Fetal and Neonatal Outcomes in Fetuses with an Estimated Fetal Weight Percentile of 10–20 in the Early Third Trimester: A Retrospective Cohort Study
Abstract
1. Introduction
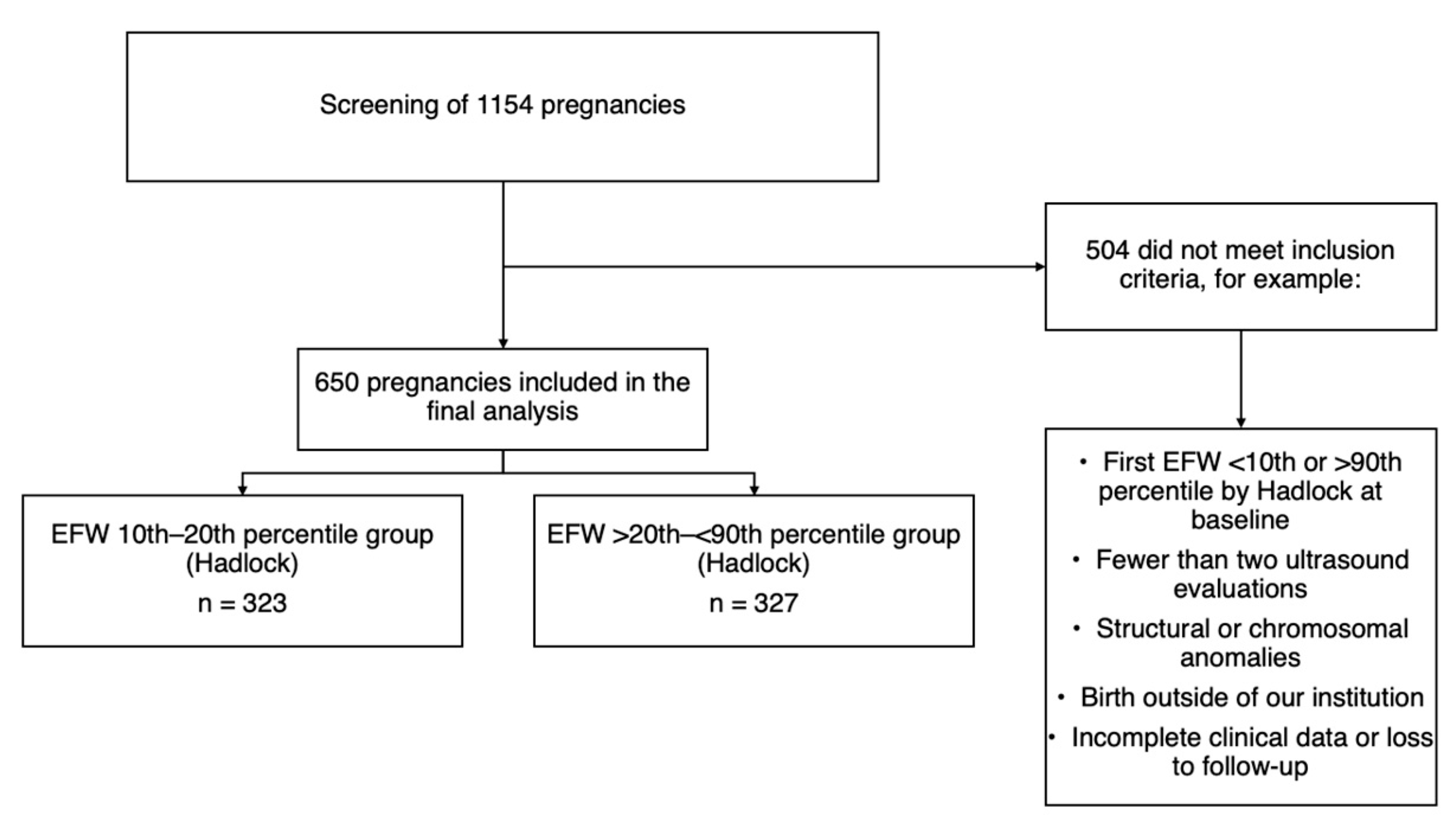
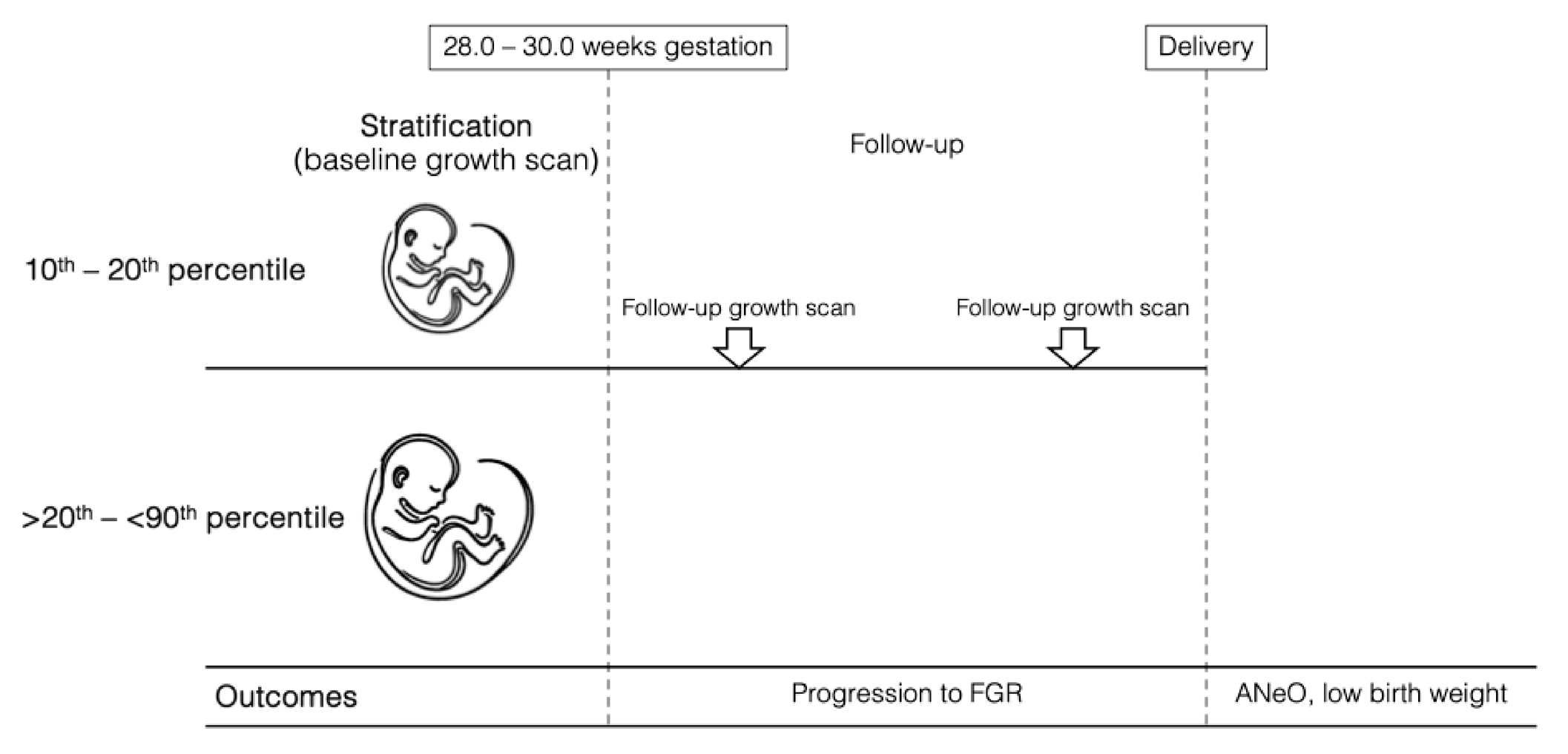
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
4.1. Research Implications
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EFW | Estimated fetal weight |
| AC | Abdominal circumference |
| FGR | Fetal growth restriction |
| LGA | Large for gestational age |
| FL | Femur length |
| HC | Head circumference |
| DBP | Biparietal diameter |
| ANeO | Adverse neonatal outcome |
| NICU | Neonatal intensive care unit |
| ACOG | American College of Obstetricians and Gynecologists |
| CXR | Chest X-ray |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| SD | Standard deviation |
| IQR | Interquartile range |
| CI | Confidence intervals |
| RGM | Ratios of geometric means |
| BMI | Body mass index |
| WHO | World Health Organization |
References
- Salomon, L.J.; Alfirevic, Z.; Da Silva Costa, F.; Deter, R.L.; Figueras, F.; Ghi, T.; Glanc, P.; Khalil, A.; Lee, W.; Napolitano, R.; et al. ISUOG Practice Guidelines: Ultrasound Assessment of Fetal Biometry and Growth. Ultrasound Obstet. Gynecol. 2019, 53, 715–723. [Google Scholar] [CrossRef]
- Martins, J.G.; Biggio, J.R.; Abuhamad, A. Society for Maternal-Fetal Medicine Consult Series #52: Diagnosis and Management of Fetal Growth Restriction: (Replaces Clinical Guideline Number 3, April 2012). Am. J. Obstet. Gynecol. 2020, 223, B2–B17. [Google Scholar] [CrossRef]
- Deter, R.L.; Lee, W.; Yeo, L.; Erez, O.; Ramamurthy, U.; Naik, M.; Romero, R. Individualized Growth Assessment: Conceptual Framework and Practical Implementation for the Evaluation of Fetal Growth and Neonatal Growth Outcome. Am. J. Obstet. Gynecol. 2018, 218, S656–S678. [Google Scholar] [CrossRef]
- Onald, D.; Ntire, I.; Teven, S.; Loom, L.B.; Asey, R.M.C.; Eveno, J.L. Birth Weight in Relation to Morbidity and Mortality among Newborn Infants. N. Engl. J. Med. 1999, 340, 1234–1238. [Google Scholar] [CrossRef]
- Futterman, I.D.; Snyder, A.; O’Hagan, K.; Siegel, M.R.; Grimes, C.L. Mode of Delivery and Neonatal Outcomes in Pregnancies with Fetal Growth Restriction and Abnormal Umbilical Artery Dopplers. Am. J. Obstet. Gynecol. MFM 2022, 4, 100497. [Google Scholar] [CrossRef]
