Conventional Diagnostic Approaches to Dermatophytosis: Insights from a Three-Year Survey at a Public Dermatology Institute in Italy (2019–2021)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Microscopy and Culture Test
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Microscopic Examination and Culture Test
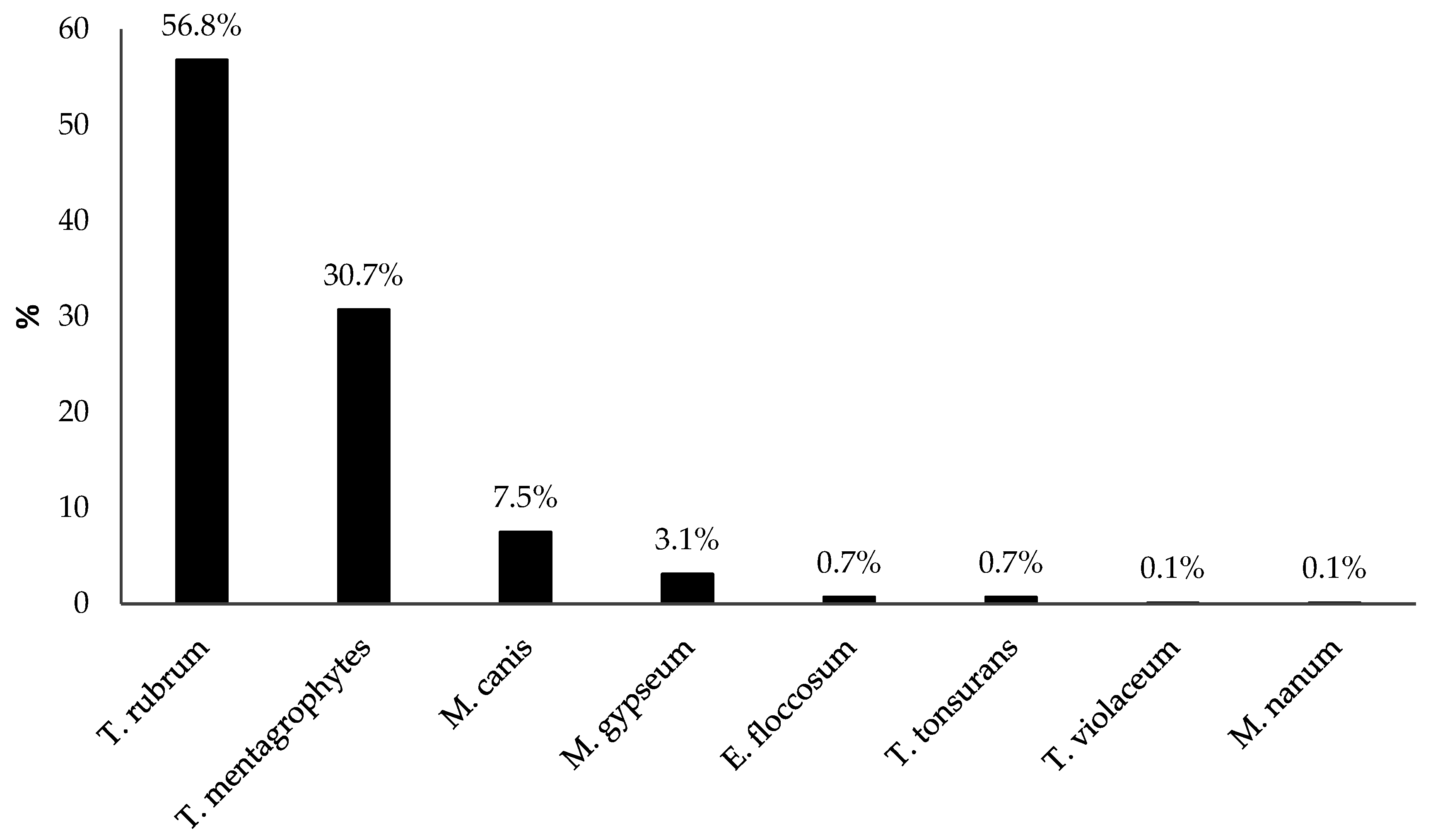
3.3. Dermatophyte Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khurana, A.; Sardana, K.; Chowdhary, A. Antifungal resistance in dermatophytes: Recent trends and therapeutic implications. Fungal Genet. Biol. 2019, 132, 103255. [Google Scholar] [CrossRef] [PubMed]
- Petrucelli, M.F.; Abreu, M.H.d.; Cantelli, B.A.M.; Segura, G.G.; Nishimura, F.G.; Bitencourt, T.A.; Marins, M.; Fachin, A.L. Epidemiology and Diagnostic Perspectives of Dermatophytoses. J. Fungi 2020, 6, 310. [Google Scholar] [CrossRef]
- Barac, A.; Stjepanovic, M.; Krajisnik, S.; Stevanovic, G.; Paglietti, B.; Milosevic, B. Dermatophytes: Update on Clinical Epidemiology and Treatment. Mycopathologia 2024, 189, 101. [Google Scholar] [CrossRef]
- Gupta, A.K.; Wang, T.; Susmita; Talukder, M.; Bakotic, W.L. Global Dermatophyte Infections Linked to Human and Animal Health: A Scoping Review. Microorganisms 2025, 13, 575. [Google Scholar] [CrossRef]
- Shah, A.A.; Mirza, R.; Sattar, A.; Khan, Y.; Khan, S.A. Unveiling onychomycosis: Pathogenesis, diagnosis, and innovative treatment strategies. Microb. Pathog. 2025, 198, 107111. [Google Scholar] [CrossRef]
- WHO. Ringworm (Tinea). Available online: https://www.who.int/news-room/fact-sheets/detail/ringworm-(tinea) (accessed on 15 July 2025).
- Coulibaly, O.; Kone, A.K.; Niaré-Doumbo, S.; Goïta, S.; Gaudart, J.; Djimdé, A.A.; Piarroux, R.; Doumbo, O.K.; Thera, M.A.; Ranque, S. Dermatophytosis among Schoolchildren in Three Eco-climatic Zones of Mali. PLoS Negl. Trop. Dis. 2016, 10, e0004675. [Google Scholar] [CrossRef] [PubMed]
- Ravid, A.; Michael, F.; Daniel, C.; Esther, S. Dermatomycoses in the Israeli defense forces-Epidemiological and clinical aspects. Mycoses 2020, 63, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Colosi, I.A.; Cognet, O.; Colosi, H.A.; Sabou, M.; Costache, C. Dermatophytes and Dermatophytosis in Cluj-Napoca, Romania-A 4-Year Cross-Sectional Study. J. Fungi 2020, 6, 154. [Google Scholar] [CrossRef]
- Carrascal-Correa, D.F.; Zuluaga, A.; González, A. Species distribution of the main aetiologic agents causing skin dermatophytosis in Colombian patients: A 23-year experience at a Mycological Reference Center. Mycoses 2020, 63, 494–499. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.; Zhi, H.; Lv, W.; Sang, B.; Zhong, Y.; Chen, X.; Xia, X.; Liu, Z. Epidemiological survey of 32,786 culture-positive dermatophytosis cases in Hangzhou from 2018 to 2023. Mycopathologia 2024, 189, 98. [Google Scholar] [CrossRef]
