Giant Left Atrial Appendage Aneurysm in a 6-Year-Old Girl with a Prothrombotic Genetic Predisposition: A Case Report and Literature Review
Abstract
1. Introduction
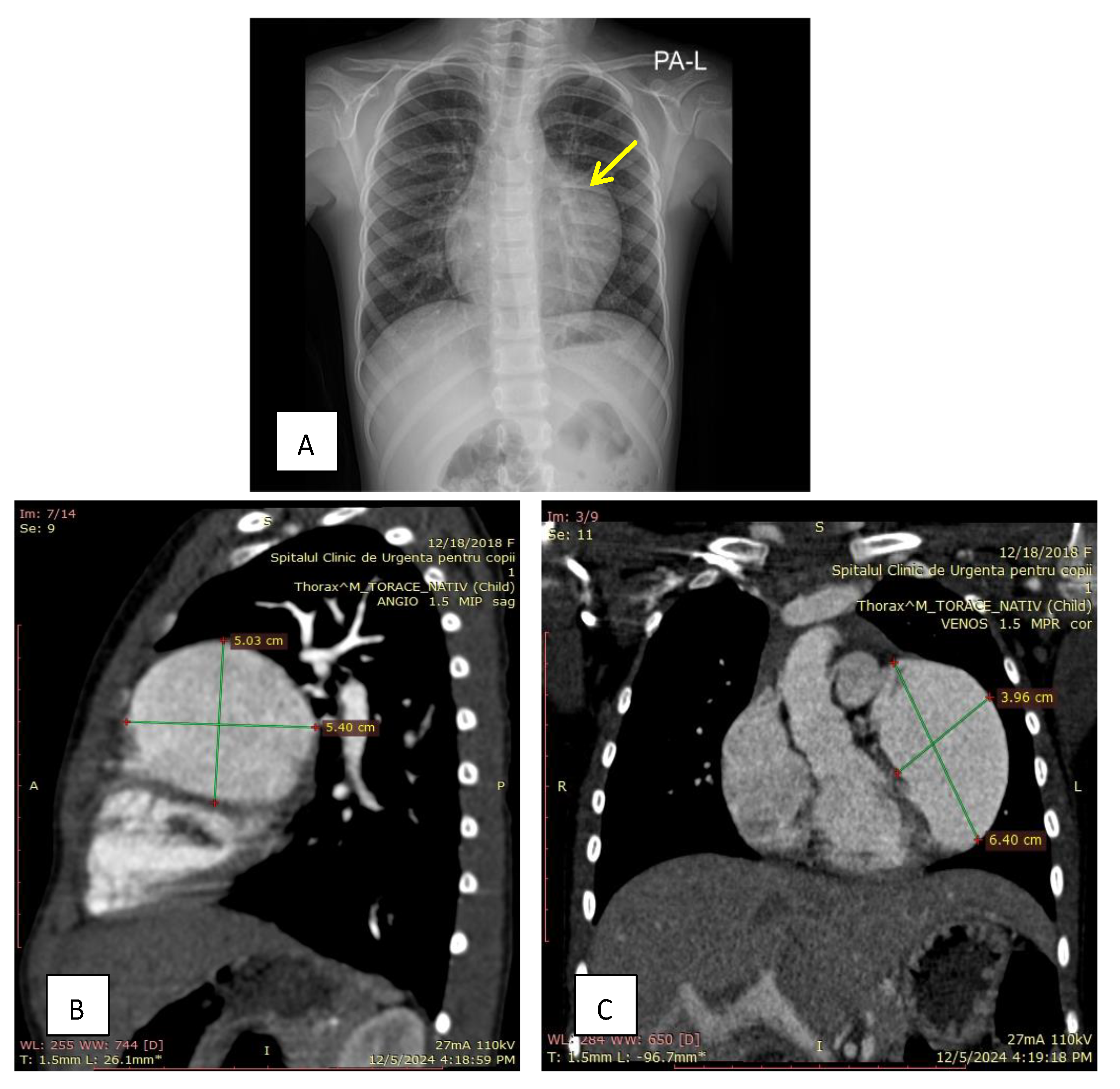
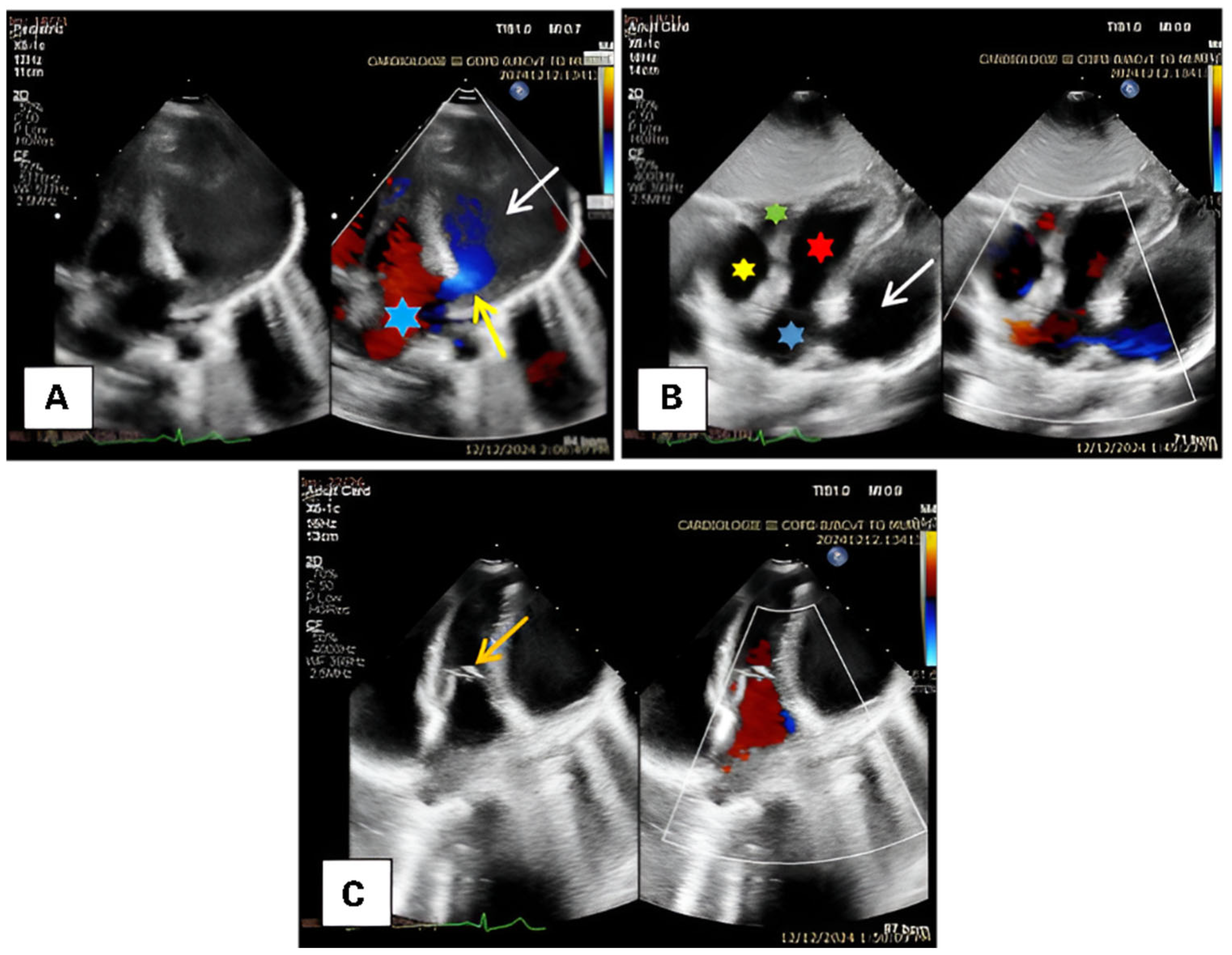
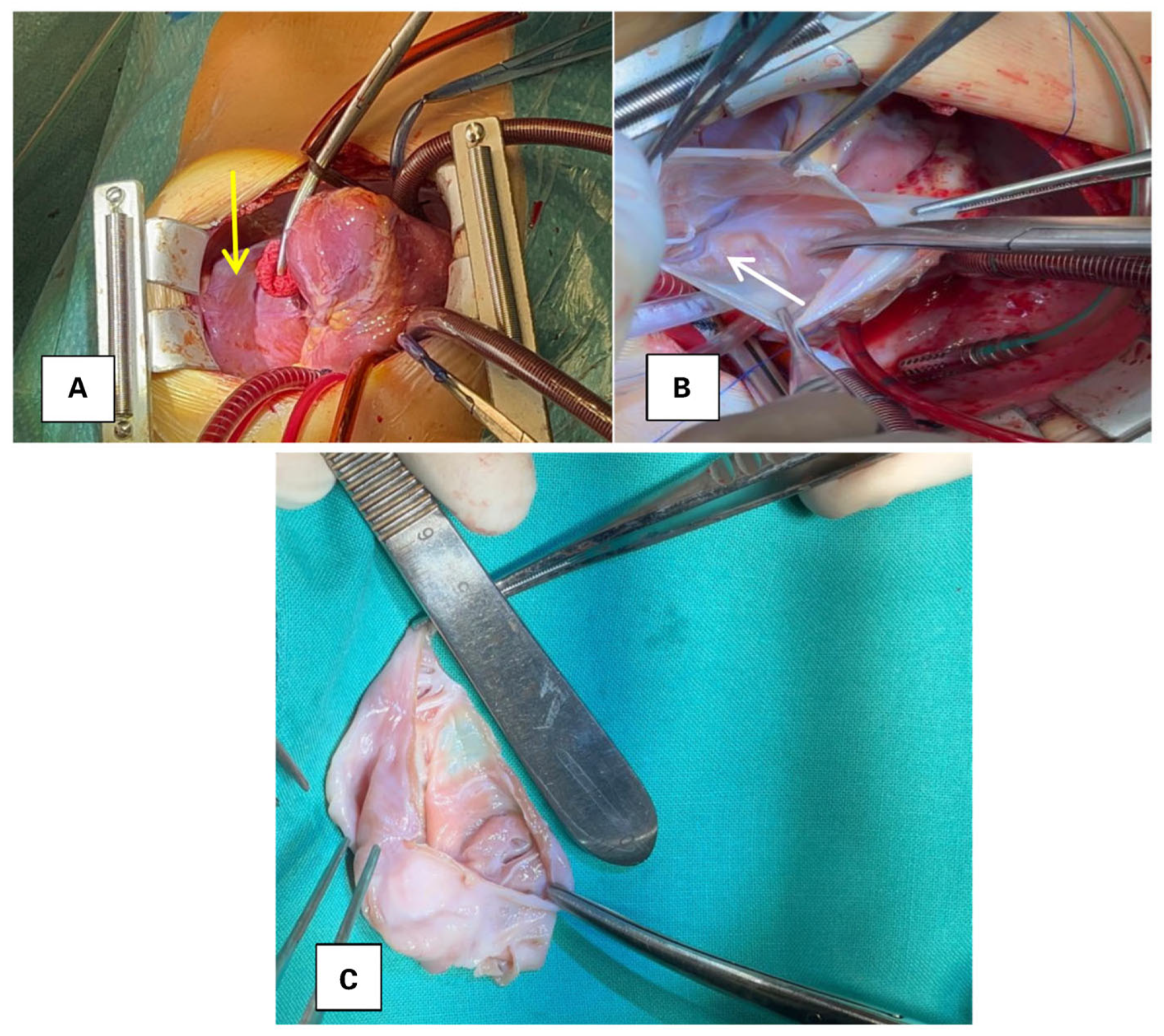
2. Case Presentation
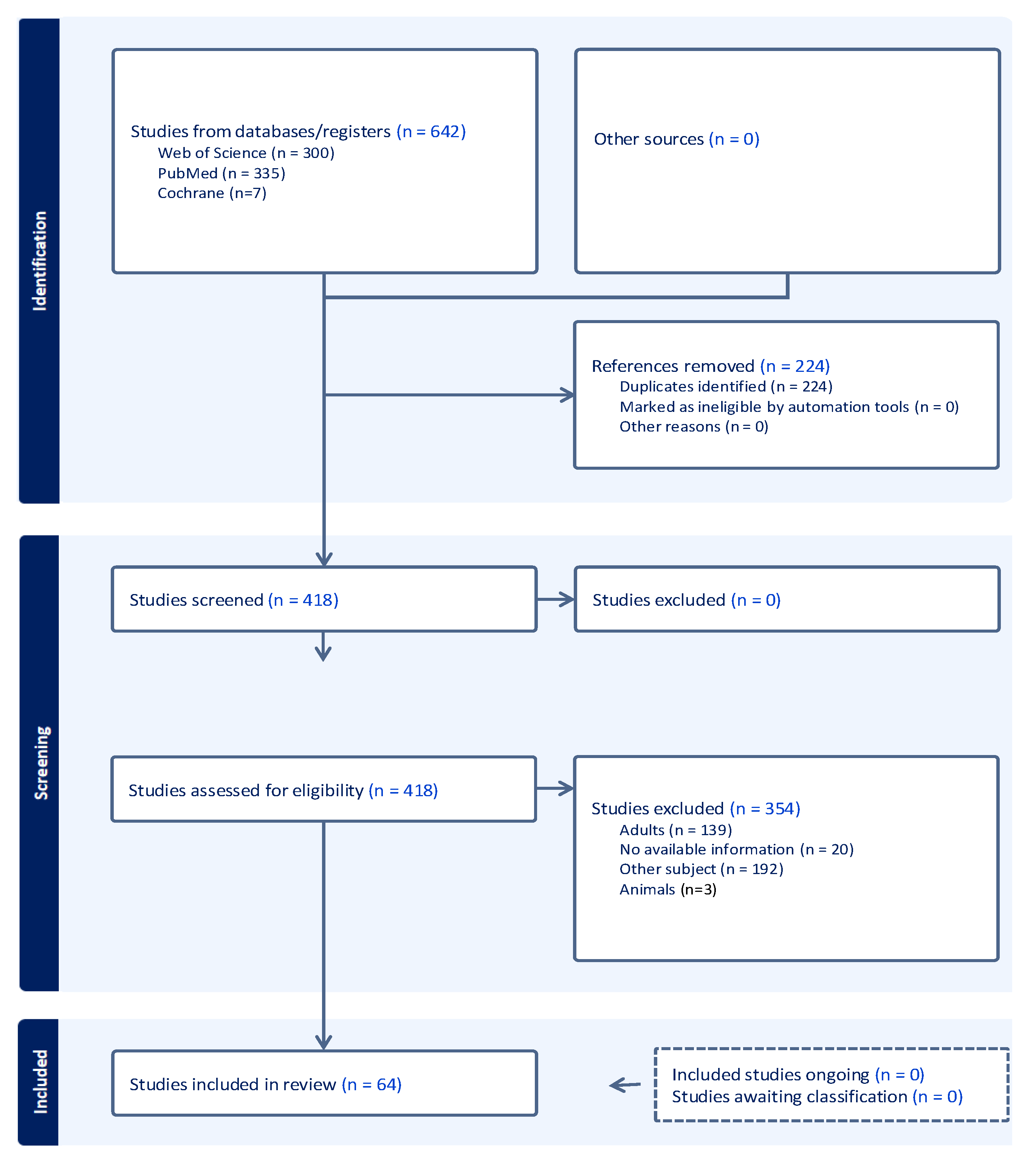
3. Materials and Methods
4. Results
5. Discussion
6. Conclusions
7. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilber, D.J. Neurohormonal Regulation and the Left Atrial Appendage: Still More to Learn. J. Am. Coll. Cardiol. 2018, 71, 145–147. [Google Scholar] [CrossRef]
- Al-Saady, N.M.; Obel, O.A.; Camm, A.J. Left atrial appendage: Structure, function, and role in thromboembolism. Heart 1999, 82, 547–554. [Google Scholar] [CrossRef]
- Parmley, L.f., Jr. Congenital atriomegaly. Circulation 1962, 25, 553–558. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Han, J.; Sun, L.; Zhang, Y.; Gu, X.; Xue, C.; Liu, X.; He, Y. Fetal atrial appendage aneurysm: Prenatal diagnosis by echocardiography and prognosis. Echocardiography 2021, 38, 1228–1234. [Google Scholar] [CrossRef]
- Daralammouri, Y.; Odeh, A.; Abuzahra, S.; Azamtta, M.; Shawahna, R. Left atrial appendage aneurysm: A descriptive systematic review of 177 cases. BMC Cardiovasc. Disord. 2024, 24, 633. [Google Scholar] [CrossRef] [PubMed]
- Asfalou, I.; Boumaaz, M.; Raissouni, M.; Sabry, M.; Benyass, A.; Zbir, E.M. Huge left atrial appendage aneurysm revealed by chronic hiccups. J. Saudi Heart Assoc. 2017, 29, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.P.; Afifi, H.Y.; Ko, W.; Horner, N.; Hahn, R. Congential giant aneurysms of the left atrial appendage: Diagnosis and management. J. Card. Surg. 1996, 11, 147–150. [Google Scholar] [CrossRef]
