Assessment of Right Ventricular Pressure in Chronic Thromboembolic Pulmonary Hypertension: Comparison of Diagnostic Modalities and Balloon Pulmonary Angioplasty Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Invasive Measurement of the Right Ventricular Pressure
2.2.1. Right Heart Catheterization
2.2.2. Pulmonary Angiography with Invasive RV Pressure Measurement
2.3. Non-Invasive Measurement of the Right Ventricular Pressure by Echocardiography
2.4. Balloon Pulmonary Angioplasty (BPA)
2.5. Clinical Assessment
2.6. Statistical Analysis
2.7. Ethical Statement
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Pulmonary Angioplasty and Therapeutic Response
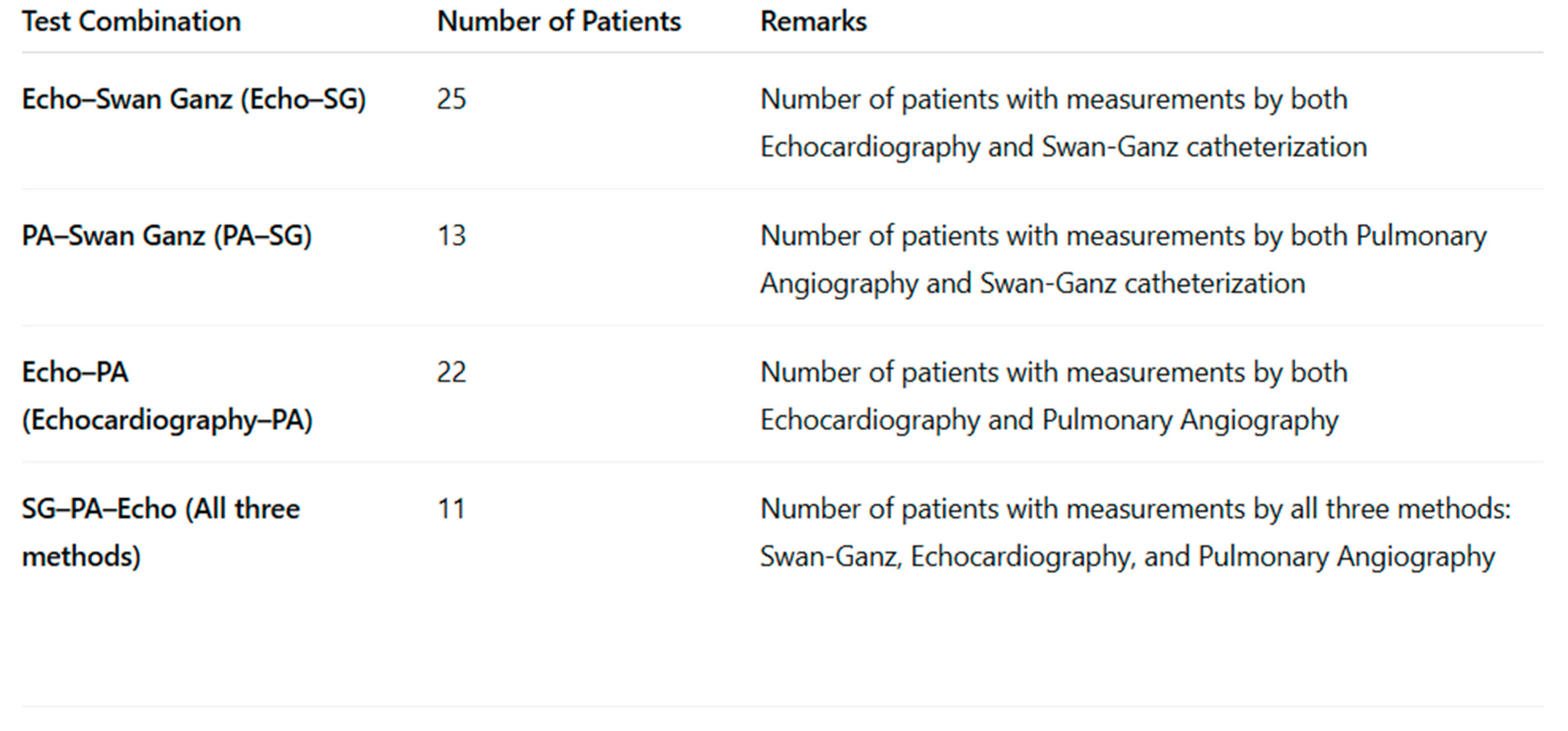
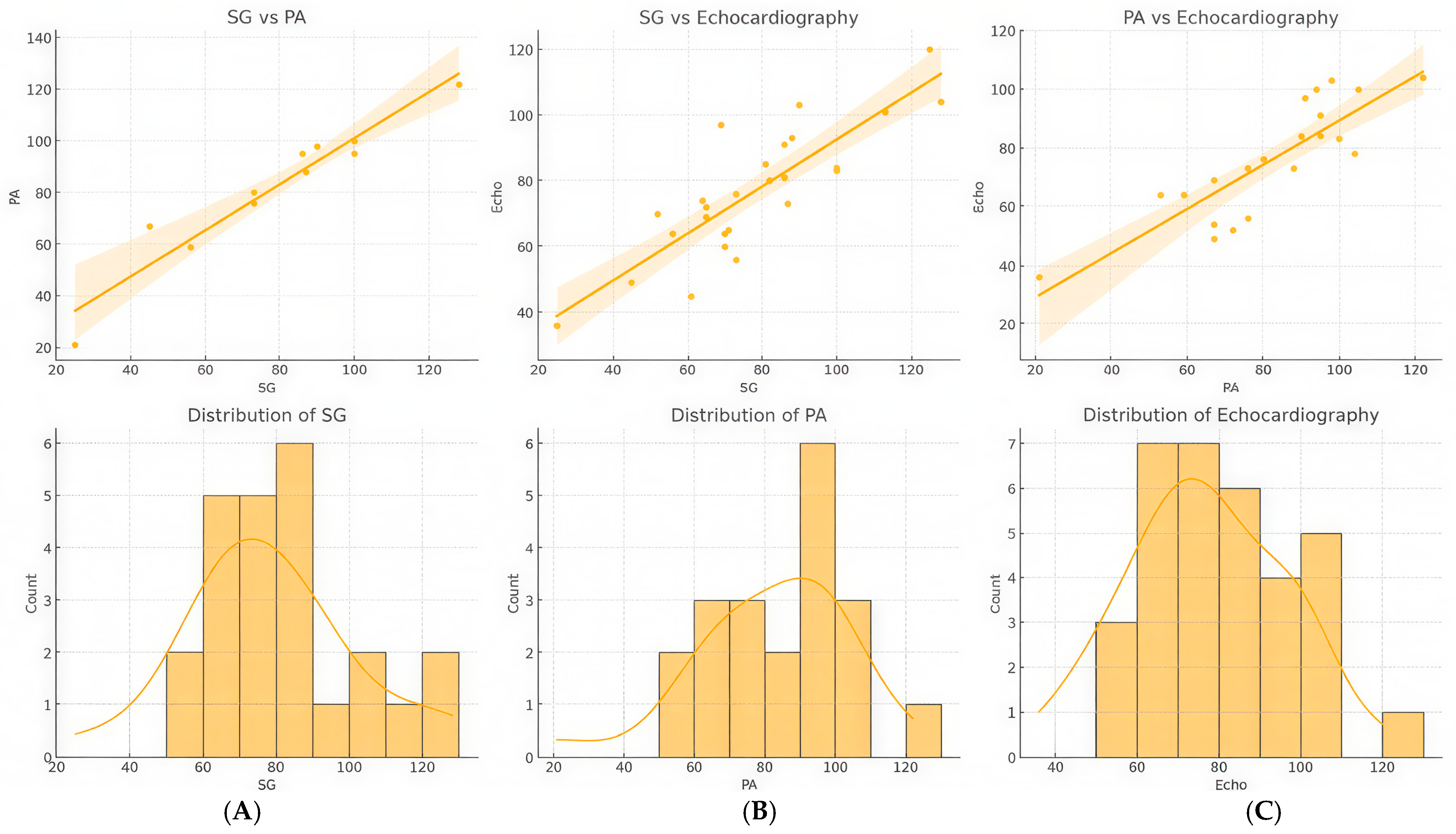
3.3. Correlation Analysis Between Right Heart Pressure Measurements Using Echocardiography, Swan-Ganz Catheterization, and Pulmonary Angiography
4. Discussion
- Clinical Interpretation:
- Limitations of the Different Pressure Measurement Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D TTE | Three-Dimensional Transthoracic Echocardiography |
| BMI | Body Mass Index |
| BP | Blood Pressure |
| BPA | Balloon Pulmonary Angioplasty |
| CI | Cardiac Index |
| CO | Cardiac Output |
| CTEPH | Chronic Thromboembolic Pulmonary Hypertension |
| ESC | European Society of Cardiology |
| IQR | Interquartile Range |
| IVC | Inferior Vena Cava |
| mPAP | Mean Pulmonary Artery Pressure |
| NT-proBNP | N-terminal pro-B-type Natriuretic Peptide |
| NYHA | New York Heart Association |
| PA | Pulmonary Angiography |
| PAP | Pulmonary Artery Pressure |
| PEA | Pulmonary Endarterectomy |
| PCWP | Pulmonary Capillary Wedge Pressure |
| PVR | Pulmonary Vascular Resistance |
| PVRI | Pulmonary Vascular Resistance Index |
| RAP | Right Atrial Pressure |
| RVD1 | Right Ventricular Diameter at Basal Level |
| RAA | Right Atrial Area |
| RV | Right Ventricular |
| SD | Standard Deviation |
| SG | Swan-Ganz Catheterization |
| sPAP | Systolic Pulmonary Artery Pressure |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| TR | Tricuspid Regurgitation |
| TRV | Tricuspid Regurgitation Velocity |
References
