Collision Tumor of Angioimmunoblastic T-Cell Lymphoma and Kaposi Sarcoma in an HIV-Negative Elderly Woman: The First Reported Case in Asia
Abstract
1. Introduction
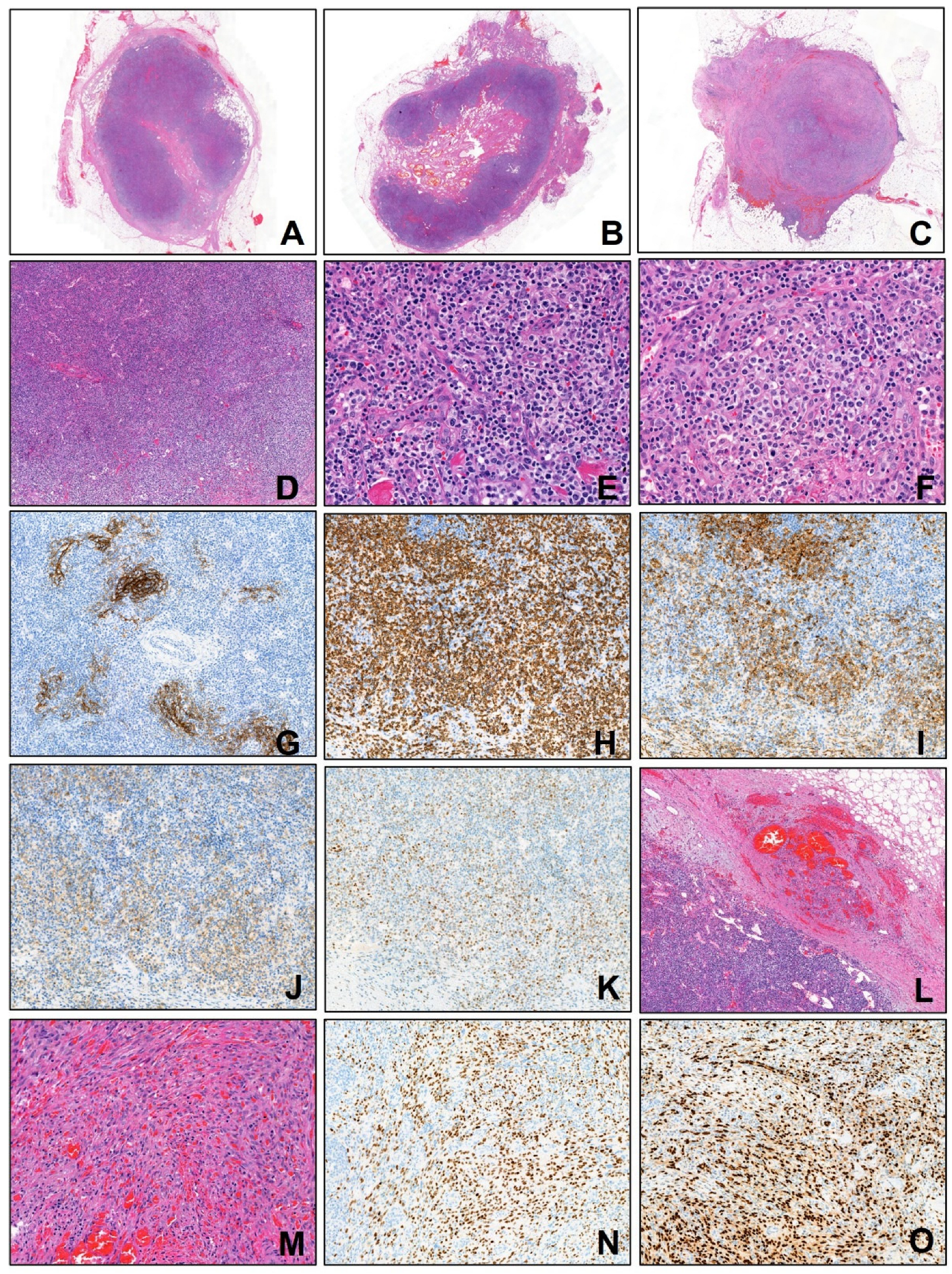
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Quintanilla-Martinez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S.; et al. The International Consensus Classification of Mature Lymphoid Neoplasms: A report from the Clinical Advisory Committee. Blood 2022, 140, 1229–1253. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, Y.; Li, T.; Liu, M.; Hong, Z.; Yin, Q.; Zheng, M. Angioimmunoblastic T-cell lymphoma: A concise overview encompassing the pathogenetic, pathological, clinical, therapeutical characteristics, and recent advances. Clin. Exp. Med. 2025, 25, 218. [Google Scholar] [CrossRef]
- Attygalle, A.; Al-Jehani, R.; Diss, T.C.; Munson, P.; Liu, H.; Du, M.Q.; Isaacson, P.G.; Dogan, A. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood 2002, 99, 627–633. [Google Scholar] [CrossRef]
- Mlika, M.; Helal, I.; Laabidi, S.; Braham, E.; El Mezni, F. Is CD10 antibody useful in the diagnosis of angioimmunoblastic T-cell lymphoma? J. Immunoass. Immunochem. 2015, 36, 510–516. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, L.; Liu, S.; Yi, S.; Xiao, M.; Zhang, Y.; Mao, X. PD-1 combined with TRBC1 and pan-T cell antibodies for robustly monitoring angioimmunoblastic T-cell lymphoma. Front. Med. 2022, 9, 962428. [Google Scholar] [CrossRef]
- Xie, Y.; Jaffe, E.S. How I Diagnose Angioimmunoblastic T-Cell Lymphoma. Am. J. Clin. Pathol. 2021, 156, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, X.; Li, H.; Lin, S.; Zang, S. Targeting TET2 as a Therapeutic Approach for Angioimmunoblastic T Cell Lymphoma. Cancers 2022, 14, 5699. [Google Scholar] [CrossRef]
- Nguyen, P.N.; Tran, N.T.B.; Nguyen, T.P.X.; Ngo, T.N.M.; Van Lai, D.; Deel, C.D.; Hassell, L.A.; Vuong, H.G. Clinicopathological Implications of RHOA Mutations in Angioimmunoblastic T-Cell Lymphoma: A Meta-analysis: RHOA mutations in AITL. Clin. Lymphoma Myeloma Leuk. 2021, 21, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.Y.; Sung, M.K.; Lee, S.H.; Kim, S.; Lee, H.; Park, S.; Kim, S.C.; Lee, B.; Rho, K.; Lee, J.E.; et al. A recurrent inactivating mutation in RHOA GTPase in angioimmunoblastic T cell lymphoma. Nat. Genet. 2014, 46, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Cesarman, E.; Damania, B.; Krown, S.E.; Martin, J.; Bower, M.; Whitby, D. Kaposi sarcoma. Nat. Rev. Dis. Primers 2019, 5, 9. [Google Scholar] [CrossRef]