- Fan, F.; Bai, S.; Tong, F.; Zheng, J.; Li, Q.; Guo, Z.; Li, X. Safe treatment of congenital left atrial appendage aneurysm using lateral thoracotomy on a 3-year-old patient. Cardiol. Young 2020, 31, 144–147. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.; Zhang, L.; He, L.; Zhang, J.; Liu, C.; Wang, J.; Lv, Q.; Shang, X.; Liu, J.; et al. Congenital left atrial appendage aneurysm: A rare case report and literature review. Medicine 2018, 97, e9344. [Google Scholar] [CrossRef] [PubMed]
- Norozi, K.; Subasri, M.; Diaz, L.A.; Honjo, O. Left atrial appendage aneurysm in pediatrics: Case study and literature review. Front. Cardiovasc. Med. 2023, 10, 1211619. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.-M.; Jiang, H.; Jin, Y.-Q.; Zhang, F.-Q.; Liu, H.-J.; Ge, H.-Y. Right Atrial Appendage Aneurysm Resection to Cure Aneurysm-Related Atrial Tachyarrhythmia. Pediatr. Cardiol. 2019, 40, 1144–1150. [Google Scholar] [CrossRef]
- Harpa, M.M.; Anitei, E.-D.; Ghiragosian, C.; Calburean, P.; Opris, D.R.; Banceu, M.C.; Arbanasi, E.M.; Suciu, H.; Al Hussein, H. Endoscopic Mitral Surgery in Noonan Syndrome-Case Report and Considerations. J. Clin. Med. 2025, 14, 583. [Google Scholar] [CrossRef]
