Screening for Cervical Cancer and Early Treatment (SCCET) Project—The Programmatic Data of Romanian Experience in Primary Screening for High-Risk HPV DNA
Abstract
1. Introduction
- (a)
- Persistent hrHPV infection is implicated in the development of CC.
- (b)
- The risk of developing CIN2+ in the long term in women with a negative hrHPV test (sensitivity ~95%) is much lower compared to those with negative cytology.
- (c)
- Testing can be performed using various cervical biological specimens.
- (d)
- Testing is objective and has greater global consistency compared to cytology.
- (e)
2. Materials and Methods
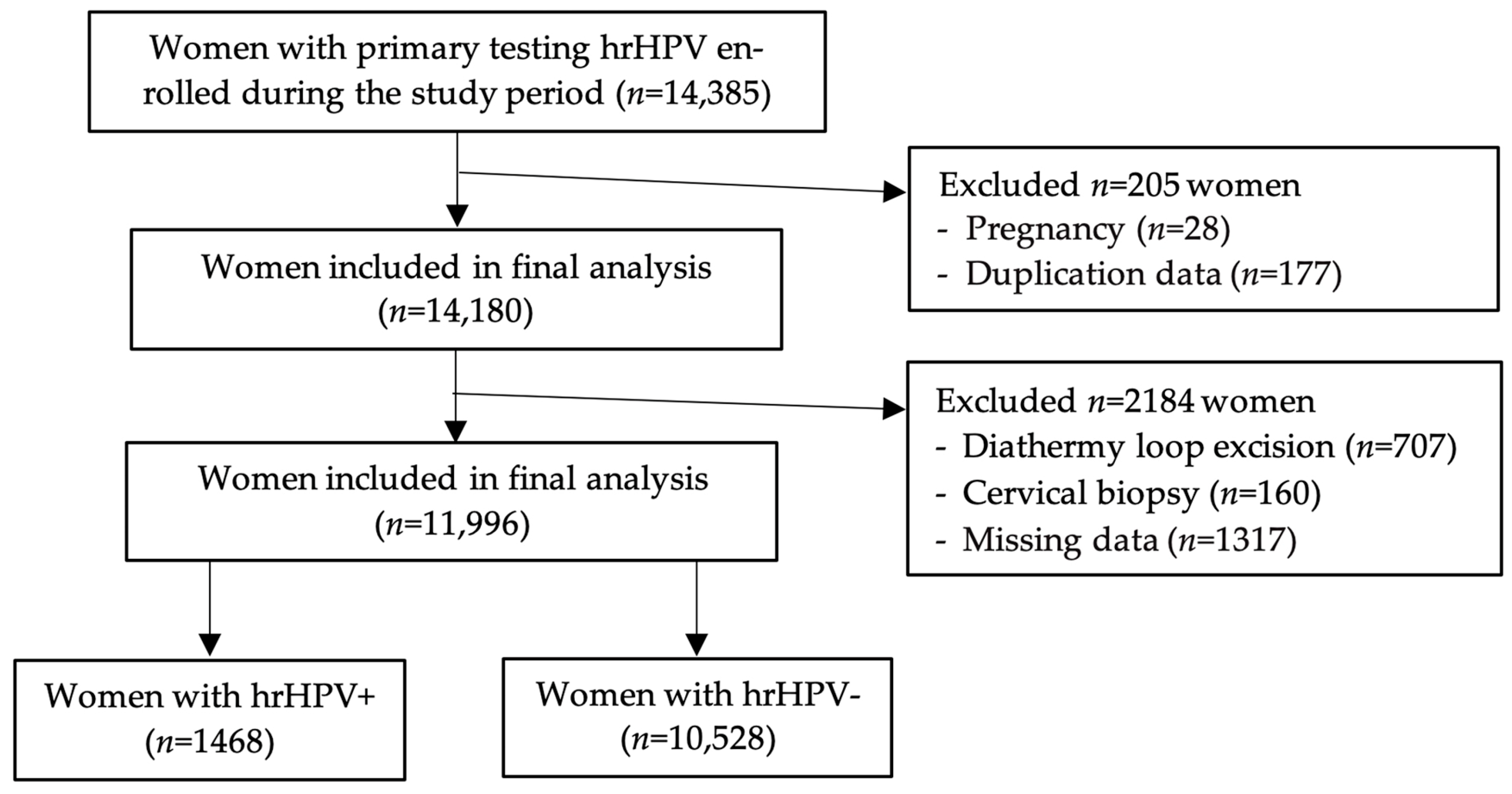
2.1. Study Design and Participant Characteristics
2.2. Ethical Approval
2.3. Clinical Evaluation and Data Collection
2.4. Statistics
3. Results
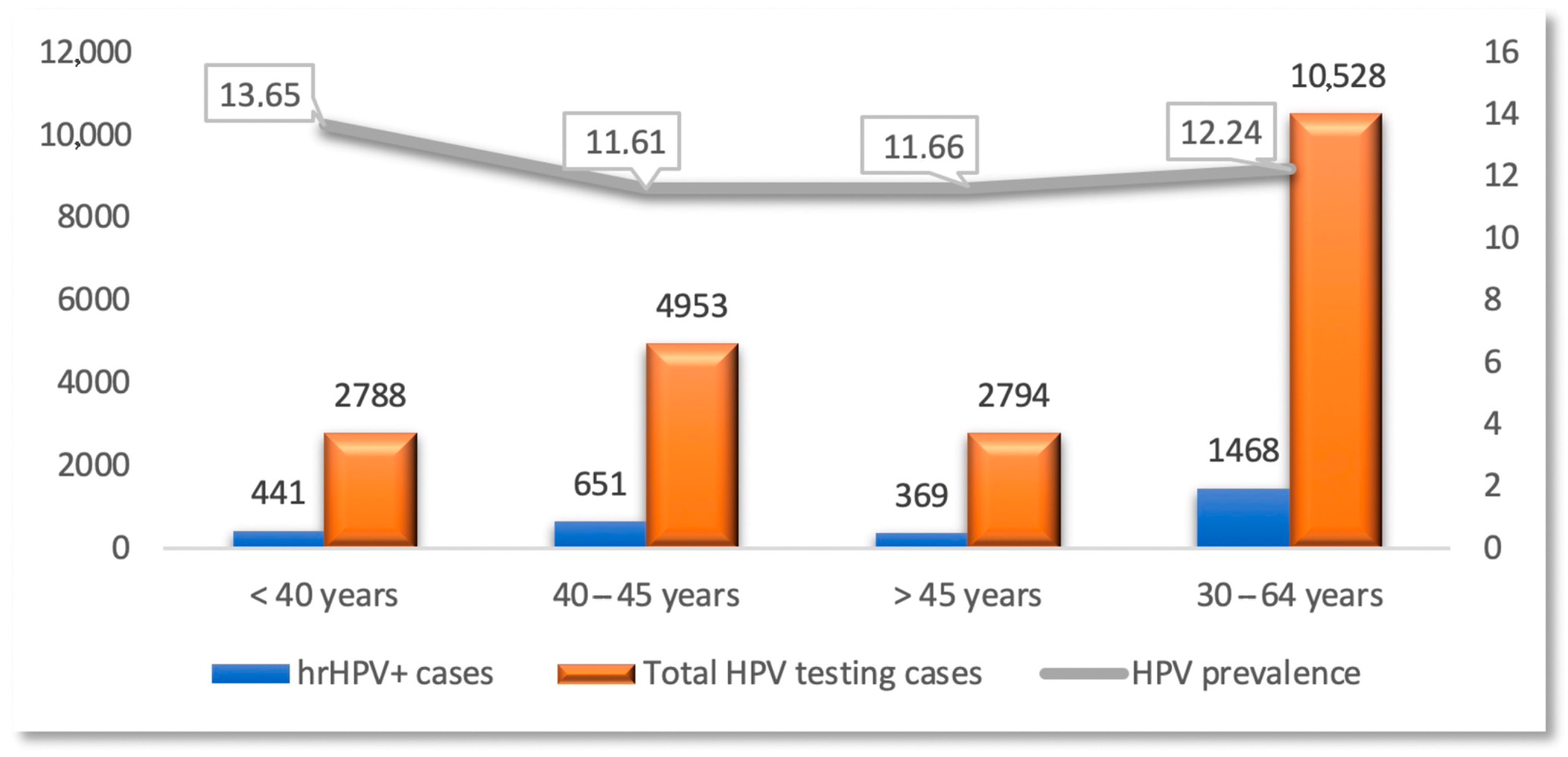