- Familiari, A.; Khalil, A.; Rizzo, G.; Odibo, A.; Vergani, P.; Buca, D.; Hidaka, N.; Di Mascio, D.; Nwabuobi, C.; Simeone, S.; et al. Adverse Intrapartum Outcome in Pregnancies Complicated by Small for Gestational Age and Late Fetal Growth Restriction Undergoing Induction of Labor with Dinoprostone, Misoprostol or Mechanical Methods: A Systematic Review and Meta-Analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 455–467. [Google Scholar] [CrossRef]
- Boers, K.E.; Van Wyk, L.; Van Der Post, J.A.M.; Kwee, A.; Van Pampus, M.G.; Spaanderdam, M.E.A.; Duvekot, J.J.; Bremer, H.A.; Delemarre, F.M.C.; Bloemenkamp, K.W.M.; et al. Neonatal Morbidity after Induction vs Expectant Monitoring in Intrauterine Growth Restriction at Term: A Subanalysis of the DIGITAT RCT. Am. J. Obstet. Gynecol. 2012, 206, 344.e1–344.e7. [Google Scholar] [CrossRef] [PubMed]
- Vasak, B.; Koenen, V.; Koster, M.P.H.; Hukkelhoven, C.W.P.M.; Franx, A.; Hanson, M.A.; Visser, G.H.A. Human Fetal Growth Is Constrained below Optimal for Perinatal Survival. Ultrasound Obstet. Gynecol. 2015, 45, 162–167. [Google Scholar] [CrossRef]
- Ganzevoort, W.; Thilaganathan, B.; Baschat, A.; Gordijn, S. Fetal Growth and Risk Assessment: Is There an Impasse? POINT. Am. J. Obstet. Gynecol. 2018, 220, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Mecacci, F.; Serena, C.; Avagliano, L.; Cozzolino, M.; Baroni, E.; Rambaldi, M.P.; Simeone, S.; Castiglione, F.; Taddei, G.L.; Bulfamante, G. Stillbirths at Term: Case Control Study of Risk Factors, Growth Status and Placental Histology. PLoS ONE 2016, 14, e0213623. [Google Scholar] [CrossRef] [PubMed]
- Pilliod, R.A.; Cheng, Y.W.; Snowden, J.M.; Doss, A.E.; Caughey, A.B. The Risk of Intrauterine Fetal Death in the Small-for-Gestational-Age Fetus. Am. J. Obstet. Gynecol. 2012, 207, 318.e1–318.e6. [Google Scholar] [CrossRef]
- Seeds, J.; Peng, T. Impaired Growth and Risk of Fetal Death: Is the Tenth Percentile the Appropriate Standard? Am. J. Obstet. Gynecol. 1998, 178, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Breart, G.; Rabarison, Y.; Plouin, P.F.; Sureau, C.; Rumeau-Rouquette, C. Risk of Fetal Growth Retardation as a Result of Maternal Hypertension. Dev. Pharmacol. Ther. 1982, 4 (Suppl. 1), 116–123. [Google Scholar] [CrossRef]
- Usher, R.; McLean, F. Intrauterine Growth of Live-Born Caucasian Infants at Sea Level: Standards Obtained from Measurements in 7 Dimensions of Infants Born between 25 and 44 Weeks of gestation. J. Pediatr. 1969, 74, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Moraitis, A.A.; Wood, A.M.; Fleming, M.; Smith, G.C.S. Birth Weight Percentile and the Risk of Term Perinatal Death. Obstet. Gynecol. 2014, 124, 274–283. [Google Scholar] [CrossRef]
