Unmasking the Placenta–Heart Axis: A Comprehensive Review of Placental Abnormalities in Congenital Heart Disease
Abstract
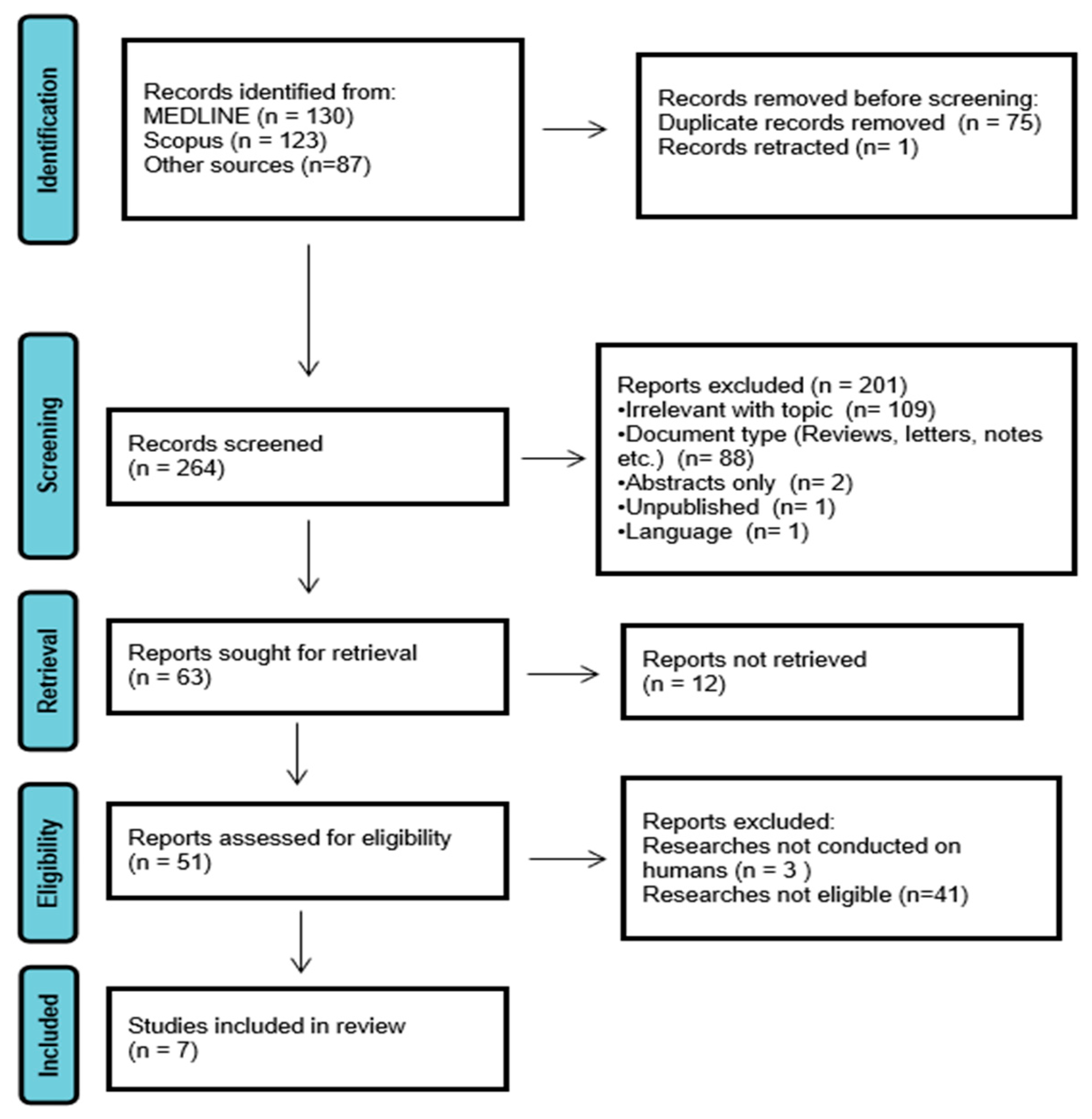
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
- Population: Human pregnancies with fetuses diagnosed with any form of CHD
- Exposure: Prenatal or postnatal imaging of the placenta (e.g., ultrasound, Doppler, and MRI)
- Outcomes: Documented placental abnormalities (e.g., reduced perfusion, altered vascular indices, and structural changes)
- Study Design: Original research including prospective or retrospective cohorts, case–control studies, cross-sectional analyses, and clinical trials
- Language and Date: Published in English between 2020 and 2025
2.3. Study Selection
3. Results
3.1. Risk of Bias Assessment
3.2. Reference Groups and Demographic Profile
3.3. Placenta Imaging Method and CHD Diagnoses
3.4. MRI Findings
3.5. Doppler Ultrasound Studies
4. Discussion
4.1. Key Findings of the Present Review
4.2. Older Literature
4.2.1. Histopathology
4.2.2. Doppler/Magnetic Resonance Imaging
4.3. Pathophysiological Considerations
4.3.1. Hemodynamics
4.3.2. Maternal Vascular Malperfusion (MVM)
4.3.3. Developmental Origins
4.3.4. Clinical Indicators for Placental Dysfunction
4.4. Limitations and Methodological Considerations
4.5. Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cromb, D.; Slator, P.J.; Hall, M.; Price, A.; Alexander, D.C.; Counsell, S.J.; Hutter, J. Advanced magnetic resonance imaging detects altered placental development in pregnancies affected by congenital heart disease. Sci. Rep. 2024, 14, 12357. [Google Scholar] [CrossRef]