3.1. Demographic Distribution of the Study Group
3.2. Risk Factors Correlated with hrHPV Infection
3.3. HPV Testing and Genotyping
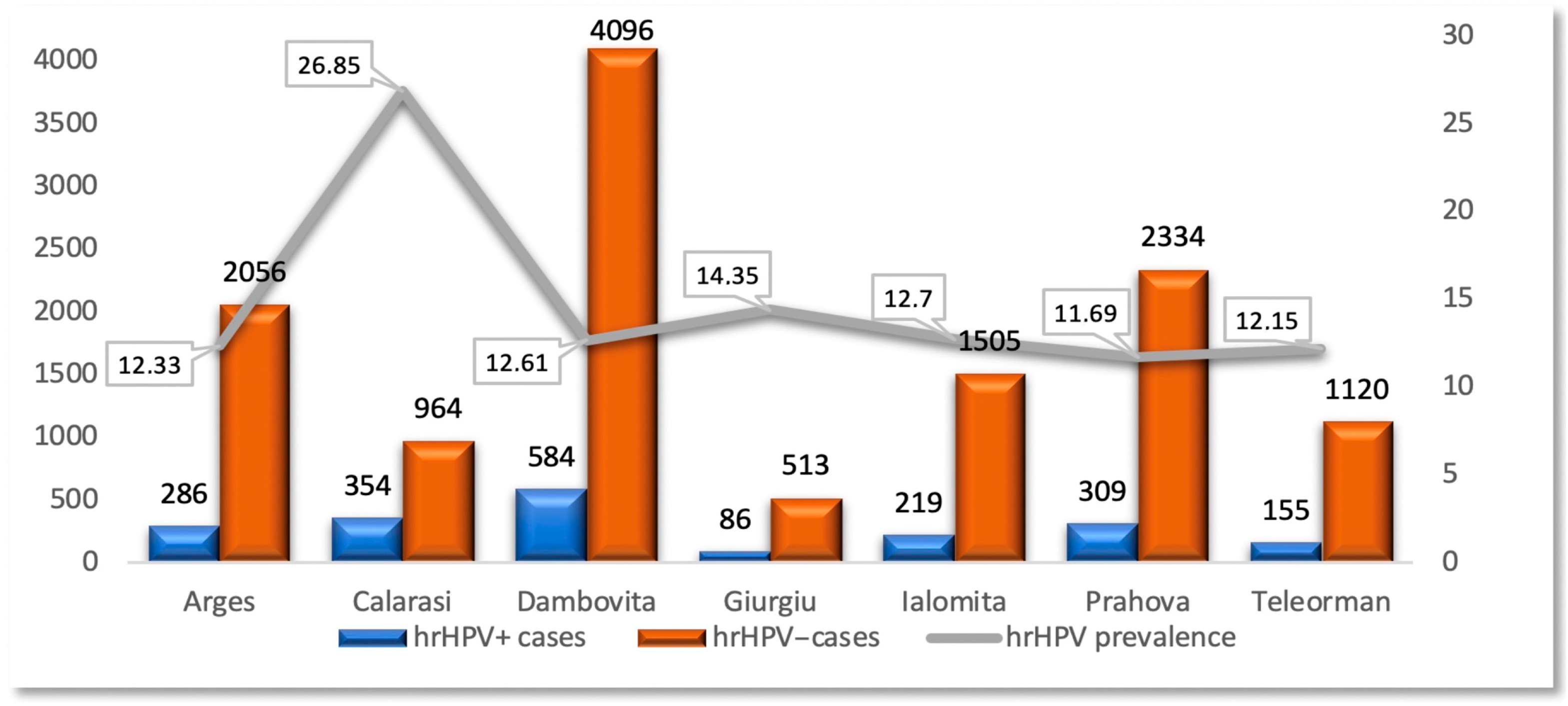
3.4. Prevalence of hrHPV Infection
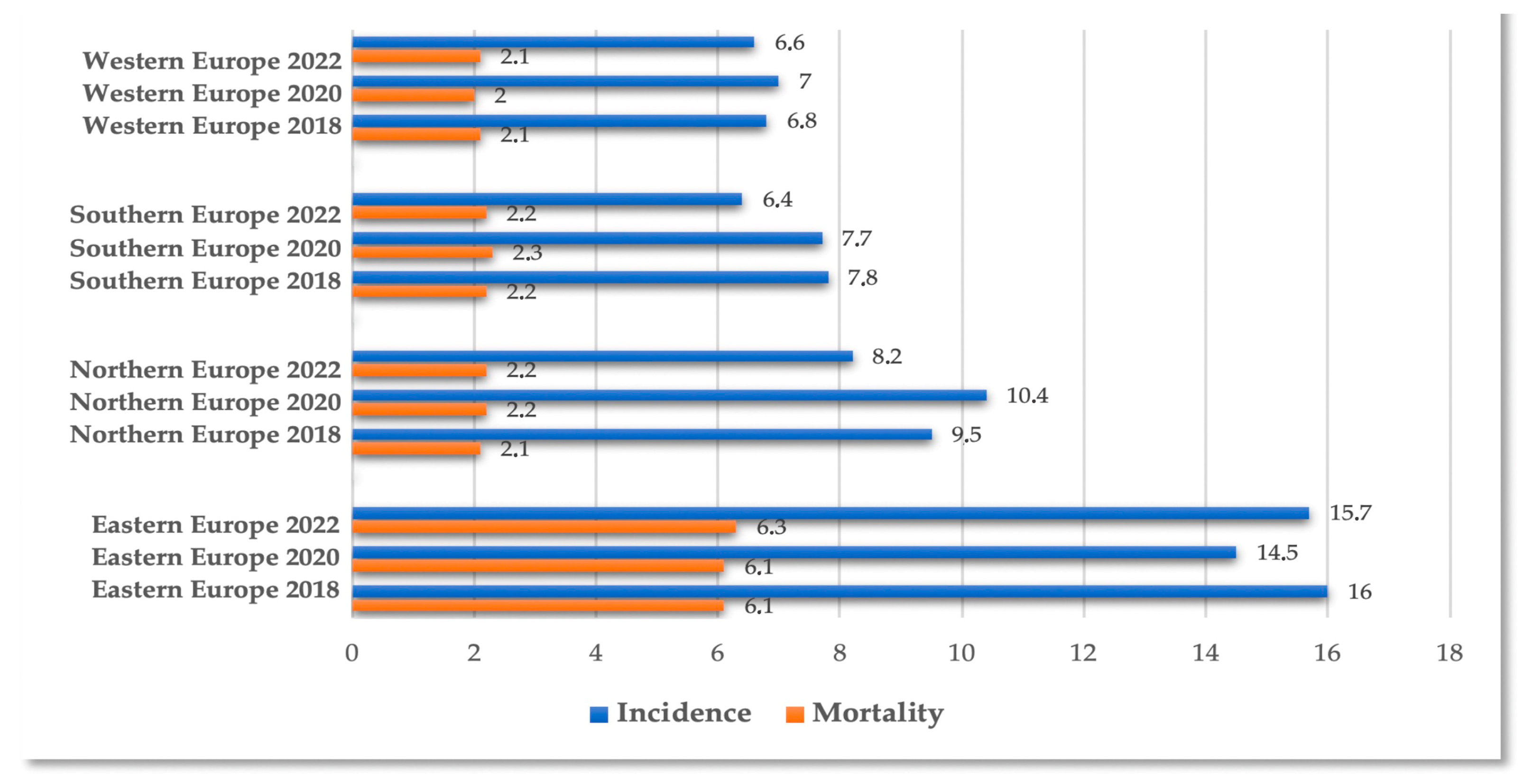
3.5. Implications for Public Health
4. Discussion
- (a)
- Risk stratification/triage for equivocal or low-grade cytological abnormalities, directing individuals to colposcopy;
- (b)
- Monitoring therapeutic success following cervical lesion removal, often referred to as a “test of cure”;
- (c)
- Primary screening for CC.
- (a)
- Enhancing women’s health through the implementation of a comprehensive screening program in the seven counties within the Muntenia Region of South Romania (Prahova, Argeș, Dâmbovița, Teleorman, Călărași, Giurgiu, Ialomița). This involves identifying susceptible