- Lees, C.C.; Stampalija, T.; Baschat, A.A.; da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and Management of Small-for-Gestational-Age Fetus and Fetal Growth Restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef]
- Tang, A.-W.; Agarwal, U. Fetal Growth Disorders. In Fetal Medicine; Kumar, B., Alfirevic, Z., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 269–285. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.B.; Martinez-Poyer, J. In Utero Analysis of Fetal Growth: A Sonographic Weight Standard. Radiology 1991, 181, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Loughna, P.; Chitty, L.; Evans, T.; Chudleigh, T. Fetal Size and Dating: Charts Recommended for Clinical Obstetric Practice. Ultrasound 2009, 17, 160–166. [Google Scholar] [CrossRef]
- Salomon, L.J.; Alfirevic, Z.; Bilardo, C.M.; Chalouhi, G.E.; Ghi, T.; Kagan, K.O.; Lau, T.K.; Papageorghiou, A.T.; Raine-Fenning, N.J.; Stirnemann, J.; et al. ISUOG Practice Guidelines: Performance of First-Trimester Fetal Ultrasound Scan. Ultrasound Obstet. Gynecol. 2013, 41, 102–113. [Google Scholar] [CrossRef]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus Definition of Fetal Growth Restriction: A Delphi Procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Papageorghiou, A.T.; Kennedy, S.H.; Salomon, L.J.; Altman, D.G.; Ohuma, E.O.; Stones, W.; Gravett, M.G.; Barros, F.C.; Victora, C.; Purwar, M.; et al. The INTERGROWTH-21 St Fetal Growth Standards: Toward the Global Integration of Pregnancy and Pediatric Care. Am. J. Obstet. Gynecol. 2018, 218, S630–S640. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.B.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of Fetal Weight with the Use of Head, Body, and Femur Measurements—A Prospective Study. Am. J. Obstet. Gynecol. 1985, 151, 333–337. [Google Scholar] [CrossRef]
- Hammami, A.; Mazer Zumaeta, A.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H. Ultrasonographic Estimation of Fetal Weight: Development of New Model and Assessment of Performance of Previous Models. Ultrasound Obstet. Gynecol. 2018, 52, 35–43. [Google Scholar] [CrossRef]
- Mendoza-Carrera, C.E.; Acevedo-Gallegos, S.; Lumbreras-Márquez, M.; Gallardo-Gaona, J.M.; Copado-Mendoza, D.Y.; Rodriguez-Sibaja, M.J. Comparison of Four Fetal Growth Charts in the Prediction of Adverse Perinatal Outcomes in a Tertiary Hospital in Mexico. Ginecol. Obstet. Mex. 2021, 89, 704–714. [Google Scholar] [CrossRef]