- Lipner, S.R.; Scher, R.K. Onychomycosis: Clinical overview and diagnosis. J. Am. Acad. Dermatol. 2019, 80, 835–851. [Google Scholar] [CrossRef]
- Segal, E.; Elad, D. Human and Zoonotic Dermatophytoses: Epidemiological Aspects. Front. Microbiol. 2021, 12, 713532. [Google Scholar] [CrossRef]
- Piorunek, M.; Kubisiak-Rzepczyk, H.; Dańczak-Pazdrowska, A.; Trafas, T.; Walkowiak, J. Superficial Zoonotic Mycoses in Humans Associated with Cats. J. Fungi 2024, 10, 244. [Google Scholar] [CrossRef]
- Kruithoff, C.; Gamal, A.; McCormick, T.S.; Ghannoum, M.A. Dermatophyte Infections Worldwide: Increase in Incidence and Associated Antifungal Resistance. Life 2023, 14, 1. [Google Scholar] [CrossRef]
- Gupta, A.K.; Wang, T.; Mann, A.; Piguet, V.; Chowdhary, A.; Bakotic, W.L. Mechanisms of resistance against allylamine and azole antifungals in Trichophyton: A renewed call for innovative molecular diagnostics in susceptibility testing. PLoS Pathog. 2025, 21, e1012913. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.A.W.; Lockhart, S.R. Scratching the surface: The rise of antifungal-resistant dermatophytes. Clin. Microbiol. Newsl. 2025, 51, 26–30. [Google Scholar] [CrossRef]
- Jabet, A.; Normand, A.; Brun, S.; Dannaoui, E.; Bachmeyer, C.; Piarroux, R.; Hennequin, C.; Moreno-Sabater, A. Trichophyton indotineae, from epidemiology to therapeutic. J. Mycol. Med. 2023, 33, 101383. [Google Scholar] [CrossRef] [PubMed]
- Pihet, M.; Le Govic, Y. Reappraisal of Conventional Diagnosis for Dermatophytes. Mycopathologia 2017, 182, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rossi, N.M.; Peres, N.T.A.; Bitencourt, T.A.; Martins, M.P.; Rossi, A. State-of-the-Art Dermatophyte Infections: Epidemiology Aspects, Pathophysiology, and Resistance Mechanisms. J. Fungi 2021, 7, 629. [Google Scholar] [CrossRef]
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008, 51, 2–15. [Google Scholar] [CrossRef]
- Heckler, I.; Sabalza, M.; Bojmehrani, A.; Venkataraman, I.; Thompson, C. The need for fast and accurate detection of dermatomycosis. Med. Mycol. 2023, 61, myad037. [Google Scholar] [CrossRef]
- Das, S.; Goyal, R.; Bhattacharya, S.N. Laboratory-based epidemiological study of superficial fungal infections. J. Dermatol. 2007, 34, 248–253. [Google Scholar] [CrossRef]
- Souza, P.R.M.; Vettorato, G.; Pinto, G.M.; Duquia, R.P.; Amaro, T.G.; Almeira Junior, H.L.; Breunig, J.d.A. Concordance between direct microscopy and fungical culture for the diagnostic of feet’s onychomycosis. An. Bras. Dermatol. 2012, 87, 157–159. [Google Scholar] [CrossRef]
