, M. A Framework for the Clinical Implementation of Optical Genome Mapping in Hematologic Malignancies. Am. J. Hematol. 2024, 99, 642–661. [Google Scholar] [CrossRef]
- Zhao, L.-P.; Dumas-Rivero, T.; Barette, L.; Aguinaga, L.; Cheffai, A.; Chauvel, C.; Dal Bello, R.; Raffoux, E.; Clappier, E.; Duchmann, M. Prognostic Significance of Monocytic-like Phenotype in Patients with AML Treated with Venetoclax and Azacytidine. Blood Adv. 2025, 9, 3556–3565. [Google Scholar] [CrossRef]
- Döhner, H.; Pratz, K.W.; DiNardo, C.D.; Wei, A.H.; Jonas, B.A.; Pullarkat, V.A.; Thirman, M.J.; Récher, C.; Schuh, A.C.; Babu, S. Genetic Risk Stratification and Outcomes among Treatment-Naive Patients with AML Treated with Venetoclax and Azacitidine. Blood 2024, 144, 2211–2222. [Google Scholar] [CrossRef]
- Issa, G.C.; Aldoss, I.; Thirman, M.J.; DiPersio, J.; Arellano, M.; Blachly, J.S.; Mannis, G.N.; Perl, A.; Dickens, D.S.; McMahon, C.M. Menin Inhibition with Revumenib for KMT2A-Rearranged Relapsed or Refractory Acute Leukemia (AUGMENT-101). J. Clin. Oncol. 2025, 43, 75–84. [Google Scholar] [CrossRef]
- Cluzeau, T.; Guolo, F.; Chiche, E.; Minetto, P.; Rahme, R.; Bertoli, S.; Fianchi, L.; Micol, J.-B.; Gottardi, M.; Peterlin, P. Long-Term Real-World Evidence of CPX-351 of High-Risk Patients with AML Identified High Rate of Negative MRD and Prolonged OS. Blood Adv. 2025, 9, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Leisch, M.; Jansko, B.; Zaborsky, N.; Greil, R.; Pleyer, L. Next Generation Sequencing in AML—on the Way to Becoming a New Standard for Treatment Initiation and/or Modulation? Cancers 2019, 11, 252. [Google Scholar] [CrossRef] [PubMed]
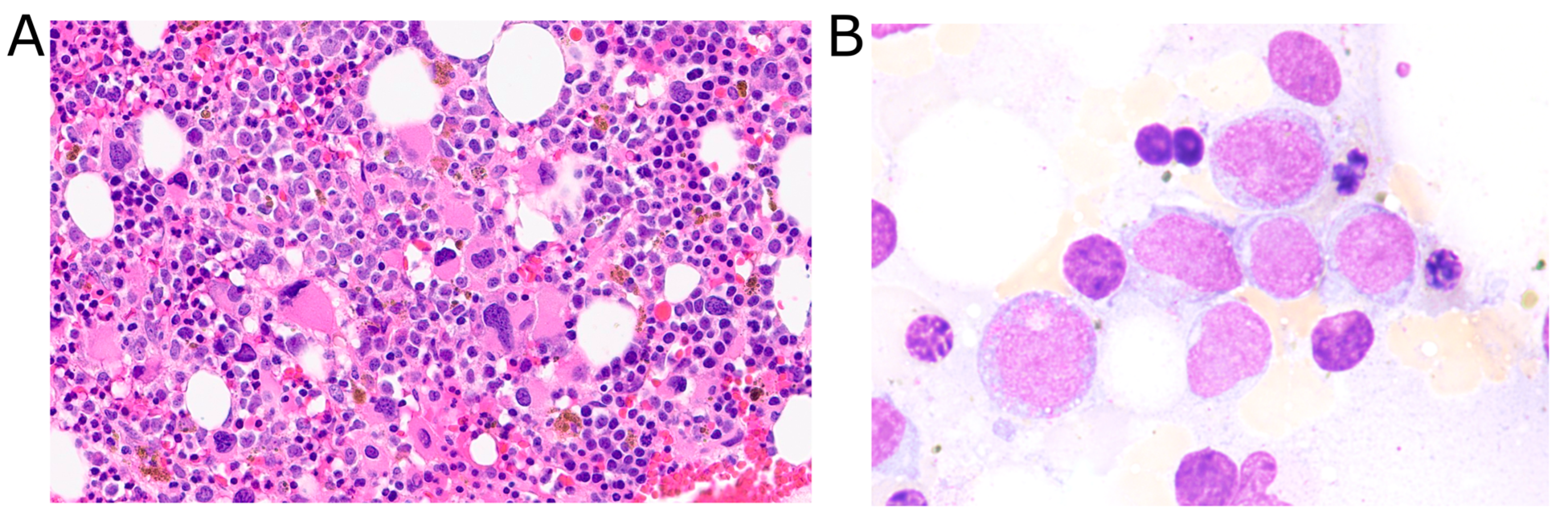
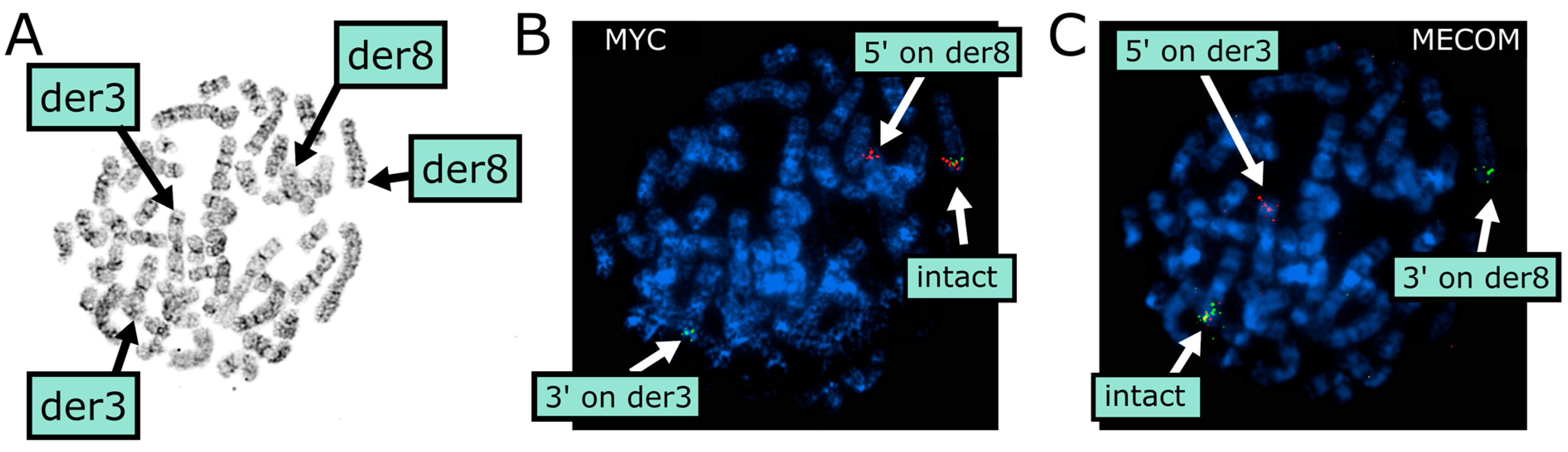
| Time | Bone Marrow Histology | Flow Cytometry | Cytogenetics |
|---|---|---|---|
| 0 months: initial diagnosis | >80–90% immature monocytes, trilineage dysplasia | Abnormal myeloid maturation, abnormal monocytes (65%) | del(12p) (100%), del(11q) (45%), KMT2A loss (13.5%), U2AF1 mutation (VAF 50%) |
| 3 months: post two cycles chemotherapy | No evidence of residual disease | No abnormal myeloid or blast populations | KMT2A loss (28.5%), KMT2A gain (5.0%) |