- Apgar, V. A Proposal for a New Method of Evaluation of the Newborn Infant. Anesth. Analg. 2015, 120, 1056–1059. [Google Scholar] [CrossRef]
- Macones, G.A. ACOG Practice Bulletin No. 106: Intrapartum Fetal Heart Rate Monitoring: Nomenclature, Interpretation, and General Management Principles. Obstet. Gynecol. 2009, 114, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Ultrasound Diagnosis and Grading Criteria of Neonatal Respiratory Distress Syndrome. J. Matern.-Fetal Neonatal Med. 2023, 36, 2206943. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. ICD-10: International Statistical Classification of Diseases and Health Related Problems: Volume 1: Tabular List (Second Edition, Tenth Revision); World Health Organization: Geneva, Switzerland, 2004; 1196p. [Google Scholar]
- Austin, P.C. Balance Diagnostics for Comparing the Distribution of Baseline Covariates between Treatment Groups in Propensity-Score Matched Samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Kabiri, D.; Romero, R.; Gudicha, D.W.; Hernandez-Andrade, E.; Pacora, P.; Benshalom-Tirosh, N.; Tirosh, D.; Yeo, L.; Erez, O.; Hassan, S.S.; et al. Prediction of Adverse Perinatal Outcomes by Fetal Biometry: A Comparison of Customized and Population-Based Standards. Ultrasound Obstet. Gynecol. 2020, 55, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Newton, H.; Cox, N. Seventy-Six Stata Tips, 2nd ed.; Newton, J.H., Cox, N.J., Eds.; Stata Press: College Station, TX, USA, 2009. [Google Scholar]
- Stampalija, T.; Wolf, H.; Mylrea-Foley, B.; Marlow, N.; Stephens, K.J.; Shaw, C.J.; Lees, C.C.; Arabin, B.; Berger, A.; Bergman, E.; et al. Reduced Fetal Growth Velocity and Weight Loss Are Associated with Adverse Perinatal Outcome in Fetuses at Risk of Growth Restriction. Am. J. Obstet. Gynecol. 2023, 228, 71.e1–71.e10. [Google Scholar] [CrossRef]
- Larsen, M.L.; Schreiber, V.; Krebs, L.; Hoei-Hansen, C.E.; Kumar, S. The Magnitude Rather than the Rate of Decline in Fetal Growth Is a Stronger Risk Factor for Perinatal Mortality in Term Infants. Am. J. Obstet. Gynecol. MFM 2023, 5, 100780. [Google Scholar] [CrossRef] [PubMed]
- Inácio, Q.A.S.; Araujo Júnior, E.; Nardozza, L.M.M.; Petrini, C.G.; Campos, V.P.; Peixoto, A.B. Perinatal Outcomes of Fetuses with Early Growth Restriction, Late Growth Restriction, Small for Gestational Age, and Adequate for Gestational Age. RBGO Gynecol. Obstet. 2019, 41, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.A.; Morales-Rosello, J.; Morlando, M.; Hannan, H.; Bhide, A.; Papageorghiou, A.; Thilaganathan, B. Is Fetal Cerebroplacental Ratio an Independent Predictor of Intrapartum Fetal Compromise and Neonatal Unit Admission? Am. J. Obstet. Gynecol. 2015, 213, 54.e1–54.e10. [Google Scholar] [CrossRef]