women among the participants through consultations with Obstetrics-Gynecology specialists, coupled with diagnostic, treatment, counseling, monitoring, and prevention services;
- (b)
- Establishing a robust personal data protection system in compliance with prevailing legislation, with stringent measures for secure data processing throughout the project;
- (c)
- Conducting information and education sessions to raise awareness and promote the necessity of participating in the screening program. This is targeted at specific groups and the general public and emphasizes the correlation between access to medical services, awareness of health status, and women’s rights to healthcare.
- (d)
- Expanding women’s access to high-quality medical services [2].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CC | Cervical cancer |
| HPV/hrHPV | Human papilloma virus/high-risk human papilloma virus |
| IUDs | Intrauterine devices |
| Pap test | Babeș–Papanicolaou smear |
| SCCUT | The Screening for Cervical Cancer and Early Treatment Project |
| STIs | Sexually transmitted infections |
| L-SIL | Low-grade squamous intraepithelial lesion |
| ASC-US | Atypical squamous cells of undetermined significance |
| ASC-H | Atypical squamous cells—cannot exclude HSIL |
| AGC | Atypical glandular cells—not otherwise specified |
| H-SIL | High-grade squamous intraepithelial lesion |
References
- The Cancer We Can Eliminate—WHO/Europe Urges Member States to Consign Cervical Cancer to History. Available online: https://www.who.int/europe/news/item/13-09-2022-the-cancer-we-can-eliminate---who-europe-urges-member-states-to-consign-cervical-cancer-to-history (accessed on 10 April 2025).