- Diao, J.; Chen, L.; Wei, J.; Shu, J.; Li, Y.; Li, J.; Zhang, S.; Wang, T.; Qin, J. Prevalence of Malnutrition in Children with Congenital Heart Disease: A Systematic Review and Meta-Analysis. J. Pediatr. 2021, 242, 39–47. [Google Scholar] [CrossRef]
- Jepson, B.M.; Metz, T.D.; Miller, T.A.; Son, S.L.; Ou, Z.; Presson, A.P.; Nance, A.; Pinto, N.M. Pregnancy loss in major fetal congenital heart disease: Incidence, risk factors and timing. Ultrasound Obstet. Gynecol. 2023, 62, 75–87. [Google Scholar] [CrossRef]
- Sun, B.Z.; Moster, D.; Harmon, Q.E.; Wilcox, A.J. Association of Preeclampsia in Term Births With Neurodevelopmental Disorders in Offspring. JAMA Psychiatry 2020, 77, 823–829. [Google Scholar] [CrossRef]
- Hoffman, J.I.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Fernandez-Campos, B.A.; Grewal, J.; Kiess, M.; Siu, S.C.; Pfaller, B.; Sermer, M.; Mason, J.; Silversides, C.K.; Haberer, K. Adverse fetal/neonatal and obstetric outcomes in pregnancies with both maternal and fetal heart disease. J. Perinatol. 2024, 44, 1424–1431. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Sáez, M.A.; Álvarez-Mon, M.A.; Torres-Carranza, D.; Álvarez-Mon, M.; Bujan, J.; García-Honduvilla, N.; Bravo, C.; et al. The pivotal role of the placenta in normal and pathological pregnancies: A focus on preeclampsia, fetal growth restriction, and maternal chronic venous disease. Cells 2022, 11, 568. [Google Scholar] [CrossRef]
- Herrick, E.J.; Bordoni, B. Embryology, Placenta. StatPearls—NCBI Bookshelf. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551634/#:~:text=The%20placenta%20can%20be%20considered,growth%20factor%2C%20and%20many%20others (accessed on 30 April 2025).