- Batash, R.; Crimí, A.; Kassem, R.; Asali, M.; Ostfeld, I.; Biz, C.; Ruggieri, P.; Schaffer, M. Classic Kaposi sarcoma: Diagnostics, treatment modalities, and genetic implications—A review of the literature. Acta Oncol. 2024, 63, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.E.; Benson, G.; Glavan, S.; Giuliano, R.; Dickson, M.A. Fifth subtype of Kaposi sarcoma in HIV-negative MSM: A retrospective single-arm cohort study from a tertiary care center in NYC from 2000 to 2022. Oncologist 2025, 30, oyaf024. [Google Scholar] [CrossRef]
- Poullot, E.; Milowich, D.; Lemonnier, F.; Bisig, B.; Robe, C.; Pelletier, L.; Letourneau, A.; Dupuy, A.; Sako, N.; Ketterer, N.; et al. Angioimmunoblastic T-cell lymphoma and Kaposi sarcoma: A fortuitous collision? Histopathology 2024, 84, 556–564. [Google Scholar] [CrossRef]
- Marinova, E.; Han, S.; Zheng, B. Germinal center helper T cells are dual functional regulatory cells with suppressive activity to conventional CD4+ T cells. J. Immunol. 2007, 178, 5010–5017. [Google Scholar] [CrossRef] [PubMed]
- Couronne, L.; Bastard, C.; Bernard, O.A. TET2 and DNMT3A mutations in human T-cell lymphoma. N. Engl. J. Med. 2012, 366, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A.; Iqbal, J.; Lemonnier, F.; Kucuk, C.; De Leval, L.; Jais, J.P.; Parrens, M.; Martin, A.; Xerri, L.; Brousset, P.; et al. IDH2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood 2012, 119, 1901–1903. [Google Scholar] [CrossRef]
- Lee, P.H.; Weng, S.W.; Liu, T.T.; You, H.L.; Liao, C.K.; Wang, M.C.; Huang, W.T. RHOA G17V mutation in angioimmunoblastic T-cell lymphoma: A potential biomarker for cytological assessment. Exp. Mol. Pathol. 2019, 110, 104294. [Google Scholar] [CrossRef]
- Mohanna, B.S.; Sanchez, L.J.; Ferrufino Ll, J.C.; Bravo, P.F.; Gotuzzo, H.E. Lymph node involvement in classic Kaposi sarcoma: Report of three cases. Rev. Med. Chile 2007, 135, 1166–1170. [Google Scholar]