- Screening Pentru Cancerul de Col Uterin și Tratament Precoce—SCCUT. Available online: https://www.insmc.ro/wp-content/uploads/2021/05/Prezentare-proiect_site-INSMC.pdf (accessed on 10 April 2025).
- OECD/European Commission. EU Country Cancer Profile: Romania 2025. In EU Country Cancer Profiles; OECD Publishing: Paris, France, 2025. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of Incidence and Mortality of Cervical Cancer in 2018: A Worldwide Analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Cervical Cancer Death Rate by Country (Female). Available online: https://www.worldlifeexpectancy.com/cause-of-death/cervical-cancer/by-country/female (accessed on 8 August 2025).
- Global Cancer Observatory. Available online: https://gco.iarc.fr/en (accessed on 14 April 2025).
- Davies-Oliveira, J.C.; Round, T.; Crosbie, E.J. Cervical Screening: The Evolving Landscape. Br. J. Gen. Pract. 2022, 72, 364–365. [Google Scholar] [CrossRef]
- Fowler, J.R.; Maani, E.V.; Dunton, C.J.; Gasalberti, D.P.; Jack, B.W. Cervical Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Mahajan, I.; Kadam, A.; McCann, L.; Ghose, A.; Wakeham, K.; Dhillon, N.S.; Stanway, S.; Boussios, S.; Banerjee, S.; Priyadarshini, A.; et al. Early Adoption of Innovation in HPV Prevention Strategies: Closing the Gap in Cervical Cancer. Ecancermedicalscience 2024, 18, 1762. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, S.F.; Salnikov, M.Y.; Zeng, P.Y.F.; Barrett, J.W.; Nichols, A.C.; Mymryk, J.S. HPV16 Intratypic Variants in Head and Neck Cancers: A North American Perspective. Viruses 2023, 15, 2411. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, H.; Zhang, L.; Qiao, Y. Cervical Cancer: Epidemiology, Risk Factors and Screening. Chin. J. Cancer Res. 2020, 32, 720–728. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide Burden of Cancer Attributable to HPV by Site, Country and HPV Type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Luvián-Morales, J.; Gutiérrez-Enríquez, S.O.; Granados-García, V.; Torres-Poveda, K. Risk Factors for the Development of Cervical Cancer: Analysis of the Evidence. Front. Oncol. 2024, 14, 1378549. [Google Scholar] [CrossRef]
- Luria, L.; Cardoza-Favarato, G. Human Papillomavirus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ojha, P.S.; Maste, M.M.; Tubachi, S.; Patil, V.S. Human Papillomavirus and Cervical Cancer: An Insight Highlighting Pathogenesis and Targeting Strategies. Virusdisease 2022, 33, 132–154. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yang, M. The Correlation Between High-Risk HPV Infection and Precancerous Lesions and Cervical Cancer. Am. J. Transl. Res. 2021, 13, 10830–10836. [Google Scholar] [PubMed]
- Browne, S.; Feemster, K.A. Human Papillomavirus: Optimizing Opportunities for Prevention. Curr. Opin. Pediatr. 2022, 34, 132–139. [Google Scholar] [CrossRef]
- Liao, C.-I.; Francoeur, A.A.; Kapp, D.S.; Caesar, M.A.P.; Huh, W.K.; Chan, J.K. Trends in Human Papillomavirus-Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw. Open 2022, 5, e222530. [Google Scholar] [CrossRef]
- Piña-Sánchez, P. Human Papillomavirus: Challenges and Opportunities for the Control of Cervical Cancer. Arch. Med. Res. 2022, 53, 753–769. [Google Scholar] [CrossRef]
- Yousefi, Z.; Aria, H.; Ghaedrahmati, F.; Bakhtiari, T.; Azizi, M.; Bastan, R.; Hosseini, R.; Eskandari, N. An Update on Human Papilloma Virus Vaccines: History, Types, Protection, and Efficacy. Front. Immunol. 2021, 12, 805695. [Google Scholar] [CrossRef]