- Sinding, M.; Peters, D.A.; Frokjaer, J.B.; Christiansen, O.B.; Petersen, A.; Uldbjerg, N.; Sorensen, A. Placental magnetic resonance imaging T2* measurements in normal pregnancies and in those complicated by fetal growth restriction. Ultrasound Obstet. Gynecol. 2016, 47, 748–754. [Google Scholar] [CrossRef]
- Cromb, D.; Steinweg, J.; Aviles Verdera, J.; van Poppel, M.P.M.; Bonthrone, A.F.; Lloyd, D.F.A.; Pushparajah, K.; Simpson, J.; Razavi, R.; Rutherford, M.; et al. T2*-Relaxometry MRI to Assess Third Trimester Placental and Fetal Brain Oxygenation and Placental Characteristics in Healthy Fetuses and Fetuses With Congenital Heart Disease. J. Magn. Reson. Imaging 2025, 61, 1246–1255. [Google Scholar] [CrossRef]
- Wardinger, J.E.; Ambati, S. Placental Insufficiency. StatPearls—NCBI Bookshelf. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563171/#:~:text=One%20of%20the%20major%20benefits,maternal%20malnutrition%20or%20calorie%20restriction (accessed on 30 April 2025).
- Leveraging New Imaging Tools to Monitor Placental Dysfunction. 2023. Available online: https://www.nichd.nih.gov/research/supported/human-placenta-project/findings/developing-imaging/new-imaging-tools-monitor-placental-dysfunction (accessed on 30 April 2025).
- Courtney, J.A.; Cnota, J.F.; Jones, H.N. The Role of Abnormal Placentation in Congenital Heart Disease; Cause, Correlate, or Consequence? Front. Physiol. 2018, 9, 1045. [Google Scholar] [CrossRef]
- Albalawi, A.; Brancusi, F.; Askin, F.; Ehsanipoor, R.; Wang, J.; Burd, I.; Sekar, P. Placental Characteristics of Fetuses With Congenital Heart Disease. J. Ultrasound Med. 2017, 36, 965–972. [Google Scholar] [CrossRef]
- Barros, T.; Ferreira, B.D.; Moleiro, M.L.; Guedes-Martins, L. Preeclampsia and fetal congenital heart defects. Curr. Cardiol. Rev. 2022, 18, 80–91. [Google Scholar] [CrossRef]
- Rychik, J.; Goff, D.; McKay, E.; Mott, A.; Tian, Z.; Licht, D.J.; Gaynor, J.W. Characterization of the Placenta in the Newborn with Congenital Heart Disease: Distinctions Based on Type of Cardiac Malformation. Pediatr. Cardiol. 2018, 39, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Snoep, M.C.; Aliasi, M.; van der Meeren, L.E.; Jongbloed, M.R.M.; DeRuiter, M.C.; Haak, M.C. Placenta morphology and biomarkers in pregnancies with congenital heart disease—A systematic review. Placenta 2021, 112, 189–196. [Google Scholar] [CrossRef]
- Jones, H.N.; Powell, T.L.; Jansson, T. Regulation of placental nutrient transport—A review. Placenta 2007, 28, 763–774. [Google Scholar] [CrossRef]
- Nijman, M.; Van Der Meeren, L.E.; Nikkels, P.G.J.; Stegeman, R.; Breur, J.M.P.J.; Jansen, N.J.G.; Ter Heide, H.; Steenhuis, T.J.; De Heus, R.; Bekker, M.N.; et al. Placental pathology contributes to impaired volumetric brain development in neonates with congenital heart disease. J. Am. Heart Assoc. 2024, 13, e033189. [Google Scholar] [CrossRef]
- Josowitz, R.; Linn, R.; Rychik, J. The Placenta in Congenital Heart Disease: Form, Function and Outcomes. Neoreviews 2023, 24, e569–e582. [Google Scholar] [CrossRef]
- Gagnon, R. Placental insufficiency and its consequences. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110 (Suppl. S1), S99–S107. [Google Scholar] [CrossRef]
- Jacobwitz, M.; Kapse, K.; Ngwa, J.; De Asis-Cruz, J.; Wu, Y.; Donofrio, M.T.; McDermott, C.; du Plessis, A.; Limperopoulos, C.; Andescavage, N. Placental and Fetal In Utero Growth Among Fetuses With Congenital Heart Disease. JAMA Netw. Open 2025, 8, e257217. [Google Scholar] [CrossRef]