- Lazzi, S.; Bellan, C.; Amato, T.; Palummo, N.; Cardone, C.; D’Amuri, A.; De Luca, F.; Beyanga, M.; Facchetti, F.; Tosi, P.; et al. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 infection in reactive lymphoid tissues: A model for KSHV/HHV-8-related lymphomas? Hum. Pathol. 2006, 37, 23–31. [Google Scholar] [CrossRef]
- Sukpanichnant, S.; Sivayathorn, A.; Visudhiphan, S.; Ngowthammatas, W. Multicentric Castleman’s disease, non-Hodgkin’s lymphoma, and Kaposi’s sarcoma: A rare simultaneous occurrence. Asian Pac. J. Allergy Immunol. 2002, 20, 127–133. [Google Scholar] [PubMed]
- Pinto, L.W.; Nunes, E.P. Simultaneous lymph node involvement by Castleman disease and Kaposi sarcoma. Rev. Bras. Hematol. Hemoter. 2011, 33, 73–76. [Google Scholar] [PubMed][Green Version]
- Ismayilov, R.; Cinar, O.E.; Ozdede, M.; Ozogul, E.; Malkan, U.Y.; Uner, A.; Gullu, I.H. Coexistence of HHV-8-Associated Plasmacytic Multicentric Castleman Disease, Kaposi’s Sarcoma, and Multiple Myeloma in a HIV-Negative Patient. Eur. J. Case Rep. Intern. Med. 2024, 11, 004876. [Google Scholar] [CrossRef]
- Carbone, A. KSHV/HHV-8 associated Kaposi’s sarcoma in lymph nodes concurrent with Epstein-Barr virus associated Hodgkin lymphoma. J. Clin. Pathol. 2005, 58, 626–628. [Google Scholar] [CrossRef]
- Kankaya, D.; Kaygusuz, G.; Kuzu, I.; Savas, B.; Bakanay, S.M.; Ozcan, M. Simultaneous occurrence of Kaposi’s sarcoma and nodular lymphocyte predominant subtype of Hodgkin’s lymphoma in the same lymph node. Turk. J. Haematol. 2009, 26, 201–203. [Google Scholar]
- Ashman, D.; Pantanowitz, L. Kaposi Sarcoma With Coexisting Intravascular Lymphoma. Int. J. Surg. Pathol. 2019, 27, 62–63. [Google Scholar] [CrossRef]
- Nador, R.G.; Cesarman, E.; Chadburn, A.; Dawson, D.B.; Ansari, M.Q.; Sald, J.; Knowles, D.M. Primary effusion lymphoma: A distinct clinicopathologic entity associated with the Kaposi’s sarcoma-associated herpes virus. Blood 1996, 88, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Licci, S.; D’Antonio, A.; Boscaino, A.; Morelli, L.; Piscioli, F.; Abbate, I.; Perrone Donnorso, R.; Del Nonno, F. Non-Hodgkin lymphomas concurrent with HHV8-associated Kaposi’s sarcoma in the same lymph node in AIDS and non-AIDS patients. Acta Haematol. 2007, 118, 47–52. [Google Scholar] [CrossRef]
- Nakashima, S.; Ohara, S.; Imai, Y.; Nakano, H.; Uchida, T.; Inoue, M.; Hagihara, M. Diffuse large B-cell lymphoma concurrent with Kaposi’s sarcoma in the same lymph node in a human immunodeficiency virus-negative patient. Rinsho Ketsueki 2024, 65, 74–77. [Google Scholar]