- Calcagni, G.; Gagliostro, G.; Limongelli, G.; Unolt, M.; De Luca, E.; Digilio, M.C.; Baban, A.; Albanese, S.B.; Ferrero, G.B.; Baldassarre, G.; et al. Atypical cardiac defects in patients with RASopathies: Updated data on CARNET study. Birth Defects Res. 2020, 112, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.R.; Hakim, F.A.; Ghimire, S.; Ghimire, S.; Giri, S.; Pandit, A.; Bhandari, Y.; Bhandari, N.; Pathak, R.; Karmacharya, P.; et al. Left atrial appendage aneurysm: A systematic review of 82 cases. Echocardiography 2014, 31, 1312–1318. [Google Scholar] [CrossRef]
- Saygi, M.; Ergul, Y.; Guzeltas, A. Combination of congenital left atrial appendage aneurysm in an infant with transposition of the great arteries: A previously unreported association. Cardiol. Young 2015, 25, 1377–1378. [Google Scholar] [CrossRef]
- Chowdhury, U.K.; Seth, S.; Govindappa, R.; Jagia, P.; Malhotra, P. Congenital left atrial appendage aneurysm: A case report and brief review of literature. Heart Lung Circ. 2009, 18, 412–416. [Google Scholar] [CrossRef]
- Fakhri, G.; Obeid, M.; El Rassi, I.; Tabbakh, A.; Bitar, F.; Alameddine, M.; Arabi, M. Large congenital left atrial wall aneurysm: An updated and comprehensive review of the literature. Echocardiography 2020, 37, 965–970. [Google Scholar] [CrossRef]
- Zhang, X.; Li, P.; Cao, Y.; Li, X.; Duan, X.; Bai, S.; Wang, F. Left atrial appendage aneurysm in pediatrics. Echocardiography 2020, 37, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Rengifo, L.M.; Hazle, M.A.; Kincaid, E.H.; Ootaki, Y. Thoracoscopic Resection of Left Atrial Appendage Aneurysm in a 16-Year-Old Boy. Ann. Thorac. Surg. 2021, 112, e451–e453. