Colon Capsule Endoscopy as a Promising Diagnostic Tool in Colorectal Cancer: A Systematic Review and Network Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Statistical Analysis
2.1.1. Meta-Analysis
2.1.2. NMA
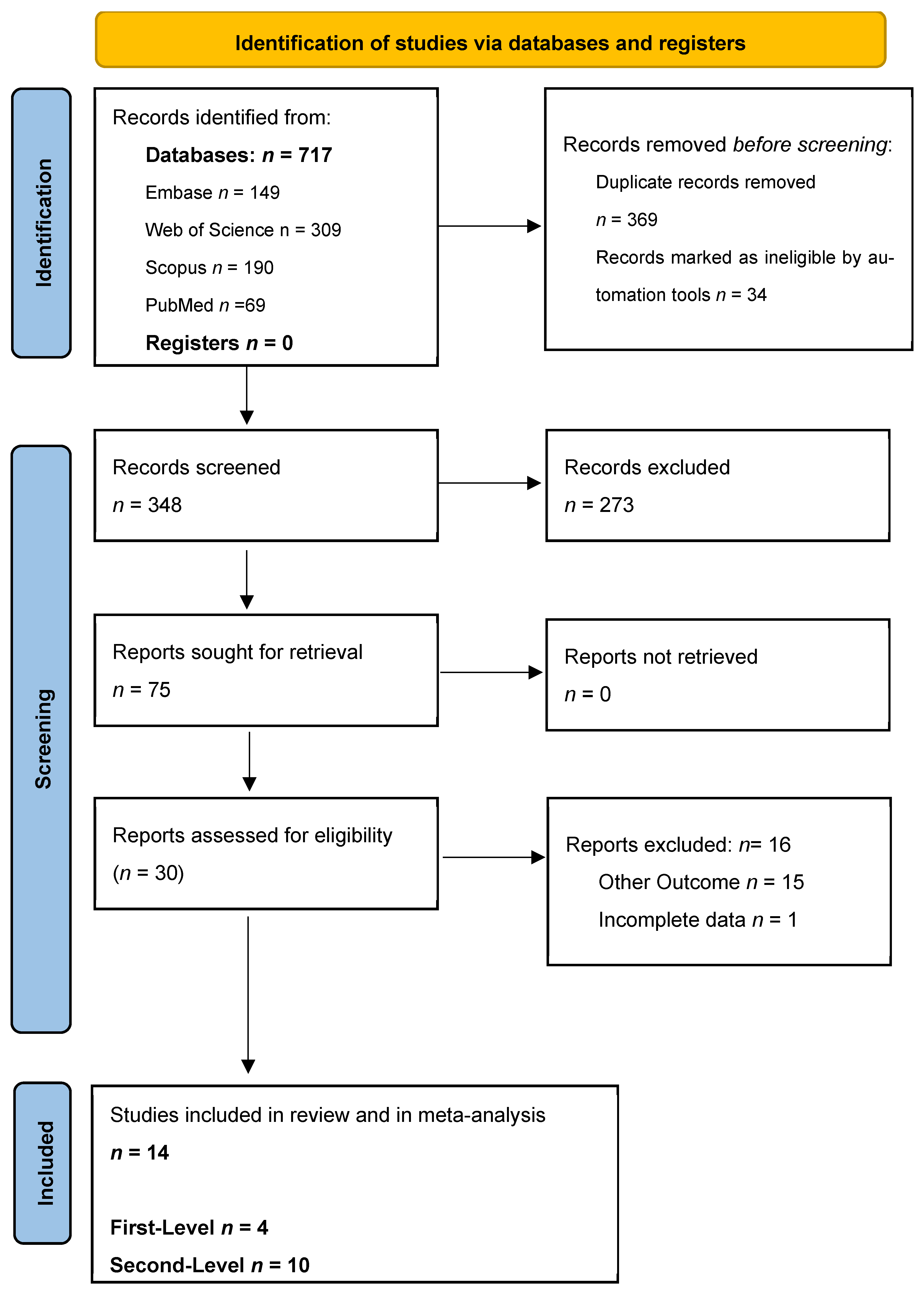
3. Results
4. Systematic Review Results
4.1. First Level: CCE as a Triage Test in a General Population Undergoing Screening for Colon Cancer
4.2. Second-Level Evaluation: Diagnostic Accuracy of CCE in FIT-Positive or High-Risk Populations
4.3. Mixed-Population Studies
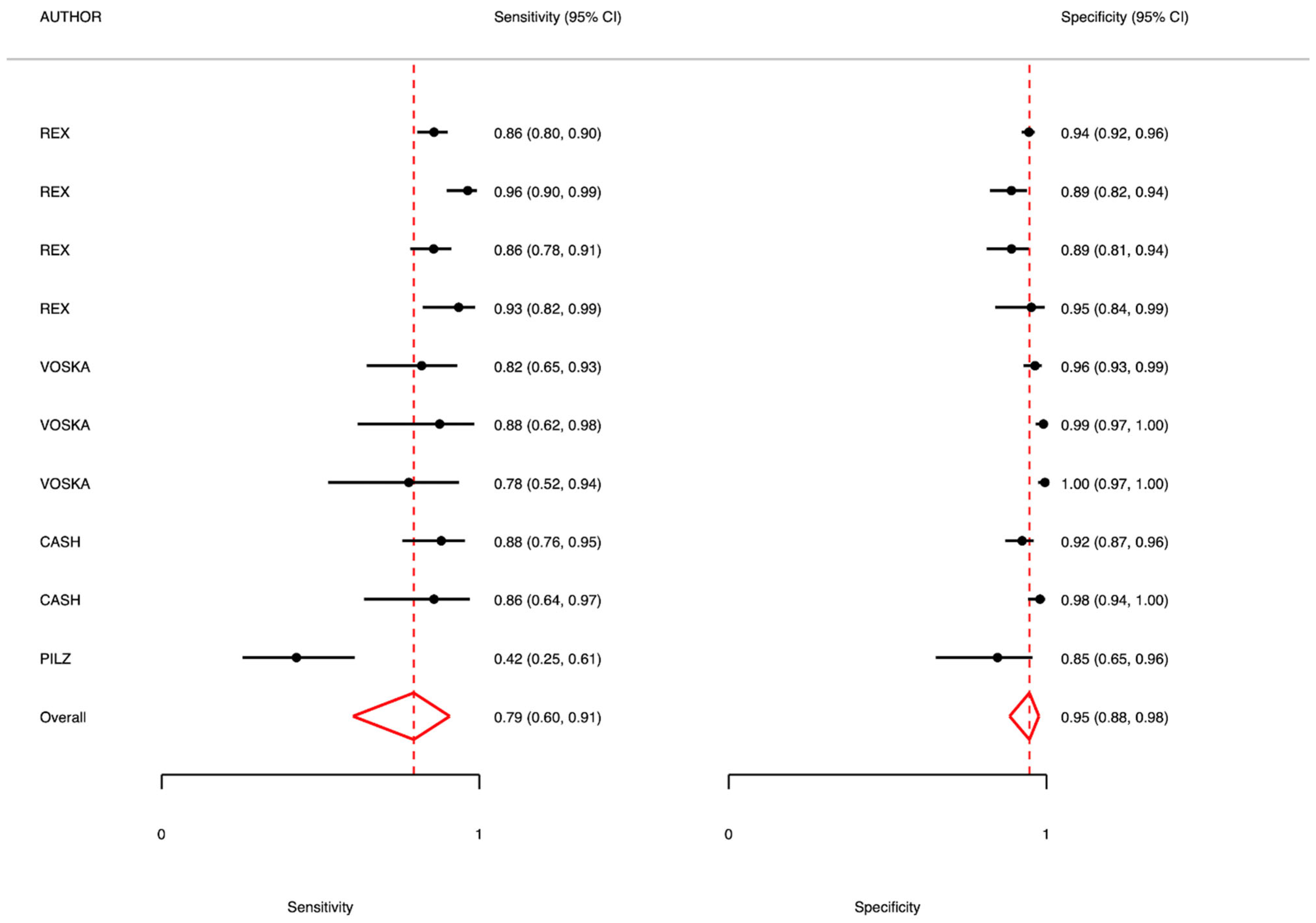
5. Colon Capsule as First-Level Diagnostic Test: Meta-Analysis Results
5.1. Adenomas 6–9 mm
5.2. Adenomas >9 mm
5.3. Polyps 6–9 mm
6. Second-Level Testing—Overall Analysis
6.1. Polyps and Adenomas 6–9 mm
6.2. Polyps and Adenomas >9 mm
6.3. FIT-Positive Population
6.4. Mixed Cases
7. Discussion
8. Limitations
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Screening, Diagnosis and Care; European Commission Initiative on Breast and Colorectal cancer European Commission Initiative on Colorectal Cancer (ECICC). Guidelines and Quality Assurance Standards for Colorectal Cancer Screening; European Commission: Brussels, Belgium, 2022; Available online: https://cancer-screening-and-care.jrc.ec.europa.eu/en (accessed on 31 May 2025).
- International Agency for Research on Cancer, World Health Organization. Available online: https://www.iarc.who.int/cancer-topics/ (accessed on 31 May 2025).