- Iliodromiti, S.; Mackay, D.F.; Smith, G.C.S.; Pell, J.P.; Sattar, N.; Lawlor, D.A.; Nelson, S.M. Customised and Noncustomised Birth Weight Centiles and Prediction of Stillbirth and Infant Mortality and Morbidity: A Cohort Study of 979,912 Term Singleton Pregnancies in Scotland. PLoS Med. 2017, 14, e1002228. [Google Scholar] [CrossRef]
- Bligard, K.H.; Odibo, A.O. Fetal Growth Restriction. In Queenan’s Management of High-Risk Pregnancy: An Evidence-Based Approach; Spong, C.Y., Lockwood, C.J., Eds.; WILEY Online Library: Hoboken, NJ, USA, 2023; pp. 392–398. [Google Scholar] [CrossRef]
- Leal, C.R.V.; Rezende, K.P.; de Macedo, E.D.C.P.; Rezende, G.D.C.; Corrêa Júnior, M.D. Comparison between Protocols for Management of Fetal Growth Restriction. RBGO Gynecol. Obstet. 2023, 45, 96–103. [Google Scholar] [CrossRef]
- Chen, W.; Wei, Q.; Liang, Q.; Song, S.; Li, J. Diagnostic Capacity of SFlt-1/PlGF Ratio in Fetal Growth Restriction: A Systematic Review and Meta-Analysis. Placenta 2022, 127, 37–42. [Google Scholar] [CrossRef]
- Whigham, C.A.; MacDonald, T.M.; Walker, S.P.; Hannan, N.J.; Tong, S.; Kaitu’u-Lino, T.J. The Untapped Potential of Placenta-Enriched Molecules for Diagnostic and Therapeutic Development. Placenta 2019, 84, 28–31. [Google Scholar] [CrossRef]
- Fewell, Z.; Davey Smith, G.; Sterne, J.A.C. The Impact of Residual and Unmeasured Confounding in Epidemiologic Studies: A Simulation Study. Am. J. Epidemiol. 2007, 166, 646–655. [Google Scholar] [CrossRef] [PubMed]
| Full Cohort | 10th–20th Centile | >20th–<90th Centile | Standardized Difference a | |
|---|---|---|---|---|
| N = 650 | N = 323 | N = 327 | ||
| Age, years, median (IQR) | 29 [24, 34] | 28 [24, 33] | 30 [25, 35] | −0.251 |
| Gravidity, median (IQR) | 2 [1, 3] | 2 [1, 3] | 2 [2, 3] | −0.245 |
| Pregestational weight, kg, median (IQR) | 69.7 [60, 80] | 68 [57, 79] | 71 [63, 82] | −0.310 |
| Pregestational BMI, kg/m2, median (IQR) | 27.9 [24.6, 31.6] | 27.8 [23.8, 31.3] | 28.3 [25.2, 32.4] | −0.213 |
| Hypertensive disorders of pregnancy, n (%) | 0.101 | |||
| None | 579 (89.1) | 276 (85.4) | 303 (92.7) | |
| Preeclampsia | 33 (5.1) | 28 (8.7) | 5 (1.5) | |
| Gestational hypertension | -- | -- | -- | |
| Chronic hypertension | 38 (5.8) | 19 (5.9) | 19 (5.8) | |
| History of preeclampsia, n (%) | 74 (11.4) | 36 (11.1) | 38 (11.6) | −0.014 |