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, X.; Liu, F.; Song, L. Left atrial appendage aneurysm. Interact. Cardiovasc. Thorac. Surg. 2019, 30, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Harada, M.; Moritake, H. Left atrial appendage aneurysm enlarged in the neonatal period. Cardiol. Young 2022, 33, 1433–1435. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; Nakano, T.; Kado, H. Expansion of a Huge Compressive Left Atrial Appendage Aneurysm in a 29-Day-Old Infant. Ann. Thorac. Surg. 2020, 110, e521–e523. [Google Scholar] [CrossRef] [PubMed]
- Oztunc, F.; Murt, N.U.; Dedeoglu, R.; Karagozlu, F.; Madazli, R. Perinatal Outcomes of Fetuses with Prenatally Diagnosed Atrial Appendage Aneurysm. Pediatr. Cardiol. 2023, 45, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, T.; Kato, K.; Sato, K.; Osawa, T.; Okawa, H.; Sakurai, H. An adult case of giant congenital left atrial wall aneurysm. J. Cardiol. Cases 2023, 28, 181–184. [Google Scholar] [CrossRef]
- Lu, W.-Y.; Huang, S.-C.; Chen, S.-J.; Jhuang, J.-Y.; Chen, Y.-C.; Wang, J.-K.; Wu, M.-H.; Chen, C.-A. Giant congenital left atrial aneurysm in an 11-year-old boy. Circulation 2012, 125, 1818–1820. [Google Scholar] [CrossRef]
- Victor, S.; Nayak, V.M. Aneurysm of the left atrial appendage. Tex. Heart Inst. J. 2001, 28, 111–118. [Google Scholar]
- Chan, Y.J.; Ly Ly, N.T.; Hai, N.M.; Jan, S.L. Case Report: One heart with two lobes: A rare infantile congenital giant left atrial appendage aneurysm. Front. Pediatr. 2023, 11, 1302182. [Google Scholar] [CrossRef]
- Făgărășan, A.; Săsăran, M.O.; Gozar, L.; Toma, D.; Șuteu, C.; Ghiragosian-Rusu, S.; Al-Akel, F.C.; Szabo, B.; Huțanu, A. Circulating Matrix Metalloproteinases for Prediction of Aortic Dilatation in Children with Bicuspid Aortic Valve: A Single-Center, Observational Study. Int. J. Mol. Sci. 2024, 25, 10538. [Google Scholar] [CrossRef]