| 10 months: relapse | 14% blasts, decreased erythropoiesis, dysplastic megakaryocytes | Mild increase in myeloblasts (8%) | t(3;8), del(11), del(12), MYC (68%) and MECOM (18%) rearrangements, KMT2A loss (91.5%), ETV6 loss, U2AF1 mutation (VAF 31.5%) |
| 15 months: post salvage reinduction | Minimal residual disease | Residual AML blasts 2–3% | t(3;8), del(11), MYC (16.5%) and MECOM (30%) rearrangements, KMT2A loss (93.5%) |
| Source | N Patients | Abnormalities Identified | Age | Sex | Treatment(s) | Outcome(s) |
|---|---|---|---|---|---|---|
| Liu and Tirado 2018 [8] | 1 | t(3;8)(q26.2;q23) | 68 | Male | Ruxolitinib | Death |
| Tang et al. 2019 [9] | 20 | t(3;8)(q26.2;q24), various gene mutations | Range: 21–79 Median: 61 | 12 male, 8 female | Various | Death 90%, AWD 10% |
| Smith et al. 2020 [10] | 2 | Both: t(3;8)(q26.2;q24.1) Case 1: der(5), IGH/CCND1 fusion Case 2: del(7), del(13q14), loss of ASXL1, CSF3R, ETV6, and U2AF1 | 60; 68 | Both male | NS; Rituximab | Death; AWD |
| McGinnis et al. 2024 [11] | NS (at least 1) | MECOM::MYC rearrangement | NS | NS | NS | NS |
| Present case | 1 | t(3;8)(q26.2;q24.3), del(11)(q23), del(12)(p13), ETV6 loss, U2AF1 c.101C>T (S34F) mutation | 70 | Male | Venetoclax + azacytidine | In remission |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murgas, K.A.; Materum, P., III; Li, L.Z.; Rocha, J.; Schuster, M.; Ahmed, T.; Tirado, C.A. A Relapsed AML Case Featuring MYC and MECOM Rearrangements. Diagnostics 2025, 15, 2410. https://doi.org/10.3390/diagnostics15182410
Murgas KA, Materum P III, Li LZ, Rocha J, Schuster M, Ahmed T, Tirado CA. A Relapsed AML Case Featuring MYC and MECOM Rearrangements. Diagnostics. 2025; 15(18):2410. https://doi.org/10.3390/diagnostics15182410
Chicago/Turabian StyleMurgas, Kevin A., Pons Materum, III, Luke Z. Li, Jacob Rocha, Michael Schuster, Tahmeena Ahmed, and Carlos A. Tirado. 2025. "A Relapsed AML Case Featuring MYC and MECOM Rearrangements" Diagnostics 15, no. 18: 2410. https://doi.org/10.3390/diagnostics15182410
APA StyleMurgas, K. A., Materum, P., III, Li, L. Z., Rocha, J., Schuster, M., Ahmed, T., & Tirado, C. A. (2025). A Relapsed AML Case Featuring MYC and MECOM Rearrangements. Diagnostics, 15(18), 2410. https://doi.org/10.3390/diagnostics15182410