- Rajagopalan, V.; Schmithorst, V.; El-Ali, A.; Reynolds, W.; Lee, V.; Wallace, J.; Weinberg, J.; Johnson, J.; Votava-Smith, J.; Adibi, J.; et al. Associations between Maternal Risk Factors and Intrinsic Placental and Fetal Brain Functional Properties in Congenital Heart Disease. Int. J. Mol. Sci. 2022, 23, 15178. [Google Scholar] [CrossRef]
- Steinweg, J.K.; Hui, G.T.Y.; Pietsch, M.; Ho, A.; van Poppel, M.P.; Lloyd, D.; Colford, K.; Simpson, J.M.; Razavi, R.; Pushparajah, K.; et al. T2* placental MRI in pregnancies complicated with fetal congenital heart disease. Placenta 2021, 108, 23–31. [Google Scholar] [CrossRef]
- Josowitz, R.; Ho, D.Y.; Shankar, S.; Mondal, A.; Zavez, A.; Linn, R.L.; Tian, Z.; Gaynor, J.W.; Rychik, J. Congenital Heart Disease Fetuses Have Decreased Mid-Gestational Placental Flow, Placental Malperfusion Defects, and Impaired Growth. JACC Adv. 2025, 4, 101559. [Google Scholar] [CrossRef] [PubMed]
- Ordas, P.; Rodriguez, R.; Herrero, B.; Deiros, L.; Gomez, E.; Llurba, E.; Bartha, J.L.; Antolin, E. Longitudinal changes in fetal head biometry and fetoplacental circulation in fetuses with congenital heart defects. Acta Obstet. Gynecol. Scand. 2022, 101, 987–995. [Google Scholar] [CrossRef]
- Andescavage, N.N.; Limperopoulos, C. Placental abnormalities in congenital heart disease. Transl. Pediatr. 2021, 10, 2148–2156. [Google Scholar] [CrossRef]
- Afacan, O.; Estroff, J.A.; Yang, E.; Barnewolt, C.E.; Connolly, S.A.; Parad, R.B.; Mulkern, R.V.; Warfield, S.K.; Gholipour, A. Fetal Echoplanar Imaging: Promises and Challenges. Top. Magn. Reson. Imaging 2019, 28, 245–254. [Google Scholar] [CrossRef]
- Miremberg, H.; Gindes, L.; Schreiber, L.; Raucher Sternfeld, A.; Bar, J.; Kovo, M. The association between severe fetal congenital heart defects and placental vascular malperfusion lesions. Prenat. Diagn. 2019, 39, 962–967. [Google Scholar] [CrossRef]
- Jones, H.N.; Olbrych, S.K.; Smith, K.L.; Cnota, J.F.; Habli, M.; Ramos-Gonzales, O.; Owens, K.J.; Hinton, A.C.; Polzin, W.J.; Muglia, L.J.; et al. Hypoplastic left heart syndrome is associated with structural and vascular placental abnormalities and leptin dysregulation. Placenta 2015, 36, 1078–1086. [Google Scholar] [CrossRef]
- Leon, R.L.; Mir, I.N.; Herrera, C.L.; Sharma, K.; Spong, C.Y.; Twickler, D.M.; Chalak, L.F. Neuroplacentology in congenital heart disease: Placental connections to neurodevelopmental outcomes. Pediatr. Res. 2022, 91, 787–794. [Google Scholar] [CrossRef]
- You, W.; Andescavage, N.N.; Kapse, K.; Donofrio, M.T.; Jacobs, M.; Limperopoulos, C. Hemodynamic Responses of the Placenta and Brain to Maternal Hyperoxia in Fetuses with Congenital Heart Disease by Using Blood Oxygen-Level Dependent MRI. Radiology 2020, 294, 141–148. [Google Scholar] [CrossRef]
- Sun, L.; Macgowan, C.K.; Sled, J.G.; Yoo, S.J.; Manlhiot, C.; Porayette, P.; Grosse-Wortmann, L.; Jaeggi, E.; McCrindle, B.W.; Kingdom, J.; et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation 2015, 131, 1313–1323. [Google Scholar] [CrossRef]
- Linask, K.K. The heart-placenta axis in the first month of pregnancy: Induction and prevention of cardiovascular birth defects. J. Pregnancy 2013, 2013, 320413. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Development of the Human Placenta and Fetal Heart: Synergic or Independent? Front. Physiol. 2018, 9, 373. [Google Scholar] [CrossRef]