- Aboul-Ella, H.; Hamed, R.; Abo-Elyazeed, H. Recent trends in rapid diagnostic techniques for dermatophytosis. Int. J. Vet. Sci. Med. 2020, 8, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Begum, J.; Mir, N.A.; Lingaraj, M.C.; Buyamayum, B.; Dev, K. Recent advances in the diagnosis of dermatophytosis. J. Basic Microbiol. 2020, 60, 293–303. [Google Scholar] [CrossRef]
- Anton, A.; Plinet, M.; Peyret, T.; Cazaudarré, T.; Pesant, S.; Rouquet, Y.; Tricoteaux, M.A.; Bernier, M.; Bayette, J.; Fournier, R.; et al. Rapid and Accurate Diagnosis of Dermatophyte Infections Using the DendrisCHIP® Technology. Diagnostics. 2023, 13, 3430. [Google Scholar] [CrossRef] [PubMed]
- L’Ollivier, C.; Cassagne, C.; Normand, A.; Bouchara, J.; Contet-Audonneau, N.; Hendrickx, M.; Fourquet, P.; Coulibaly, O.; Piarroux, R.; Ranque, S. A MALDI-TOF MS procedure for clinical dermatophyte species identification in the routine laboratory. Med. Mycol. 2013, 51, 713–720. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, F.; Sun, X.; Gao, H.; Lin, S.; Zeng, Y. The qualitative accuracy of clinical dermatophytes via matrix-assisted laser desorption ionization-time of flight mass spectrometry: A meta-analysis. Med. Mycol. 2021, 59, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gupta, P.; Tiwari, K.; Suvirya, S.; Verma, P.; Banerjee, G. A Comparative Evaluation of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) and Conventional Methods for the Diagnosis of Dermatophytes. Cureus 2025, 17, e78344. [Google Scholar] [CrossRef]
- Asticcioli, S.; Di Silverio, A.; Sacco, L.; Fusi, I.; Vincenti, L.; Romero, E. Dermatophyte infections in patients attending a tertiary care hospital in northern Italy. New Microbiol. 2008, 31, 543–548. [Google Scholar]
- Vena, G.A.; Chieco, P.; Posa, F.; Garofalo, A.; Bosco, A.; Cassano, N. Epidemiology of dermatophytoses: Retrospective analysis from 2005 to 2010 and comparison with previous data from 1975. New Microbiol. 2012, 35, 207–213. [Google Scholar] [PubMed]
- Seebacher, C.; Bouchara, J.; Mignon, B. Updates on the epidemiology of dermatophyte infections. Mycopathologia 2008, 166, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Panasiti, V.; Devirgiliis, V.; Borroni, R.G.; Mancini, M.; Curzio, M.; Rossi, M.; Bottoni, U.; Calvieri, S. Epidemiology of dermatophytic infections in Rome, Italy: A retrospective study from 2002 to 2004. Med. Mycol. 2007, 45, 57–60. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A.; Liguori, G.; Cafarchia, C.; Triggiano, F.; Ciccarese, G.; Poli, M.A.; Ambrogio, F.; Bonamonte, D.; Cassano, N.; Vena, G.A.; et al. Cutaneous Infections Caused by Trichophyton indotineae: Case Series and Systematic Review. J. Clin. Med. 2025, 14, 1280. [Google Scholar] [CrossRef]