- Culver, D.L.; Bezante, G.P.; Schwarz, K.Q.; Meltzer, R.S. Transesophageal echocardiography in the diagnosis of acquired aneurysms of the left atrial appendage. Clin. Cardiol. 1993, 16, 149–151. [Google Scholar] [CrossRef]
- Cujec, B.; Bharadwaj, B.; Orchard, R.C.; Lopez, J.F. Transesophageal echocardiography in the diagnosis of left atrial appendage aneurysm. J. Am. Soc. Echocardiogr. 1990, 3, 408–411. [Google Scholar] [CrossRef]
- Sauza-Sosa, J.C.; De la Cruz-Reyna, E.L.; Velazquez-Gutierrez, C.N. An Unusual Congenital Heart Disease: Giant Left Atrial Appendage. Methodist DeBakey Cardiovasc. J. 2022, 18, 106–107. [Google Scholar] [CrossRef]
- Anitei, E.-D.; Harpa, M.M.; Al Hussein, H.; Ghiragosian, C.; Stroe, V.I.; Calburean, P.; Gurzu, S.; Suciu, H. Pulmonary Valve Fibroelastoma, Still a Very Rare Cardiac Tumor: Case Report and Literature Review. Diagnostics 2025, 15, 283. [Google Scholar] [CrossRef]
- Di Salvo, G.; Al-Sehly, A.; Al Fadley, F.; Al Bulbul, Z.; Fadel, B.M.; Al Fayyadh, M.; Al Soufi, B. A rare case of giant congenital left atrial appendage aneurysm in a 4-month-old child. J. Cardiovasc. Med. 2017, 18, 723–724. [Google Scholar] [CrossRef]
- Das, N.; Tadros, S.S.; DeBrunner, M. A case of a left atrial appendage disguised as a coronary artery aneurysm. CASE 2021, 5, 305–308. [Google Scholar] [CrossRef]
- Plonska-Gosciniak, E.; Larysz, B.; Jurczyk, K.; Kasprzak, J.D. Five-chambered heart: A 20-year story of left atrial appendage aneurysm. Eur. Heart J. 2009, 30, 1014. [Google Scholar] [CrossRef]