- Leon, R.L.; Bitar, L.; Rajagopalan, V.; Spong, C.Y. Interdependence of placenta and fetal cardiac development. Prenat. Diagn. 2024, 44, 846–855. [Google Scholar] [CrossRef]
- Kwee, L.; Baldwin, H.S.; Shen, H.M.; Stewart, C.L.; Buck, C.; Buck, C.A.; Labow, M.A. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development 1995, 121, 489–503. [Google Scholar] [CrossRef]
- Courtney, J.A.; Wilson, R.L.; Cnota, J.; Jones, H.N. Conditional mutation of HAND1 in the mouse placenta disrupts placental vascular development resulting in fetal loss in both early and late pregnancy. Int. J. Mol. Sci. 2021, 22, 9532. [Google Scholar] [CrossRef] [PubMed]
- Fresch, R.; Courtney, J.; Brockway, H.; Wilson, R.L.; Jones, H. HAND1 knockdown disrupts trophoblast global gene expression. Physiol. Rep. 2023, 11, e15553. [Google Scholar] [CrossRef]
- Ge, C.J.; Mahle, A.C.; Burd, I.; Jelin, E.B.; Sekar, P.; Jelin, A.C. Fetal CHD and perinatal outcomes. Cardiol. Young 2020, 30, 686–691. [Google Scholar] [CrossRef]
- Desmond, A.; Imany-Shakibai, H.; Wong, D.; Kwan, L.; Satou, G.; Sklansky, M.; Afshar, Y. Prenatal congenital heart disease and placental phenotypes. JACC Adv. 2023, 2, 100383. [Google Scholar] [CrossRef]
- Sorensen, A.; Hutter, J.; Seed, M.; Grant, P.E.; Gowland, P. T2*-weighted placental MRI: Basic research tool or emerging clinical test for placental dysfunction? Ultrasound Obstet. Gynaecol. 2020, 55, 293–302. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. The human placenta: New perspectives on its formation and function during early pregnancy. Proc. R. Soc. B 2023, 290, 20230191. [Google Scholar] [CrossRef]
| Study | Selection | Comparability | Outcome | Total Score |
|---|---|---|---|---|
| Cromb et al. [1] | 3 | 1 | 2 | 6 |
| Cromb et al. [10] | 2 | 1 | 2 | 5 |
| Jacobwitz et al. [22] | 2 | 1 | 2 | 5 |
| Rajagopalan et al. [23] | 3 | 1 | 2 | 6 |
| Steinweg et al. [24] | 3 | 1 | 2 | 6 |
| Josowitz et al. [25] | 3 | 1 | 2 | 6 |
| Ordas et al. [26] | 3 | 1 | 2 | 6 |
| Authors | Year | Study Design | Number of Participants (n) | Gestational Age at Scan | Congenital Heart Defects | Type of Image Study | Results |
|---|---|---|---|---|---|---|---|
| Cromb et al. [1] | 2024 | Prospective | CHD: 12 Controls: 36 | CHD: 28.7–34.0 Controls: 26.9–32.6 | CoA ToF TGA HLHS TA | Combined diffusion-relaxation MRI | Subjects with CHD had a significantly lower mean T2*. Volume and mean ADC (Apparent Diffusion Coefficient) values did not significantly differ across groups. |
| Cromb et al. [10] | 2025 | Retrospective | CHD: 51 Controls: 30 | CHD: 30.9–32.9 Controls: 31.9–36.7 | CoA TGA HLHS TOF CAT IAA AS APV MA PS DORV TAPVD AVSD | T2*-Relaxometry MRI | While placental volume and maximal placental thickness did not substantially change between groups, the CHD group’s placental T2* value and placental texture did. The CHD group’s placental morphology was noticeably better. |
| Jacobwitz et al. [22] | 2025 | Retrospective | CHD: 53 Controls: 55 | CHD: 32.16 (mean) Controls: 28.11 (mean) | d-TGA HLHS TOF HRH VSD Truncus arteriosus Truncus with IAA Ebstein’s anomaly CAVC DORV AA and VSD Other | MRI | Throughout gestation, the CHD cohort’s placental volumes were noticeably lower than those of the control cohort. |