- Delcroix, M.; Torbicki, A.; Gopalan, D.; Sitbon, O.; Klok, F.A.; Lang, I.; Jenkins, D.; Kim, N.H.; Humbert, M.; Jais, X.; et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2021, 57, 2002828. [Google Scholar] [CrossRef]
- Teerapuncharoen, K.; Bag, R. Chronic Thromboembolic Pulmonary Hypertension. Lung 2022, 200, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Bazmpani, M.A.; Arvanitaki, A.; Toumpourleka, M.; Pitsiou, G.; Panagiotidou, E.; Mouratoglou, S.A.; Sianos, G.; Hadjimiltiades, S.; Pitsis, A.; Mayer, E.; et al. Epidemiology and management of chronic thromboembolic pulmonary hypertension: Experience from two expert centers. Hell. J. Cardiol. 2018, 59, 16–23. [Google Scholar] [CrossRef]
- Shahidi, P.; Mentzel, L.; Blazek, S.; Sulimov, D.; Thiele, H.; Fengler, K. From Pulmonary Embolism to Chronic Thromboembolic Pulmonary Hypertension: A Pathophysiological Approach. Rev. Cardiovasc. Med. 2024, 25, 402. [Google Scholar] [CrossRef]
- Matthews, D.T.; Hemnes, A.R. Current concepts in the pathogenesis of chronic thromboembolic pulmonary hypertension. Pulm. Circ. 2016, 6, 145–154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonderman, D.; Wilkens, H.; Wakounig, S.; Schäfers, H.-J.; Jansa, P.; Lindner, J.; Simkova, I.; Martischnig, A.M.; Dudczak, J.; Sadushi, R.; et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2009, 33, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Ghani, H.; Pepke-Zaba, J. Chronic Thromboembolic Pulmonary Hypertension: A Review of the Multifaceted Pathobiology. Biomedicines 2024, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Labrada, L.; Vaidy, A.; Vaidya, A. Right ventricular assessment in pulmonary hypertension. Curr. Opin. Pulm. Med. 2023, 29, 348–354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simonneau, G.; Torbicki, A.; Dorfmüller, P.; Kim, N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160112. [Google Scholar] [CrossRef]
- Correale, M.; Chirivì, F.; Bevere, E.M.L.; Tricarico, L.; D’Alto, M.; Badagliacca, R.; Brunetti, N.D.; Vizza, C.D.; Ghio, S. Endothelial Function in Pulmonary Arterial Hypertension: From Bench to Bedside. J. Clin. Med. 2024, 13, 2444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simeone, B.; Maggio, E.; Schirone, L.; Rocco, E.; Sarto, G.; Spadafora, L.; Bernardi, M.; D’Ambrosio, L.; Forte, M.; Vecchio, D.; et al. Chronic Thromboembolic Pulmonary Hypertension: The Diagnostic Assessment. Front. Cardiovasc. Med. 2024, 11, 1439402. [Google Scholar] [CrossRef]