| Diabetes, n (%) | −0.070 | |||
| None | 610 (93.8) | 305 (94.5) | 305 (93.3) | |
| Pregestational | 5 (0.8) | 4 (1.2) | 1 (0.3) | |
| Gestational | 35 (5.4) | 14 (4.3) | 21 (6.4) | |
| Tobacco exposure, n (%) | 34 (5.2) | 25 (7.7) | 9 (2.8) | 0.224 |
| APS or SLE, n (%) | 22 (3.4) | 16 (5.0) | 6 (1.8) | 0.172 |
| History of stillbirth, n (%) | 8 (1.2) | 4 (1.2) | 4 (1.2) | 0.001 |
| Full Cohort | 10th–20th Centile | >20th–<90th Centile | p-Value | |
|---|---|---|---|---|
| N = 650 | N = 323 | N = 327 | ||
| Gestational age, first growth scan, median (IQR) | 28.5 [28.0, 29.6] | 28.4 [27.6, 29.5] | 28.5 [28.1, 30.0] | 0.005 |
| EFW, first growth scan, median (IQR) | 1239.5 [1098, 1418] | 1132 [1036, 1288] | 1332 [1213, 1531] | <0.001 |
| EFW centile, first growth scan, median (IQR) | 21 [15, 43] | 15 [12, 18] | 42 [30, 58] | <0.001 |
| Gestational age, last growth scan, median (IQR) | 36 [34.3, 36.6] | 36.0 [34.1, 36.6] | 36.0 [34.4, 36.6] | 0.760 |
| EFW, last growth scan, median (IQR) | 2457 [2221, 2708] | 2355 [2047, 2579] | 2606 [2350, 2823] | <0.001 |
| EFW centile, last growth scan, median (IQR) | 25 [11, 43] | 16.0 [7.0, 26.6] | 37.0 [21.0, 51.0] | <0.001 |
| Total number of growth scans performed, median (IQR) | 3 [2, 4] | 3 [2, 4] | 3 [2, 3] | 0.001 |
| Latency between last growth scan and delivery, days, median (IQR) | 15 [7, 26] | 13 [5, 25] | 17 [9, 27] | <0.001 |
| Full Cohort | 10th–20th Centile | >20th–<90th Centile | p-Value | |
|---|---|---|---|---|
| N = 650 | N = 323 | N = 327 | ||
| Final fetal growth diagnosis in the last ultrasound before delivery, n (%) | <0.001 | |||
| Fetal growth restriction | 89 (13.7) | 68 (21.1) | 21 (6.4) | |
| Small for gestational age | 70 (10.8) | 53 (16.4) | 17 (5.2) | |
| 10th–20th centile | 121 (18.6) | 86 (26.6) | 35 (10.7) | |
| >20th centile | 370 (56.9) | 116 (35.9) | 254 (77.7) | |
| Gestational age at fetal growth restriction diagnosis, weeks, median (IQR) a | 35.4 [33.0, 37.0] | 35.0 [32.5, 36.5] | 36.4 [35.0, 37.0] | 0.043 |
| Gestational age at small for gestational age at diagnosis, weeks, median (IQR) a | 36.0 [34.6, 36.5] | 35.4 [34.2, 36.1] | 36.6 [36.1, 37.0] | <0.001 |
| Birth weight classification b, n (%) | <0.001 | |||
| Extremely low | 1 (0.2) | 1 (0.3) | 0 (0.0) | |
| Very low | -- | -- | -- | |
| Low | 108 (16.6) | 89 (27.6) | 19 (5.8) | |
| Adequate | 538 (82.7) | 233 (72.1) | 305 (93.3) | |
| High | 3 (0.5) | 0 (0.0) | 3 (0.9) |
| Full Cohort | 10th–20th Centile | >20th–<90th Centile | Crude Effect (95% CI) | Adjusted Effect (95% CI) a | p-Value a | |