- Bretthauer, M.; Kalager, M. Principles, effectiveness and caveats in screening for cancer. Br. J. Surg. 2013, 100, 55–65. [Google Scholar] [CrossRef]
- Altobelli, E.; D’Aloisio, F.; Angeletti, P.M. Colorectal cancer screening in countries of European Council outside of the EU-28. World J. Gastroenterol. 2016, 22, 4946–4957. [Google Scholar] [CrossRef]
- Altobelli, E.; Rapacchietta, L.; Marziliano, C.; Campagna, G.; Profeta, V.F.; Fagnano, R. Differences in colorectal cancer surveillance epidemiology and screening in the WHO European Region. Oncol. Lett. 2019, 17, 2531–2542. [Google Scholar] [CrossRef]
- Schwarz, S.; Hornschuch, M.; Pox, C.; Haug, U. Polyp detection rate and cumulative incidence of post-colonoscopy colorectal cancer in Germany. Int. J. Cancer. 2023, 152, 1547–1555. [Google Scholar] [CrossRef]
- Hosoe, N.; Limpias Kamiya, K.J.L.; Hayashi, Y.; Sujino, T.; Ogata, H.; Kanai, T. Current status of colon capsule endoscopy. Dig. Endosc. 2021, 33, 529–537. [Google Scholar] [CrossRef]
- Iddan, G.; Meron, G.; Glukhovsky, A.; Swain, P. Wireless capsule endoscopy. Nature 2000, 405, 417. [Google Scholar] [CrossRef]
- Eliakim, R.; Fireman, Z.; Gralnek, I.M.; Yassin, K.; Waterman, M.; Kopelman, K.; Lachter, K.; Koslowsky, B.; Adler, S.N. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: Results of the first multicenter, prospective, comparative study. Endoscopy 2006, 38, 963–970. [Google Scholar] [CrossRef]
- Eliakim, R.; Yassin, K.; Niv, Y.; Metzger, Y.; Lachter, J.; Gal, E.; Sapoznikov, B.; Konikoff, F.; Leichtmann, G.; Fireman, Z.; et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy 2009, 41, 1026–1231. [Google Scholar] [CrossRef]
- Vuik, F.E.R.; Nieuwenburg, S.A.V.; Moen, S.; Spada, C.; Senore, C.; Hassan, C.; Pennazio, C.; Rondonotti, E.; Pecere, S.; Kuipers, E.J.; et al. Colon capsule endoscopy in colorectal cancer screening: A systematic review. Endoscopy 2021, 53, 815–824. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, 372. [Google Scholar]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Salanti, G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: Many names, many benefits, many concerns for the next generation evidence synthesis tool. Res. Synth. Methods 2012, 3, 80–97. [Google Scholar] [CrossRef]
- Rex, D.K.; Adler, S.N.; Aisenberg, J.; Burch, W.C., Jr.; Carretero, C.; Chowers, Y.; Fein, S.A.; Fern, S.E.; Fernandez-Urien Sainz, I.; Fich, A.; et al. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterology 2015, 148, 948–957. [Google Scholar] [CrossRef]
- Voska, M.; Zavoral, M.; Grega, T.; Majek, O.; Martinek, J.; Tacheci, I.; Benes, M.; Vojtechova, G.; Drastich, P.; Bures, J.; et al. Accuracy of Colon Capsule Endoscopy for Colorectal Neoplasia Detection in Individuals Referred for a Screening Colonoscopy. Gastroenterol. Res. Pract. 2019, 2019, 5975438. [Google Scholar] [CrossRef]
- Pilz, J.B.; Portmann, S.; Peter, S.; Beglinger, C.; Degen, L. Colon Capsule Endoscopy compared to Conventional Colonoscopy under routine screening conditions. BMC Gastroenterol. 2010, 10, 66. [Google Scholar] [CrossRef]
- Cash, B.D.; Fleisher, M.R.; Fern, S.; Rajan, E.; Haithcock, R.; Kastenberg, D.M.; Pound, D.; Papageorgiou, N.P.; Fernández-Urién, I.; Schmelkin, I.J.; et al. Multicentre, prospective, randomised study comparing the diagnostic yield of colon capsule endoscopy versus CT colonography in a screening population (the TOPAZ study). Gut 2021, 70, 2115–2122. [Google Scholar] [CrossRef]
- González-Suárez, B.; Pagés, M.; Araujo, I.K.; Romero, C.; Rodríguez de Miguel, C.; Ayuso, J.R.; Pozo, À.; Vila-Casadesús, M.; Serradesanferm, A.; Ginès, À.; et al. Colon capsule endoscopy versus CT colonography in FIT-positive colorectal cancer screening subjects: A prospective randomized trial-the VICOCA study. BMC Med. 2020, 18, 255. [Google Scholar] [CrossRef]