| Rajagopalan et al. [23] | 2022 | Prospective | CHD: 58 Controls: 114 | CHD: 33.62 (mean) Controls: 32.27 (mean) | HLHS TGA ToF DORV TA, PA CoA AVC VSD ASD | MRI (pBOLD) | The inherent spatiotemporal pBOLD signal variance did not significantly differ between the CHD and non-CHD groups. The association between gestational age and intrinsic pBOLD spatial variance showed a negative interaction with CHD status, while the association between gestational age and temporal variance showed a significant interaction with CHD status. |
| Steinweg et al. [24] | 2021 | Prospective cross-sectional observational study | CHD: 69 Controls: 37 | CHD: 31.3 (mean) Controls: 31.2 (mean) | HLHS CoA RSOL TGA VR Other | MRI | The placenta as a whole showed short T2* values, with extra and quicker deterioration from the lobules’ center to their perimeter. In RSOL, there was an increase in heterogeneity. In comparison to our control cohort, the CHD cohort seemed to exhibit advanced lobularity, increased granularity within the lobules at a given GA, and generally lower signal intensity across the placenta. |
| Josowitz et al. [25] | 2025 | Prospective | CHD: 38 Controls: 36 | CHD: 22.3–26.8 Controls: 20.02–22.0 | SV ToF d-TGA | US/ Doppler | There was no difference in absolute UVVF between patients and controls; however, in comparison to controls, UVVF was considerably lower in all cases and the SV subgroup when it was indexed to fetal weight (UVVF/Wt). When comparing cases to controls, the mean UA pulsatility index (UAPI) was higher and the mean uterine artery PI (UtAPI) was lower. There was no difference between CPR and middle cerebral artery PI (MCAPI). |
| Ordás et al. [26] | 2022 | Prospective | CHD: 71 Controls: 1773 | CHD: 33.27 ± 5.512 Controls: N/A | Subgroup I: TGA, HLHS, AoAD Subgroup II: TOF, VSD, DORV, AVSD, TA, TRUN, PS, L-TGA | US/ Doppler | Higher UtA-PI in CHD-affected subjects than controls |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerede, A.; Orgianelis, I.; Stavros, S.; Potiris, A.; Drakaki, E.; Tsimpoukis, I.; Papasozomenou, P.; Domali, E.; Nikolettos, N.; Chatzakis, C.; et al. Unmasking the Placenta–Heart Axis: A Comprehensive Review of Placental Abnormalities in Congenital Heart Disease. Diagnostics 2025, 15, 2159. https://doi.org/10.3390/diagnostics15172159
Gerede A, Orgianelis I, Stavros S, Potiris A, Drakaki E, Tsimpoukis I, Papasozomenou P, Domali E, Nikolettos N, Chatzakis C, et al. Unmasking the Placenta–Heart Axis: A Comprehensive Review of Placental Abnormalities in Congenital Heart Disease. Diagnostics. 2025; 15(17):2159. https://doi.org/10.3390/diagnostics15172159
Chicago/Turabian StyleGerede, Angeliki, Ilias Orgianelis, Sofoklis Stavros, Anastasios Potiris, Eirini Drakaki, Ioannis Tsimpoukis, Panagiota Papasozomenou, Ekaterini Domali, Nikolaos Nikolettos, Christos Chatzakis, and et al. 2025. "Unmasking the Placenta–Heart Axis: A Comprehensive Review of Placental Abnormalities in Congenital Heart Disease" Diagnostics 15, no. 17: 2159. https://doi.org/10.3390/diagnostics15172159
APA StyleGerede, A., Orgianelis, I., Stavros, S., Potiris, A., Drakaki, E., Tsimpoukis, I., Papasozomenou, P., Domali, E., Nikolettos, N., Chatzakis, C., & Eleftheriades, M. (2025). Unmasking the Placenta–Heart Axis: A Comprehensive Review of Placental Abnormalities in Congenital Heart Disease. Diagnostics, 15(17), 2159. https://doi.org/10.3390/diagnostics15172159