|---|---|---|---|---|---|---|
| N = 650 | N = 323 | N = 327 | ||||
| Oligohydramnios, n (%) | 28 (4.3) | 22 (6.8) | 6 (1.8) | 3.71 (1.52, 9.04) b | 2.91 (1.17, 7.22) b | 0.021 |
| Gestational age at the time of delivery, weeks, median (IQR) | 38.2 [37.4, 39.1] | 38.1 [37.2, 39.1] | 38.3 [37.6, 39.1] | 0.98 (0.98, 0.99) c | 0.99 (0.98, 0.99) c | 0.001 |
| Induction of labor, n (%), indications (not mutually exclusive) | 83 (15.7) | 42 (15.2) | 41 (16.4) | 0.92 (0.62, 1.37) b | 0.86 (0.57, 1.30) b | 0.494 |
| Term pregnancy (i.e., 40 weeks’ gestation) | 45 (8.8) | 19 (7.1) | 26 (10.7) | -- | -- | |
| Hypertensive disorders of pregnancy | 20 (3.9) | 9 (3.4) | 11 (4.5) | -- | -- | |
| Pregestational or gestational diabetes | 0 (0) | 0 (0) | 0 (0) | -- | -- | |
| Premature rupture of membranes | 11 (2.2) | 7 (2.6) | 4 (1.6) | -- | -- | |
| Fetal growth restriction | 8 (1.6) | 6 (2.2) | 2 (0.8) | -- | -- | |
| Oligohydramnios | 5 (1.0) | 4 (1.5) | 1 (0.4) | -- | -- | |
| Delivery mode, n (%) | ||||||
| Cesarean delivery | 456 (70.2) | 240 (74.3) | 216 (66.1) | 1.12 (1.01, 1.24) b | 1.13 (1.02, 1.25) b | 0.019 |
| Birth weight, grams, median (IQR) | 2862.5 [2595, 3146] | 2736 [2455, 2985] | 3020 [2765, 3295] | 0.88 (0.86, 0.91) c | 0.90 (0.87, 0.92) c | <0.001 |
| Five-minute Apgar, median (IQR) | 9 [9, 9] | 9 [9, 9] | 9 [9, 9] | 0.99 (0.99, 1.00) c | 0.99 (0.99, 1.00) c | 0.150 |
| Meconium-stained amniotic fluid, n (%) | 15 (2.3) | 13 (4.0) | 2 (0.6) | 6.58 (1.49, 28.96) b | 4.89 (1.07, 22.27) b | 0.040 |
| Neonatal jaundice, n (%) | 203 (31.2) | 115 (35.6) | 88 (26.9) | 1.32 (1.04, 1.66) b | 1.33 (1.05, 1.69) b | 0.016 |
| Necrotizing enterocolitis, n (%) | 3 (0.5) | 2 (0.6) | 1 (0.3) | 2.02 (0.18, 22.26) b | 1.60 (0.12, 19.79) b | 0.714 |
| Neonatal sepsis, n (%) | ||||||
| Early | 3 (0.5) | 2 (0.6) | 1 (0.3) | 2.02 (0.18, 22.26) b | 25.87 (5.63, 118.76) b | <0.001 |
| Late | 1 (0.2) | 0 (0.0) | 1 (0.3) | -- | -- | |
| Mechanical ventilation, n (%) | 11 (1.7) | 5 (1.5) | 6 (1.8) | 0.84 (0.25, 2.73) b | 1.02 (0.31, 3.38) b | 0.964 |
| Continuous positive airway pressure, n (%) | 37 (5.7) | 20 (6.2) | 17 (5.2) | 1.19 (0.63, 2.23) b | 1.13 (0.61, 2.09) b | 0.681 |
| Umbilical cord pH ≤ 7.1, n (%) | 11 (1.7) | 5 (1.5) | 6 (1.8) | 0.84 (0.25, 2.73) b | 0.79 (0.25, 2.46) b | 0.688 |
| Five-minute Apgar ≤ 7, n (%) | 5 (0.8) | 3 (0.9) | 2 (0.6) | 1.51 (0.25, 9.04) b | 1.72 (0.34, 8.62) b | 0.505 |