- Pioche, M.; Ganne, C.; Gincul, R.; De Leusse, A.; Marsot, J.; Balique, J.; Fond, A.; Bretagnolle, M.; Henry, L.; Billaud, Y.; et al. Colon capsule versus computed tomography colonography for colorectal cancer screening in patients with positive fecal occult blood test who refuse colonoscopy: A randomized trial. Endoscopy 2018, 50, 761–769. [Google Scholar] [CrossRef]
- Rondonotti, E.; Borghi, C.; Mandelli, G.; Radaelli, F.; Paggi, S.; Amato, A.; Imperiali, G.; Terreni, N.; Lenoci, N.; Terruzzi, V.; et al. Accuracy of capsule colonoscopy and computed tomographic colonography in individuals with positive results from the fecal occult blood test. Clin. Gastroenterol. Hepatol. 2014, 12, 1303–1310. [Google Scholar] [CrossRef]
- Holleran, G.; Leen, R.; O’Morain, C.; McNamara, D. Colon capsule endoscopy as possible filter test for colonoscopy selection in a screening population with positive fecal immunology. Endoscopy 2014, 46, 473–478. [Google Scholar]
- Pecere, S.; Senore, C.; Hassan, C.; Riggi, E.; Segnan, N.; Pennazio, M.; Sprujievnik, T.; Rondonotti, E.; Baccarin, A.; Quintero, E.; et al. Accuracy of colon capsule endoscopy for advanced neoplasia. Gastrointest. Endosc. 2020, 91, 406–414. [Google Scholar] [CrossRef]
- Kobaek-Larsen, M.; Kroijer, R.; Dyrvig, A.K.; Buijs, M.M.; Steele, R.J.C.; Qvist, N.; Baatrup, G. Back-to-back colon capsule endoscopy and optical colonoscopy in colorectal cancer screening individuals. Color. Dis. 2018, 20, 479–485. [Google Scholar] [CrossRef]
- Spada, C.; Hassan, C.; Marmo, R.; Petruzziello, L.; Riccioni, M.E.; Zullo, A.; Cesaro, P.; Pilz, J.; Costamagna, G. Meta-analysis shows colon capsule endoscopy is effective in detecting colorectal polyps. Clin. Gastroenterol. Hepatol. 2010, 8, 516–522. [Google Scholar] [CrossRef]
- Morgan, D.R.; Malik, P.R.; Romeo, D.P. Initial US evaluation of second-generation capsule colonoscopy for detecting colon polyps. BMJ Open Gastroenterol. 2016, 3, e000089. [Google Scholar] [CrossRef]
- Parodi, A.; Vanbiervliet, G.; Hassan, C.; Hebuterne, X.; De Ceglie, A.; Filiberti, R.A.; Spada, C.; Conio, M. Colon capsule endoscopy to screen for colorectal neoplasia in those with family histories of colorectal cancer. Gastrointest. Endosc. 2018, 87, 695–704. [Google Scholar] [CrossRef]
- Raffle, A.E.; Mackie, A.; Muir Gray, J.A. Screening: Evidence and Practice, 2nd ed.; Oxford University Press: Cary, NC, USA, 2019. [Google Scholar]
- Lee, J.K.; Liles, E.G.; Bent, S.; Levin, T.R.; Corley, D.A. Accuracy of fecal immunochemical tests for colorectal cancer: Systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 171. [Google Scholar] [CrossRef]
- Imperiale, T.F.; Gruber, R.N.; Stump, T.E.; Emmett, T.W.; Monahan, P.O. Performance Characteristics of Fecal Immunochemical Tests for Colorectal Cancer and Advanced Adenomatous Polyps: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2019, 170, 319–329. [Google Scholar] [CrossRef]
- Andermann, A.; Blancquaert, I.; Beauchamp, S.; Déry, V. Revisiting Wilson and Jungner in the genomic age: A review of screening criteria over the past 40 years. Bull. World Health Organ. 2008, 86, 317–319. [Google Scholar] [CrossRef]
- Rex, D.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Levin, T.R.; Lieberman, D.; Robertson, D.J. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2017, 112, 1016–1030. [Google Scholar] [CrossRef]
- Turvill, J.; Haritakis, M.; Pygall, S.; Bryant, E.; Cox, H.; Forshaw, G.; Musicha, C.; Allgar, V.; Logan, R.; McAlindon, M. Multicentre Study of 10,369 Symptomatic Patients Comparing the Diagnostic Accuracy of Colon Capsule Endoscopy, Colonoscopy and CT Colonography. Aliment. Pharmacol. Ther. 2025, 61, 1532–1544. [Google Scholar] [CrossRef]
- Scottish Health Technologies Group. Colon Capsule Endoscopy (CCE-2) for the Detection of Colorectal Polyps and Cancer in Adults. Available online: https://shtg.scot/media/2430/20240109-cce-update-v10.pdf (accessed on 30 June 2025).