- Torres-Poveda, K.; Ruiz-Fraga, I.; Madrid-Marina, V.; Chavez, M.; Richardson, V. High Risk HPV Infection Prevalence and Associated Cofactors: A Population-Based Study in Female ISSSTE Beneficiaries Attending the HPV Screening and Early Detection of Cervical Cancer Program. BMC Cancer 2019, 19, 1205. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, J.; Cui, X.; Ma, J.; Wang, C.; Piao, H. Risk Factors Associated with Human Papillomavirus Infection, Cervical Cancer, and Precancerous Lesions in Large-Scale Population Screening. Front. Microbiol. 2022, 13, 914516. [Google Scholar] [CrossRef]
- Wentzensen, N.; Clarke, M.A. Cervical Cancer Screening-Past, Present, and Future. Cancer Epidemiol. Biomark. Prev. 2021, 30, 432–434. [Google Scholar] [CrossRef]
- Melnikow, J.; Henderson, J.T.; Burda, B.U.; Senger, C.A.; Durbin, S.; Weyrich, M.S. Screening for Cervical Cancer with High-Risk Human Papillomavirus Testing: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 320, 687–705. [Google Scholar] [CrossRef]
- Bruni, L.; Serrano, B.; Roura, E.; Alemany, L.; Cowan, M.; Herrero, R.; Poljak, M.; Murillo, R.; Broutet, N.; Riley, L.M.; et al. Cervical Cancer Screening Programmes and Age-Specific Coverage Estimates for 202 Countries and Territories Worldwide: A Review and Synthetic Analysis. Lancet Glob. Health 2022, 10, e1115–e1127. [Google Scholar] [CrossRef]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summary Report 22 October 2021. Available online: https://hpvcentre.net/statistics/reports/XWX.pdf (accessed on 11 April 2025).
- eClinicalMedicine. Global Strategy to Eliminate Cervical Cancer as a Public Health Problem: Are We on Track? EClinicalMedicine 2023, 55, 101842. [Google Scholar] [CrossRef]
- Pimple, S.A.; Mishra, G.A. Global Strategies for Cervical Cancer Prevention and Screening. Minerva Ginecol. 2019, 71, 313–320. [Google Scholar] [CrossRef]
- Bouvard, V.; Wentzensen, N.; Mackie, A.; Berkhof, J.; Brotherton, J.; Giorgi-Rossi, P.; Kupets, R.; Smith, R.; Arrossi, S.; Bendahhou, K.; et al. The IARC Perspective on Cervical Cancer Screening. N. Engl. J. Med. 2021, 385, 1908–1918. [Google Scholar] [CrossRef]
- Ilisiu, M.B.; Hashim, D.; Andreassen, T.; Støer, N.C.; Nicula, F.; Weiderpass, E. HPV Testing for Cervical Cancer in Romania: High-Risk HPV Prevalence among Ethnic Subpopulations and Regions. Ann. Glob. Health 2019, 85, 89. [Google Scholar] [CrossRef] [PubMed]
- IARC. Cervical Cancer Screening; IARC: Lyon, France, 2022. [Google Scholar]
- Todor, R.D.; Bratucu, G.; Moga, M.A.; Candrea, A.N.; Marceanu, L.G.; Anastasiu, C.V. Challenges in the Prevention of Cervical Cancer in Romania. Int. J. Environ. Res. Public Health 2021, 18, 1721. [Google Scholar] [CrossRef] [PubMed]
- Radoi, V.E.; Bohiltea, C.L.; Bohiltea, R.E.; Albu, D.N. Cell Free Fetal DNA Testing in Maternal Blood of Romanian Pregnant Women. Iran. J. Reprod. Med. 2015, 13, 623–626. [Google Scholar]
- Koussouri, A.; Baraquin, A.; Desmarets, M.; Diallo, K.; Puget, L.; Lepiller, Q.; Prétet, J.-L. Comparison of High-Risk HPV Detection by the AmpFire® HPV Screening 16/18/HR Technique (Atila Biosystems) and the Hybrid Capture 2 Test (Qiagen). Mol. Biol. Rep. 2024, 51, 52. [Google Scholar] [CrossRef] [PubMed]
- von Karsa, L.; Arbyn, M.; De Vuyst, H.; Dillner, J.; Dillner, L.; Franceschi, S.; Patnick, J.; Ronco, G.; Segnan, N.; Suonio, E.; et al. European Guidelines for Quality Assurance in Cervical Cancer Screening. Summary of the Supplements on HPV Screening and Vaccination. Papillomavirus Res. 2015, 1, 22–31. [Google Scholar] [CrossRef]