- Lang, I.M.; Madani, M. Update on chronic thromboembolic pulmonary hypertension. Circulation 2014, 130, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Baratella, E.; Caforio, G.; Confalonieri, P.; Wade, B.; Marrocchio, C.; Geri, P.; Pozzan, R.; Andrisano, A.G.; Cova, M.A.; et al. Chronic Thromboembolic Pulmonary Hypertension: An Update. Diagnostics 2022, 12, 235. [Google Scholar] [CrossRef]
- Grünig, E.; Peacock, A.J. Imaging the heart in pulmonary hypertension: An update. Eur. Respir. Rev. 2015, 24, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.; Jenkins, D.; Lindner, J.; D’armini, A.; Kloek, J.; Meyns, B.; Ilkjaer, L.B.; Klepetko, W.; Delcroix, M.; Lang, I.; et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. J. Thorac. Cardiovasc. Surg. 2011, 141, 702–710. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; D’ARmini, A.M.; Grimminger, F.; Hoeper, M.M.; Jansa, P.; Kim, N.H.; Mayer, E.; Simonneau, G.; Wilkins, M.R.; Fritsch, A.; et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N. Engl. J. Med. 2013, 369, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Mukherjee, M.; Rudski, L.G.; Addetia, K.; Afilalo, J.; D’aLto, M.; Freed, B.H.; Friend, L.B.; Gargani, L.; Grapsa, J.; Hassoun, P.M.; et al. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults and Special Considerations in Pulmonary Hypertension: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2025, 38, 141–186. [Google Scholar] [CrossRef]
- Seyyedi, S.R.; Mozafari, M.; Sharif-Kashani, B.; Sadr, M.; Emami, H.; Mehrazmay, A. Correlation of Echocardiographic and Right Heart Catheterization Estimations of Pulmonary Artery Systolic Pressure. Tanaffos 2022, 21, 78–84. [Google Scholar] [PubMed] [PubMed Central]
- Rich, J.D.; Shah, S.J.; Swamy, R.S.; Kamp, A.; Rich, S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: Implications for clinical practice. Chest 2011, 139, 988–993. [Google Scholar] [CrossRef]