- Baatrup, G.; Bjørsum-Meyer, T.; Kaalby, L.; Schelde-Olesen, B.; Kobaek-Larsen, M.; Koulaouzidis, A.; Kroijer, R.; Al-Najami, I.; Buch, N.; Høgh, A.; et al. Choice of colon capsule or colonoscopy versus default colonoscopy in FIT positive patients in the Danish screening programme: A parallel group randomised controlled trial. Gut 2025, e333687. [Google Scholar] [CrossRef]
- Hotta, N.; Ohmiya, N.; Hiraga, H.; Nakaji, K.; Osawa, S.; Omori, T.; Mitsufuji, S.; Hosoe, N.; Nouda, S.; Kobayashi, T.; et al. Nationwide multicenter prospective study on the usefulness, safety, and acceptability of colon capsule endoscopy in Japan. Gastrointest. Endosc. 2025, 101, 1051–1063. [Google Scholar] [CrossRef]

| Author, Year, Country | Study Population | Results | ||||
|---|---|---|---|---|---|---|
| Study Setting | Sample Size, Gender (% Males), Age | Outcome | Diagnostic Accuracy: Se, Sp, VPN, VPP, LR+LR− | Other Results | ||
| Colon Capsule | ||||||
| Dimension | ||||||
| 6–9 mm | >9 mm | |||||
| Rex 2015 USA [15] | Prospective cohort study | 885 44% male mean age 57 y. range not reported | Polyps and Adenomas | Polyps SE 88 (82–93) SP 82 (80–83) PPV 86 (83–88) NPV 95 (94–96) LR+ 14.5 LR− 0.14 Adenomas SE 81 (77–84) SP 93 (91–95) PPV 85 (82–89) NPV 89 (86–93) LR+ 5.35 LR− 0.11 | Polyps SE 92 (82–97) SP 95 (94–95) PPV 96 (93–98) NPV 89 (86–92) LR+ 28.33 LR− 0.15 Adenomas SE 80 (74–86) SP 97 (96–98) PPV 93 (89–97) NPV 95 (91–98) LR+ 11.88 LR− 0.05 | Colonoscopy result frequency (%) Polyps 6–9 mm 28% >9 mm 11% Adenomas 6–9 mm 16% >9 mm 6% |
| Voska 2019 Czech Republic [16] | Prospective cohort study | 225 men, 53% mean age: 59 years; range: 50–81 years | Polyps and Adenomas | Polyps SE 79 (62–91) SP 97 (80–83) PPV 82 (75–90) NPV 96 (95–98) LR+ 26.34 LR− 0.22 | Polyps SE 88 (62–98) SP 97 (80–83) PPV 87 (75–90) NPV 99 (99–100) LR+ 88 LR− 0.12 Adenomas SE 100 SP 98 (80–83) PPV 76 (65–88) NPV 100 (100–100) LR+ 50 LR− 0 | Colonoscopy result frequency (%) Polyps 6–9 mm 15% >9.7% Adenomas >9.5% |
| Pilz 2010 Switzerland [17] | Prospective cohort study | 59 | Polyps and Adenomas | SE 79 (61–90) SP 54 (35–70) PPV 71 (62–80) NPV 66 (54–79) LR+ 2.08 LR− 0.65 | Colonoscopy result frequency (%) 50% (n = 28) | |
| Cash 2020 USA [18] | Prospective randomized study | 320 | Polyps | Polyps 6–9 mm SE 79.2 (66.4–82.2) SP 96.3 (89.1–92.2) PPV 88 (83–93) NPV 93 (90–95) LR+ 19.75 LR− 0.22 Polyps >9 mm SE 85.7 (64.5–95.9) SP 98.2 (93.3–99.0) PPV 86 (78–94) NPV 98 (97–99) LR+ 43 LR− 0.14 | Adenomas not reported | Colonoscopy result frequency (%) Polyps 6–9 mm 27.6% Polyps > 9 mm 10.3% Adenomas 6–9 mm 17.6% Adenomas > 10 mm 6.1% |
| Author, Year, Country | Study Population | Main Results | Other Results | |||
|---|---|---|---|---|---|---|
| Study Setting | Sample Size | Outcome | Diagnostic Accuracy | |||
| FIT+ | ||||||