- Raccagni, A.R.; Castagna, A.; Nozza, S. When is it a sexually transmitted infection? Intimate contact transmission of pathogens not traditionally defined as STIs. Curr. Opin. Infect. Dis. 2025, 38, 65–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, W.; Liu, W.; Li, X.; Liang, G. Epidemiological and Clinical Profile Analysis of Trichophyton mentagrophytes ITS Genotype VII Infected Dermatomycosis: An Emerging Sexually Transmitted Pathogen. Mycoses 2025, 68, e70075. [Google Scholar] [CrossRef]

| Microscopic Examination | Fungal Culture Test, n (%) | |||
|---|---|---|---|---|
| Negative | Dermatophytes | Other Fungi | Total | |
| Negative | 2315 (96.5) | 82 (3.4) | 3 (0.1) | 2400 (74.8) |
| Positive | 184 (22.8) | 585 (72.4) | 39 (4.8) | 808 (25.2) |
| Total | 2499 (77.9) | 667 (20.8) | 42 (1.3) | 3208 (100) |
| Body Site | Dermatophytes n (%) | Other Fungi a n (%) |
|---|---|---|
| Toenails, n = 1356 | 216 (15.9) | 4 (0.3) |
| Feet (skin), n = 487 | 163 (33.5) | 1 (0.2) |
| Fingernails, n = 335 | 8 (2.4) | 31 (9.3) |
| Torso, n = 210 | 34 (16.2) | 1 (0.5) |
| Genitals, n = 176 | 64 (36.4) | 4 (2.3) |
| Lower limbs, n = 158 | 41 (25.9) | 0 (0.0) |
| Upper limbs, n = 125 | 44 (35.2) | 0 (0.0) |
| Face, n = 100 | 26 (26.0) | 0 (0.0) |
| Scalp, n = 96 | 25 (26.0) | 0 (0.0) |
| Hands (skin), n = 94 | 20 (21.3) | 1 (1.1) |
| Buttocks, n = 64 | 24 (37.5) | 0 (0.0) |
| Perianal region, n = 7 | 2 (28.6) | 0 (0.0) |
| Total, n = 3208 | 667 (20.8) | 42 (1.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliani, E.; Donà, M.G.; Giglio, A.; Abril, E.; Sperati, F.; Pimpinelli, F.; Latini, A. Conventional Diagnostic Approaches to Dermatophytosis: Insights from a Three-Year Survey at a Public Dermatology Institute in Italy (2019–2021). Diagnostics 2025, 15, 2245. https://doi.org/10.3390/diagnostics15172245
Giuliani E, Donà MG, Giglio A, Abril E, Sperati F, Pimpinelli F, Latini A. Conventional Diagnostic Approaches to Dermatophytosis: Insights from a Three-Year Survey at a Public Dermatology Institute in Italy (2019–2021). Diagnostics. 2025; 15(17):2245. https://doi.org/10.3390/diagnostics15172245
Chicago/Turabian StyleGiuliani, Eugenia, Maria Gabriella Donà, Amalia Giglio, Elva Abril, Francesca Sperati, Fulvia Pimpinelli, and Alessandra Latini. 2025. "Conventional Diagnostic Approaches to Dermatophytosis: Insights from a Three-Year Survey at a Public Dermatology Institute in Italy (2019–2021)" Diagnostics 15, no. 17: 2245. https://doi.org/10.3390/diagnostics15172245
APA StyleGiuliani, E., Donà, M. G., Giglio, A., Abril, E., Sperati, F., Pimpinelli, F., & Latini, A. (2025). Conventional Diagnostic Approaches to Dermatophytosis: Insights from a Three-Year Survey at a Public Dermatology Institute in Italy (2019–2021). Diagnostics, 15(17), 2245. https://doi.org/10.3390/diagnostics15172245








