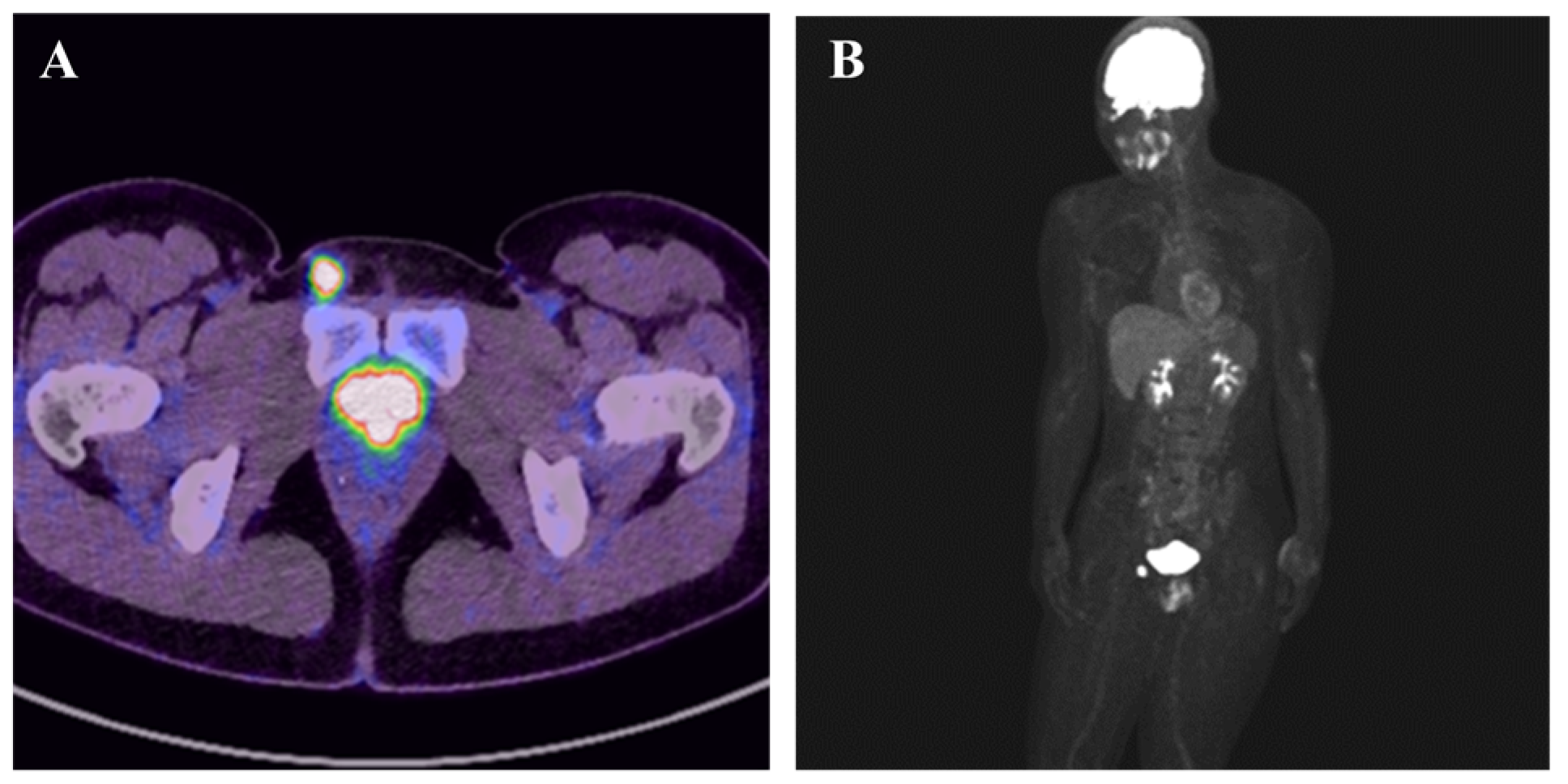
Proximal Epithelioid Sarcoma Mimicking Inguinal Inflammation
Abstract


Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRI | Magnetic resonance imaging |
| ES | Epithelioid sarcoma |
| CT | Computed tomography |
| IHC | Immunohistochemistry |
| DWI | Diffusion-weighted imaging |
| ADC | Apparent diffusion coefficient |
References
- Oda, Y.; Dal Cin, P.; Le Loarer, F.; Nielsen, T.O. Epithelioid Sarcoma. In WHO Classification of Tumours, Soft Tissue and Bone Tumours, 5th ed.; IARC Press: Lyon, France, 2020; pp. 294–296. [Google Scholar]
- Czarnecka, A.M.; Sobczuk, P.; Kostrzanowski, M.; Spalek, M.; Chojnacka, M.; Szumera-Cieckiewicz, A.; Rutkowski, P. Epithelioid Sarcoma-From Genetics to Clinical Practice. Cancers 2020, 12, 2112. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.; Zaidia, S.; Suha, Y.; Joshi, G.; Smith, N.L.; Khan, S.A. An Index of Inguinal and Inguinofemoral Masses in Women: Critical Considerations for Diagnosis. Transl. Res. Anat. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Tateishi, U.; Hasegawa, T.; Kusumoto, M.; Yokoyama, R.; Moriyama, N. Radiologic manifestations of proximal-type epithelioid sarcoma of the soft tissues. AJR Am. J. Roentgenol. 2002, 179, 973–977. [Google Scholar] [CrossRef] [PubMed]
- McCarville, M.B.; Kao, S.C.; Dao, T.V.; Gaffney, C.; Coffin, C.M.; Parham, D.M.; Hayes-Jordan, A.; Spunt, S.L. Magnetic resonance and computed tomography imaging features of epithelioid sarcoma in children and young adults with pathological and clinical correlation: A report from Children’s Oncology Group study ARST0332. Pediatr. Radiol. 2019, 49, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.L.; Kaste, S.; Jenkins, J.J.; Hewan-Lowe, K.; Spence, J.V.; Gupta, M.; Monson, D.; Fletcher, B.D. Epithelioid sarcoma: Clinical, MR imaging and pathologic findings. Skelet. Radiol. 