| Gonzales-Suarez Spain 2020 [19] | RCT | n = 286 mean age 59 y (50–81 y) men 53% | Polyps and adenomas | 6 mm (any neoplastic lesion) SE 96. (91.1–100) SP 88.2 (79.6–95.3) PPV 90.2 (83.5–96.1) NPV 95.2 (89.2–100) AUC 92.4 (87.5–96.5) LR+ 8.04 LR− 0.04 | >9 mm (any neoplastic lesion) SE 97.3 (91.1–100) SP 95.3 (90.7–99.0) PPV 87.8 (76.7–97.3) NPV 99.0 (96.8–100) AUC 95.5 (92.4–99.3) LR+ 20.4 LR− 0.02 | CT colonoscopy 6 mm (any neoplastic lesion) SE 79.3 (68.6–88.8) SP 96.3 (91.1–100) PPV 93.8 (85.7–100) NPV 86.9 (80.0–93.2) AUC 89.3 (83.6–93.6) 10 mm (any neoplastic lesion) SE 90.0 (83.9–93.9) SP 99.0 (95.6–100) PPV 97.3 (93.1–100) NPV 96.1 (91.5–98.2) AUC 96.4 (91.9–98.4) |
| Pioche 2018 France [20] | RCT | n = 78 mean age and gender not reported on effective performed exams | Adenomas and cancers | >9 mm (any neoplastic lesion) SE: 60.0 (36.1–80.9) SP 40.0 (19.1–63.9) PPV 80.0 NPV 20.0 LR+ 1.0 LR− 1.0 | CT colonography SE 28.6 (11.3–52.2) SP 53.6 (33.9–72.5) PPV: 46.4 NPV: 53.6 | |
| Rondonotti 2014 Italy [21] | Prospective cohort study | n = 50 mean age 59.2 +5.8 y male 58% | Polyps | 6–9 mm SE 88.2 (62.2–97.9) SP 87.8 (70.8–96.0) PPV 79 (73–81) NPV 94 (90–94) LR+ 3.7 (1.52–9.2) LR− 0.06 (0.01–0.26) | ≥9 mm SE 92.8 (64.1–99.6) SP 91.6 (76.4–97.8) PPV 81 (76–83) NPV 0.97 (0.95–0.98) LR+ 4.3 (1.5–12.3) LR− 0.03 (0.004–0.20) | CT colonography 6–9 mm SE 88.2 (62.2–97.9) SP84.8 (67.3–94.3) LR+ 3.0 (1.34–6.67) LR− 0.07 (0.01–0.27) ≥9 mm SE 78.6 (48.8–94.3) SP 91.7 (76.4–97.8) LR+ 3.7 (1.3–10.4) LR− 0.09 (0.03–0.20) |
| Holleran Ireland 2013 [22] | Prospective cohort study | n = 62 mean age 62.5 + 5.8 y. male 55% | Polyps | ≥9 mm SE 97 (84–100) SP 86 (0.68–0.96) PPV 97 (95–99) NPV 86 (82–87) LR+ 23.11 LR− 0.11 | Colonoscopy results on significant lesions were the same as CCE | |
| Pecere Italy 2019 [23] | Prospective cohort study | n = 222 mean age 66 y. male 56.3% | Adenomas | 6–9 mm SE 90.0 SP 66.1 PPV 57 (54–58) NPV 92.9 (91–93) LR+ 2.6 LR− 0.15 | ≥9 mm SE 76.7 SP 90.7 PPV 80.7 (78–82) NPV 88.4 (86–89) LR+ 8.2 LR− 0.25 | |
| Kobaek-Larsen 2018 [24] | Prospective cohort study | n = 253 | Polyps | >9 mm SE 87 (78–91) SP 92 (89–95) PPV 86 (84–87) NPV 92 (91–93) LR+ 10.71 LR− 0.14 | ||
| MIXED CASES | ||||||
| Spada 2010 Italy [25] | Prospective cohort study | n = 117 mean age 60 +9 y male 61.5% | Neoplasia and adenomas | 6–9 mm SE 84 (74–95) SP 64 (52–76) PPV 54 (50–55) NPV 89 (86–89) LR+ 2.3 LR− 0.24 | >9 mm SE 88 (76–99) SP 95 (90–100) PPV 88 (84–88) NPV 95 (93–95) LR+ 17.06 LR− 0.13 | 6–9 mm 41% ≥9 mm 29% |
| Eliakim 2009 Israel [10] | Prospective cohort study | n = 104 mean age 49.8 (range 18–57) | 6–9 mm Se 89 (70–97) Sp 76 (72–78 PPV 46 (41–46) NPV 97 (95–97) LR+ 3.7 LR− 0.14 | >9 mm SE 88 (56–98) SP 89 (86–90) PPV 41 (36–46) NPV 97 (95–97) LR+ 7.8 LR− 0.14 | 6–9 mm 19% ≥9 mm 8% | |
| Morgan USA 2016 [26] | Prospective cohort study | n = 50 mean age 60.2 y (range 32–70) male 45% | Polyps | 6–9 MM SE 93.3 (66.0–97.7) SP 80 (62.5–90.9) PPV 67 (60–68) NPV 97 (94–97) LR+ 4.6 LR− 0.08 | >9 mm SE 100 (56.1–100.0) SP 93.0 (79.9–98.2) PPV 70 (64–71) NPV 98 (95–98) LR+ 12.5 LR− 0.13 | 6–9 mm 30% ≥9 mm 14% |