| NICU admission, n (%) | 146 (22.5) | 99 (30.7) | 47 (14.4) | 2.13 (1.56, 2.91) b | 2.06 (1.50, 2.84) b | <0.001 |
| Hypoglycemia, n (%) | 24 (3.7) | 15 (4.6) | 9 (2.8) | 1.68 (0.74, 3.80) b | 1.51 (0.65, 3.52) b | 0.332 |
| Emergency C-section due to non-reassuring fetal state, n (%) | 101 (15.5) | 64 (19.8) | 37 (11.3) | 1,75 (1,20, 2,54) b | 1.56 (1.05, 2.31) b | 0.026 |
| Respiratory distress syndrome, n (%) | 115 (17.7) | 67 (20.7) | 48 (14.7) | 1.41 (1.00, 1.98) b | 1.41 (0.99, 1.99) b | 0.050 |
| Intraventricular hemorrhage, n (%) | 5 (0.8) | 3 (0.9) | 2 (0.6) | 1.51 (0.25, 9.04) b | 1.52 (0.17, 13.67) b | 0.705 |
| Hypoxic ischemic encephalopathy, n (%) | 5 (0.8) | 1 (0.3) | 4 (1.2) | 0.25 (0.02, 2.25) b | 0.29 (0.02, 2.97) b | 0.300 |
| Perinatal death, n (%) | 0 (0) | 0 (0.0) | 0 (0.0) | -- | -- | |
| Composite outcome, n (%) d | 244 (37.5) | 148 (45.8) | 96 (29.4) | 1.56 (1.27, 1.91) b | 1.51 (1.22, 1.86) b | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendez-Piña, M.A.; Lumbreras-Marquez, M.I.; Acevedo-Gallegos, S.; Velazquez-Torres, B.; Rodriguez-Sibaja, M.J.; Camarena-Cabrera, D.M.; Gallardo-Gaona, J.M. Fetal and Neonatal Outcomes in Fetuses with an Estimated Fetal Weight Percentile of 10–20 in the Early Third Trimester: A Retrospective Cohort Study. Diagnostics 2025, 15, 2251. https://doi.org/10.3390/diagnostics15172251
Mendez-Piña MA, Lumbreras-Marquez MI, Acevedo-Gallegos S, Velazquez-Torres B, Rodriguez-Sibaja MJ, Camarena-Cabrera DM, Gallardo-Gaona JM. Fetal and Neonatal Outcomes in Fetuses with an Estimated Fetal Weight Percentile of 10–20 in the Early Third Trimester: A Retrospective Cohort Study. Diagnostics. 2025; 15(17):2251. https://doi.org/10.3390/diagnostics15172251
Chicago/Turabian StyleMendez-Piña, Miguel A., Mario I. Lumbreras-Marquez, Sandra Acevedo-Gallegos, Berenice Velazquez-Torres, Maria J. Rodriguez-Sibaja, Dulce M. Camarena-Cabrera, and Juan M. Gallardo-Gaona. 2025. "Fetal and Neonatal Outcomes in Fetuses with an Estimated Fetal Weight Percentile of 10–20 in the Early Third Trimester: A Retrospective Cohort Study" Diagnostics 15, no. 17: 2251. https://doi.org/10.3390/diagnostics15172251
APA StyleMendez-Piña, M. A., Lumbreras-Marquez, M. I., Acevedo-Gallegos, S., Velazquez-Torres, B., Rodriguez-Sibaja, M. J., Camarena-Cabrera, D. M., & Gallardo-Gaona, J. M. (2025). Fetal and Neonatal Outcomes in Fetuses with an Estimated Fetal Weight Percentile of 10–20 in the Early Third Trimester: A Retrospective Cohort Study. Diagnostics, 15(17), 2251. https://doi.org/10.3390/diagnostics15172251