- Valentino, M.A.; Al Danaf, J.; Morris, R.; Tecce, M.A. Giant left atrial appendage aneurysm: A case of mistaken identity. J. Cardiol. Cases 2017, 15, 129–131. [Google Scholar] [CrossRef] [PubMed]
- DiBardino, D.J.; Aggarwal, A.; Knudson, J.D. Off-pump snare technique for congenital left atrial appendage aneurysm. Cardiol. Young 2013, 24, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.R.; Zvaigzne, C.G.; Disler, D.; Giuffre, R.M.; Rebeyka, I.M.; Patton, D.J. Giant left atrial appendage aneurysm in a neonate. World J. Pediatr. Congenit. Heart Surg. 2012, 3, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Kothandam, S.; Ramasamy, R. Planning and execution of catheter closure of a giant left atrial appendage aneurysm causing recurrent cardioembolism. Ann. Pediatr. Cardiol. 2020, 13, 353–356. [Google Scholar] [CrossRef]
- Kiaii, B.; Doll, N.; Kuehl, M.; Mohr, F.W. Minimal invasive endoscopic resection of a giant left atrial appendage aneurysm. Ann. Thorac. Surg. 2004, 77, 1437–1438. [Google Scholar] [CrossRef]
- Burke, R.P.; Mark, J.B.; Collins, J.J., Jr.; Cohn, L.H. Improved surgical approach to left atrial appendage aneurysm. J. Card. Surg. 1992, 7, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Kim, H.J.; Ju, M.H.; Lee, J.W. The Treatment of Left Atrial Appendage Aneurysm by a Minimally Invasive Approach. Korean J. Thorac. Cardiovasc. Surg. 2018, 51, 146–148. [Google Scholar] [CrossRef] [PubMed]

| Arrhythmia Status | No Thrombus, n (%) | Thrombus, n (%) | Fisher’s Exact p Value |
|---|---|---|---|