| Parodi Italy 2017 [27] | Prospective cohort study | n = 177 mean age 57 y. (26–82) male 45.3% | Polyps | 6–9 mm SE 91 (81–96) SP 88 (81–93) PPV 75 (72–75) NPV 98 (97–98) LR+ 16.7 LR− 0.17 | >9 mm SE 89 (72–96) SP 95 (90–97) PPV 77 (74–78) NPV 95 (94–96) LR+ 7.34 LR− 0.12 | OC diagnosed 243 lesions, of which 151 (62.1%) < 6 mm, 92 (37.9%) ≥ 6 mm and 41 (16.9%) ≥ 10 mm |
| Author | Bowel Preparation Method |
|---|---|
| Rex [15] | Senna (12 mg × 4 tablets, 2 days prior), clear liquids (day before), 2 L PEG-ELS (evening + morning), oral sulfate solution (SUPREP), bisacodyl if delayed capsule transit. |
| Voska [16] | PEG with sodium phosphate booster (details limited). |
| Pilz [17] | Low-fiber diet (3 days), 4 L PEG (Cololyt®), domperidone, sodium phosphate (Colophos®) boosters, bisacodyl suppository. |
| Cash [18] | Split-dose PEG-based regimen; details in Supplementary Materials. |
| Gonzales-Suarez [19] | Low-residue diet for 3 days prior. Day -1: 2 L PEG (PM). Day 0: 2 L PEG (AM), then capsule ingestion. Booster 1: 40 mL sodium phosphate + 1 L water. Booster 2 (if no excretion after 2 h): 20 mL sodium phosphate + 1 L water. |
| Pioche [20] | 2-day prep: Day -2: 4 L PEG + water; Day -1: 3 L PEG + clear liquids; Day 0: 1 L PEG + domperidone + sodium phosphate (55 mL) + bisacodyl suppository. |
| Rondonotti [21] | Day -3/-2: low-fiber diet; Day -1: clear liquids + Macrogol 3350 + ascorbic acid (2 L), bisacodyl 20 mg at night; Day 0: Macrogol 3350 + ascorbic acid (1 L) before capsule, then metoclopramide IV, NaP booster (30 mL + 1 L), then 15 mL + 500 mL. |
| Holleran [22] | PEG-based regimen (not fully specified). |
| Pecere [23] | Split-dose PEG + sodium phosphate and gastrografin boosters. |
| Kobaek-Larsen [24] | Day -2: 1000 mg magnesium oxide + 2 L water (AM), 1000 mg magnesium oxide (PM). Day -1: clear liquids, 1 L Moviprep + 2 L water (PM). Day 0: 1 L Moviprep + 1 L water (AM), 20 mg domperidone orally. Booster 1: ¾ L Moviprep + 1 L water. Booster 2: ¼ L Moviprep + ¼ L water, 10 mg bisacodyl suppository if no excretion. |
| Spada [25] | Senna (4 tablets, 2 days prior), 4 L split PEG, sodium phosphate boosters guided by data recorder, bisacodyl suppository if capsule not expelled. |
| Eliakim [9] | PEG, sodium phosphate, bisacodyl suppositories. |
| Morgan [26] | Magnesium citrate as booster—shown to result in inadequate cleansing. |
| Parodi [27] | Day -1: 2 L PEG–electrolyte solution (PM). Day 0: 2 L PEG at 6:00 AM, capsule ingestion after 10 h fasting. Booster 1: 40 mL sodium phosphate + 125 mL water + 1 L water. Booster 2 (after 2 h): 20 mL sodium phosphate + 125 mL water + 1 L water. If no excretion: 10 mg bisacodyl suppository. |
| Sensibility | Specificity | PPV | NPV | |
|---|---|---|---|---|
| First-level studies | ||||