- Madani, M.; Mayer, E.; Fadel, E.; Jenkins, D.P. Pulmonary Endarterectomy. Patient Selection, Technical Challenges, and Outcomes. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 3), S240-7. [Google Scholar] [CrossRef] [PubMed]
- Saouti, N.; Morshuis, W.J.; Heijmen, R.H.; Snijder, R.J. Long-term outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J. Thorac. Cardiovasc. Surg. 2012, 144, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Faccioli, E.; Verzeletti, V.; Perazzolo Marra, M.; Boscolo, A.; Schiavon, M.; Navalesi, P.; Rea, F.; Dell’Amore, A. Pulmonary Endarterectomy for Chronic Thromboembolic Pulmonary Hypertension: A Systematic Review of the Most Updated Literature. J. Clin. Med. 2022, 11, 6976. [Google Scholar] [CrossRef]
- Galié, N.; Humbert, M.; Vachiéry, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, M.; Lang, I.; Pepke-Zaba, J.; Jansa, P.; D’Armini, A.M.; Snijder, R.; Bresser, P.; Torbicki, A.; Mellemkjaer, S.; Lewczuk, J.; et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. Circulation 2016, 133, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.; Yang, J.H.; Park, T.K.; Chang, S.A.; Jung, D.S.; Cho, Y.S.; Kim, S.M.; Kim, T.J.; Park, H.Y.; Choi, S.H.; et al. Impact of Balloon Pulmonary Angioplasty on Hemodynamics and Clinical Outcomes in Patients with Chronic Thromboembolic Pulmonary Hypertension: The Initial Korean Experience. J. Korean Med. Sci. 2018, 33, e24. [Google Scholar] [CrossRef] [PubMed]
- Suntharalingam, J.; Goldsmith, K.; Toshner, M.; Doughty, N.; Sheares, K.K.; Hughes, R.; Jenkins, D.; Pepke-Zaba, J. Role of NT-proBNP and 6MWD in chronic thromboembolic pulmonary hypertension. Respir. Med. 2007, 101, 2254–2262. [Google Scholar] [CrossRef][Green Version]



| Parameter | Mean ± SD | Range |
|---|---|---|
| BMI | 28.39 ± 5.00 | 20.86–37.11 |
| Systolic BP (mmHg) | 125.56 ± 17.39 | 104–153 |
| Age at Diagnosis (years) | 61.41 ± 14.34 | 21–81 |
| NYHA Class | 2.76 ± 0.83 | 1–4 |
| 6MWT Distance | 330.63 ± 152.17 | 42.0–548.0 |
| NT-proBNP (pg/mL) | 4820.97 ± 10,288.15 | 82.0–43,722.0 |
| PAPs (mmHg)-echo | 72.07 ± 22.25 | 43.0–128.0 |
| PAPs (mmHg)-BPA | 88.10 ± 11.90 | 76.0–104.0 |
| PAPs (mmHg)-SG | 77.69 ± 23.49 | 41.0–111.0 |