- Varsano, S.; Manor, Y.; Steiner, Z.; Griffel, B.; Klajman, A. Kaposi’s sarcoma and angioimmunoblastic lymphadenopathy. Cancer 1984, 54, 1582–1585. [Google Scholar] [CrossRef]
- Abe, Y.; Matsubara, D.; Gatanaga, H.; Oka, S.; Kimura, S.; Sasao, Y.; Saitoh, K.; Fujii, T.; Sato, Y.; Sata, T.; et al. Distinct expression of Kaposi’s sarcoma-associated herpesvirus-encoded proteins in Kaposi’s sarcoma and multicentric Castleman’s disease. Pathol. Int. 2006, 56, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, D.M.; Brown, J.A.; Shahsafaei, A.; Freeman, G.J. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am. J. Surg. Pathol. 2006, 30, 802–810. [Google Scholar] [CrossRef] [PubMed]

| Case | Age/Sex | Ethnicity | Biopsy Site | Skin Lesion (KS) | Clinical Status | HIV Status |
|---|---|---|---|---|---|---|
| 1 | 76/M | Caucasian (Switzerland) | Inguinal LN | - | Stage IV; R-CHOP | Negative |
| 2 | 49/M | African (Cameroon) | Axillary LN | - | Stage IV; CHOP | Negative |
| 3 | 70/M | Comorian (Mayotte) | Inguinal LN | + | Stage IV; Palliative care | Negative |
| This case | 81/F | Asian (Korea) | Inguinal LN | - | Stage IV; Palliative care | Negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-W.; Kim, J.-M. Collision Tumor of Angioimmunoblastic T-Cell Lymphoma and Kaposi Sarcoma in an HIV-Negative Elderly Woman: The First Reported Case in Asia. Diagnostics 2025, 15, 2411. https://doi.org/10.3390/diagnostics15182411
Lee M-W, Kim J-M. Collision Tumor of Angioimmunoblastic T-Cell Lymphoma and Kaposi Sarcoma in an HIV-Negative Elderly Woman: The First Reported Case in Asia. Diagnostics. 2025; 15(18):2411. https://doi.org/10.3390/diagnostics15182411
Chicago/Turabian StyleLee, Myung-Won, and Jin-Man Kim. 2025. "Collision Tumor of Angioimmunoblastic T-Cell Lymphoma and Kaposi Sarcoma in an HIV-Negative Elderly Woman: The First Reported Case in Asia" Diagnostics 15, no. 18: 2411. https://doi.org/10.3390/diagnostics15182411
APA StyleLee, M.-W., & Kim, J.-M. (2025). Collision Tumor of Angioimmunoblastic T-Cell Lymphoma and Kaposi Sarcoma in an HIV-Negative Elderly Woman: The First Reported Case in Asia. Diagnostics, 15(18), 2411. https://doi.org/10.3390/diagnostics15182411