| Absent | 47 (92.2%) | 4 (7.8%) | |
| Present | 12 (66.7%) | 6 (33.3%) | 0.0158 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suciu, H.; Anitei, E.-D.; Stroe, V.I.; Brudan, E.E.; Capilna, T.; Al Hussein, H.; Ghiragosian, S.; Calburean, P.; Veres, M.; Harpa, M.M. Giant Left Atrial Appendage Aneurysm in a 6-Year-Old Girl with a Prothrombotic Genetic Predisposition: A Case Report and Literature Review. Diagnostics 2025, 15, 2070. https://doi.org/10.3390/diagnostics15162070
Suciu H, Anitei E-D, Stroe VI, Brudan EE, Capilna T, Al Hussein H, Ghiragosian S, Calburean P, Veres M, Harpa MM. Giant Left Atrial Appendage Aneurysm in a 6-Year-Old Girl with a Prothrombotic Genetic Predisposition: A Case Report and Literature Review. Diagnostics. 2025; 15(16):2070. https://doi.org/10.3390/diagnostics15162070
Chicago/Turabian StyleSuciu, Horatiu, Emanuel-David Anitei, Valentin Ionut Stroe, Emilia Eleonora Brudan, Tudor Capilna, Hussam Al Hussein, Simina Ghiragosian, Paul Calburean, Mihaly Veres, and Marius Mihai Harpa. 2025. "Giant Left Atrial Appendage Aneurysm in a 6-Year-Old Girl with a Prothrombotic Genetic Predisposition: A Case Report and Literature Review" Diagnostics 15, no. 16: 2070. https://doi.org/10.3390/diagnostics15162070
APA StyleSuciu, H., Anitei, E.-D., Stroe, V. I., Brudan, E. E., Capilna, T., Al Hussein, H., Ghiragosian, S., Calburean, P., Veres, M., & Harpa, M. M. (2025). Giant Left Atrial Appendage Aneurysm in a 6-Year-Old Girl with a Prothrombotic Genetic Predisposition: A Case Report and Literature Review. Diagnostics, 15(16), 2070. https://doi.org/10.3390/diagnostics15162070