| Overall lesions K= 7 | 0.79 (0.60–0.91) I2 = 57.59 | 0.95 (0.88–0.98) I2 = 45.86 | 0.89 (0.82–0.91) I2 = 93.3% | 0.97 (0.96–0.97) I2 = 94.9 |
| Adenomas 6–9 mm K = 3 | 0.83 (0.76–0.88) I2 = 26.82 | 0.95 (0.93–0.97) I2 = 30.21 | 0.95 (0.93–0.97) I2 = 81.98 | 0.86 (0.84–0.88) I2 = 0.05 |
| Adenomas >9 K = 3 | 0.85 (0.78–0.90) I2 = 0.00 | 0.98 (0.97–0.99) I2 = 0.00 | 0.91 (0.84–0.98) I2 = 71.55 | 0.95 (0.89–1.02) I2 = 98.53 |
| Polyps 6–9 mm K = 2 | 0.95 (0.86–0.98) | 0.97 (0.94–0.99) | 0.86 (0.70–1.01) | - |
| Second-level studies | ||||
| Overall lesions K = 17 | 0.75 (0.65–0.83) I2 = 75.00 | 0.95 (0.92–0.97) I2 = 55.41 | 0.76 (0.73–0.74) I2 = 99.5 | 0.95 (0.95–0.62) I2 = 98.4 |
| Polyps and adenomas 6–9 mm K = 7 | 0.69 (0.55–0.80) I2 = 75.87 | 0.95 (0.92–0.97) I2 = 20.93 | 0.63 (0.68–0.70) I2 = 99.5 | 0.95 (0.95–0.96) I2 = 96.8 |
| Polyps and adenomas >9 mm K = 10 | 0.79 (0.67–0.88) I2 = 72.40 | 0.94 (0.89–0.97) I2 = 63.56 | 0.79 (0.78–0.80) I2 = 99.4 | 0.96 (0.95–0.97) I2 = 98.8 |
| FIT+ K = 6 | 0.81 (0.69–0.89) I2 = 76.54 | 0.92 (0.86–0.96) I2 = 61.87 | 0.81 (0.80–0.81) I2 = 98.5 | 0.94 (0.94–0.95) I2 = 99.7 |
| Mixed cases K = 4 | 0.66 (0.54–0.76) I2 = 63.68 | 0.96 (0.94–0.98) I2 = 23.84 | 0.70 (0.69–0.71) I2 = 99.6 | 0.96 (0.95–0.97) I2 = 97.3 |
| Baseline (COL) | |
|---|---|
| CCEIndirect | SMDSE = 0.30 (0.12; 0.47) SMDSP = −0.18 (−0.29; −0.06) SMDPPV = 0.02 (−0.13; 0.17) SMDNPV = 0.16 (−0.09; 0.40) |
| CTCDirect | SMDSE = 0.44 (0.29; 0.59) SMDSP = −0.98 (−1.07; −0.90) SMDPPV = −0.02 (−0.14; 0.10) SMDNPV = 0.10 (−0.10; 0.31) |
| Se | Sp | VPP | VPN | |
|---|---|---|---|---|
| COL | 0.9998 | 1.0000 | 0.7122 | 0.8655 |
| CCF | 0.1989 | 0.1901 | 0.4805 | 0.1773 |
| CTC | 0.0013 | 0.0006 | 0.3073 | 0.1572 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altobelli, E.; Angeletti, P.M.; Varesini, P.A.; Bianchi, Z.; Masedu, F. Colon Capsule Endoscopy as a Promising Diagnostic Tool in Colorectal Cancer: A Systematic Review and Network Meta-Analysis. Diagnostics 2025, 15, 2157. https://doi.org/10.3390/diagnostics15172157
Altobelli E, Angeletti PM, Varesini PA, Bianchi Z, Masedu F. Colon Capsule Endoscopy as a Promising Diagnostic Tool in Colorectal Cancer: A Systematic Review and Network Meta-Analysis. Diagnostics. 2025; 15(17):2157. https://doi.org/10.3390/diagnostics15172157
Chicago/Turabian StyleAltobelli, Emma, Paolo Matteo Angeletti, Paolo Angelo Varesini, Zuleyka Bianchi, and Francesco Masedu. 2025. "Colon Capsule Endoscopy as a Promising Diagnostic Tool in Colorectal Cancer: A Systematic Review and Network Meta-Analysis" Diagnostics 15, no. 17: 2157. https://doi.org/10.3390/diagnostics15172157
APA StyleAltobelli, E., Angeletti, P. M., Varesini, P. A., Bianchi, Z., & Masedu, F. (2025). Colon Capsule Endoscopy as a Promising Diagnostic Tool in Colorectal Cancer: A Systematic Review and Network Meta-Analysis. Diagnostics, 15(17), 2157. https://doi.org/10.3390/diagnostics15172157