- Maver, P.J.; Poljak, M. Primary HPV-Based Cervical Cancer Screening in Europe: Implementation Status, Challenges, and Future Plans. Clin. Microbiol. Infect. 2020, 26, 579–583. [Google Scholar] [CrossRef]
- Song, F.; Du, H.; Xiao, A.; Wang, C.; Huang, X.; Liu, Z.; Zhao, M.; Men, H.; Wu, R. Type-Specific Distribution of Cervical hrHPV Infection and the Association with Cytological and Histological Results in a Large Population-Based Cervical Cancer Screening Program: Baseline and 3-Year Longitudinal Data. J. Cancer 2020, 11, 6157–6167. [Google Scholar] [CrossRef]
- Gao, D.; Zhao, G.; Wang, X.; Juan, J.; Shi, Y.; Xu, T.; Wang, Y.; Wang, L.; Zhang, X. Association Between High-Risk Human Papillomavirus Infection and Cervical Cytology in Health Check-Up Women—23 PLADs, China, 2023. China CDC Wkly. 2025, 7, 327–333. [Google Scholar] [CrossRef]
- Tanik, E.B.; Bakir, A.; Turkmenoglu, T.T.; Erdem, G. Distribution of High-Risk Human Papillomavirus Genotypes in Cervical Smear Samples and Evaluation of ASC-US, LSIL, and HSIL Results. J. Cytol. 2025, 42, 37–42. [Google Scholar] [CrossRef]
- Ali, M.A.M.; Bedair, R.N.; Abd El Atti, R.M. Cervical High-Risk Human Papillomavirus Infection Among Women Residing in the Gulf Cooperation Council Countries: Prevalence, Type-Specific Distribution, and Correlation with Cervical Cytology. Cancer Cytopathol. 2019, 127, 567–577. [Google Scholar] [CrossRef]
- Berza, N.; Zodzika, J.; Kivite-Urtane, A.; Baltzer, N.; Curkste, A.; Pole, I.; Nygård, M.; Pärna, K.; Stankunas, M.; Tisler, A.; et al. Understanding the High-Risk Human Papillomavirus Prevalence and Associated Factors in the European Country with a High Incidence of Cervical Cancer. Eur. J. Public Health 2024, 34, 826–832. [Google Scholar] [CrossRef]
- Giubbi, C.; Martinelli, M.; Rizza, M.; Di Meo, M.L.; Njoku, R.C.; Perdoni, F.; Mannarà, G.; Musumeci, R.; Fruscio, R.; Landoni, F.; et al. Molecular Detection of Human Papillomavirus (HPV) and Other Sexually Transmitted Pathogens in Cervical and Self-Collected Specimens. Int. J. Mol. Sci. 2025, 26, 1296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, H.; Huang, X.; Wang, C.; Duan, X.; Liu, Y.; Shi, B.; Zhang, W.; Qu, X.; Wei, L.; et al. Evaluation of an Isothermal Amplification HPV Detection Assay for Primary Cervical Cancer Screening. Infect. Agent. Cancer 2020, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Koliopoulos, G.; Nyaga, V.N.; Santesso, N.; Bryant, A.; Martin-Hirsch, P.P.; Mustafa, R.A.; Schünemann, H.; Paraskevaidis, E.; Arbyn, M. Cytology versus HPV Testing for Cervical Cancer Screening in the General Population. Cochrane Database Syst. Rev. 2017, 8, CD008587. [Google Scholar] [CrossRef] [PubMed]
- Elfström, K.M.; Arnheim-Dahlström, L.; von Karsa, L.; Dillner, J. Cervical Cancer Screening in Europe: Quality Assurance and Organisation of Programmes. Eur. J. Cancer Oxf. Engl. 1990 2015, 51, 950–968. [Google Scholar] [CrossRef] [PubMed]