2002, 31, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, M.; Oebisu, N.; Takada, J.; Ieguchi, M.; Wakasa, K.; Nakamura, H. Role of FDG-PET/CT for monitoring soft tissue tumors. Oncol. Lett. 2014, 7, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Thway, K.; Jones, R.L.; Noujaim, J.; Fisher, C. Epithelioid Sarcoma: Diagnostic Features and Genetics. Adv. Anat. Pathol. 2016, 23, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Ichikawa, J.; Imada, H.; Kanno, S.; Onohara, K.; Yazawa, Y.; Tatsuno, R.; Jyubashi, T.; Torigoe, T. Indolent Multinodular Synovial Sarcoma of Peripheral Nerves Mimicking Schwannoma: A Case Report and Literature Review. Anticancer Res. 2023, 43, 5729–5736. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawasaki, T.; Watanabe, T.; Kanno, S.; Torigoe, T.; Onohara, K.; Wako, M.; Hagino, T.; Ichikawa, J. Proximal Epithelioid Sarcoma Mimicking Inguinal Inflammation. Diagnostics 2025, 15, 2246. https://doi.org/10.3390/diagnostics15172246
Kawasaki T, Watanabe T, Kanno S, Torigoe T, Onohara K, Wako M, Hagino T, Ichikawa J. Proximal Epithelioid Sarcoma Mimicking Inguinal Inflammation. Diagnostics. 2025; 15(17):2246. https://doi.org/10.3390/diagnostics15172246
Chicago/Turabian StyleKawasaki, Tomonori, Takuya Watanabe, Satoshi Kanno, Tomoaki Torigoe, Kojiro Onohara, Masanori Wako, Tetsuhiro Hagino, and Jiro Ichikawa. 2025. "Proximal Epithelioid Sarcoma Mimicking Inguinal Inflammation" Diagnostics 15, no. 17: 2246. https://doi.org/10.3390/diagnostics15172246
APA StyleKawasaki, T., Watanabe, T., Kanno, S., Torigoe, T., Onohara, K., Wako, M., Hagino, T., & Ichikawa, J. (2025). Proximal Epithelioid Sarcoma Mimicking Inguinal Inflammation. Diagnostics, 15(17), 2246. https://doi.org/10.3390/diagnostics15172246