- Fontham, E.T.H.; Wolf, A.M.D.; Church, T.R.; Etzioni, R.; Flowers, C.R.; Herzig, A.; Guerra, C.E.; Oeffinger, K.C.; Shih, Y.-C.T.; Walter, L.C.; et al. Cervical Cancer Screening for Individuals at Average Risk: 2020 Guideline Update from the American Cancer Society. CA. Cancer J. Clin. 2020, 70, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Kiff, J.M.; Cotter, M.; Munro, E.G.; Leonard, M.E.; Morgan, T.K.; Bruegl, A.S. Cervical Cancer Screening in Postmenopausal Women: Is It Time to Move Toward Primary High-Risk Human Papillomavirus Screening? J. Womens Health 2021, 30, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Hamashima, C. Emerging Technologies for Cervical Cancer Screening. Jpn. J. Clin. Oncol. 2021, 51, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Popalis, M.L.; Ramirez, S.I.; Leach, K.M.; Granzow, M.E.; Stoltzfus, K.C.; Moss, J.L. Improving Cervical Cancer Screening Rates: A Scoping Review of Resources and Interventions. Cancer Causes Control 2022, 33, 1325–1333. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Total hrHPV Testing Cases |
|---|---|
| Number of cases (n) | 11,996 |
| Age (mean ± SD) years | 42.39 ± 3.21 |
Residence (n, %)
| 5474 (45.64%) 6522 (54.36%) |
Hormonal status (n, %)
| 10,831 (90.28%) 1007 (8.39%) 158 (1.32%) |
| IUDs (n, %) | 390 (3.25%) |
| STIs (n, %) | 592 (4.93%) |
| Parameter | hrHPV Test | hrHPV Infection Types | ||||
|---|---|---|---|---|---|---|
| hrHPV− | hrHPV+ | HPV 16 | HPV 18 | Other hrHPV | p-Value | |
| Number of cases (n, %) | 10,528 (87.76%) | 1468 (12.24%) | 276 (2.3 %) | 86 (0.71%) | 1181 (9.84%) | - |
| Age (mean ± SD) | 42.41 ± 3.2 | 42.18 ± 3.27 | 41.77 ± 3.3 | 42.74 ± 3.38 | 42.17 ± 3.25 | 0.107 * |
Residence (n, %)
| 4773 (50.11%) 5755 (49.89%) | 681 (46.38%) 787 (53.62%) | 127 (46.01%) 149 (53.99%) | 44 (51.16%) 42 (48.84%) | 550 (46.57%) 631 (53.43%) | 0.534 ** |
Hormonal status (n, %)
| 9487 (90.12%) 901 (8.55%) 140 (1.33%) | 1344 (91.55%) 87 (5.93%) 37 (2.52%) | 260 (94.2%) 11 (3.99%) 5 (1.81%) | 77 (89.53%) 8 (9.3%) 1 (1.17%) | 1076 (91.11%) 89 (7.54%) 16 (1.35%) | <0.508 ** |
| IUDs (n, %) | 345 (2.87%) | 45 (3.06%) | 8 (2.89%) | 3 (3.48%) | 34 (2.87%) | N.S. |
| STIs (n, %) | 506 (4.21%) | 86 (5.85%) | 19 (6.88%) | 4 (4.65%) | 63 (5.33%) | N.S. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saveliev, G.M.; Ciuvică, A.I.; Cretoiu, D.; Varlas, V.N.; Balalau, C.; Balescu, I.; Bacalbasa, N.; Bohiltea, L.C.; Suciu, N. Screening for Cervical Cancer and Early Treatment (SCCET) Project—The Programmatic Data of Romanian Experience in Primary Screening for High-Risk HPV DNA. Diagnostics 2025, 15, 2066. https://doi.org/10.3390/diagnostics15162066
Saveliev GM, Ciuvică AI, Cretoiu D, Varlas VN, Balalau C, Balescu I, Bacalbasa N, Bohiltea LC, Suciu N. Screening for Cervical Cancer and Early Treatment (SCCET) Project—The Programmatic Data of Romanian Experience in Primary Screening for High-Risk HPV DNA. Diagnostics. 2025; 15(16):2066. https://doi.org/10.3390/diagnostics15162066
Chicago/Turabian StyleSaveliev, Gabriel Marian, Adriana Irina Ciuvică, Dragos Cretoiu, Valentin Nicolae Varlas, Cristian Balalau, Irina Balescu, Nicolae Bacalbasa, Laurentiu Camil Bohiltea, and Nicolae Suciu. 2025. "Screening for Cervical Cancer and Early Treatment (SCCET) Project—The Programmatic Data of Romanian Experience in Primary Screening for High-Risk HPV DNA" Diagnostics 15, no. 16: 2066. https://doi.org/10.3390/diagnostics15162066
APA StyleSaveliev, G. M., Ciuvică, A. I., Cretoiu, D., Varlas, V. N., Balalau, C., Balescu, I., Bacalbasa, N., Bohiltea, L. C., & Suciu, N. (2025). Screening for Cervical Cancer and Early Treatment (SCCET) Project—The Programmatic Data of Romanian Experience in Primary Screening for High-Risk HPV DNA. Diagnostics, 15(16), 2066. https://doi.org/10.3390/diagnostics15162066