| PVR 1 (dyn·s·cm−5) | 675.38 ± 369.60 | 272.0–1786.0 |
| PVRI 1 (dyn·s·cm−5·m2) | 1275.19 ± 691.34 | 518.0–3232.0 |
| PCWP 1 (mmHg) | 10.71± 3.35 | 8–20 |
| Mean Pulmonary Artery Pressure 1 | 43.23 ± 8.56 | 24.0–80.0 |
| Right Atrial Pressure 1 | 7.88 ± 4.13 | 2.0–21.0 |
| Cardiac Output 1 (CO, L/min) | 4.44 ± 1.55 | 1.2–6.9 |
| Cardiac Index 1 (CI, L/min/m2) | 2.38 ± 0.79 | 0.7–4.6 |
| Number of BPA Procedures | 3.13 ± 2.17 | 1–11 |
| Total Number of Dilatations | 12.00 ± 9.16 | 3–44 |
| % of Treated Segments | 62.96 ± 10.84 | 49.90–78.12 |
| TAPSE (mm) | 19.50 ± 5.15 | 10.0–26.0 |
| RVD1 (mm) | 41.75 ± 6.08 | 39.0–52.0 |
| RAA (mm2) | 25.16 ± 7.89 | 15.0–36.5 |
| TI Severity | II–III | I–III–IV |
| Left Atrium Diameter | 36.25 ± 5.53 | 30.0–45.0 |
| Well-Responding Group (n = 10) | Poor-Responding Group (n = 7) | p | |
|---|---|---|---|
| BMI | 27.39 ± 5.35 | 29.81 ± 4.46 | 0.364 |
| Systolic BP (mmHg) | 128.00 ± 19.33 | 121.50 ± 14.25 | 0.428 |
| Age at Diagnosis (years) | 60.30 ± 16.70 | 63.00 ± 11.162 | 0.740 |
| NYHA Class | 2.70 ± 0.82 | 2.86 ± 0.89 | 0.887 |
| 6MWT Distance | 317.80 ± 176.58 | 352.00 ± 111.64 | 0.792 |
| NT-proBNP (pg/mL) | 5077.78 ± 10,569.13 | 2598 ± 3217.74 | 0.740 |
| PAPs (mmHg)-echo | 80.00 ± 22.63 | 62.43 ± 15.96 | 0.114 |
| PAPs (mmHg)-BPA | 89.88 ± 10.40 | 81 ± 19.80 | 0.533 |
| PAPs (mmHg)-SG | 88.33 ± 23.02 | 64 ± 16.85 | 0.042 |
| PVR (dyn·s·cm−5) 2 | 793.56 ± 435.25 | 523.43 ± 172.5 | 0.210 |
| PVRI (dyn·s·cm−5·m2) 2 | 1442.67 ± 805.03 | 1059.86 ± 484.38 | 0.408 |
| PCWP (mmHg) 2 | 12.00 ± 3.50 | 8.86 ± 2.19 | 0.681 |
| Mean Pulmonary Artery Pressure 2 | 44.57 ± 10.23 | 41.67 ± 6.68 | 0.267 |
| Cardiac Output (CO, L/min) 2 | 4.21 ± 1.44 | 4.73 ± 1.73 | 0.351 |
| Cardiac Index (CI, L/min/m2) | 2.31 ± 0.77 | 2.47 ± 0.88 | 0.606 |
| Number of BPA Procedures | 3.56 ± 2.96 | 2.37 ± 1.19 | 0.042 |
| Total Number of Dilatations | 14.60 ± 10.35 | 8.29 ± 5.13 | 0.193 |
| % of Treated Segments | 69.24 ± 19.14 | 45.47 ± 30.15 | 0.161 |
| TAPSE (mm) | 18.33 ± 3.84 | 21.00 ± 6.48 | 0.470 |
| RVD1 (mm) | 45.00 ± 3.08 | 37.57 ± 6.13 | 0.023 |
| RAA (mm2) | 26.88 ± 6.54 | 22.96 ± 9.40 | 0.299 |
| TI Severity | II–III | II–III | |
| Left Atrium Diameter | 36.89 ± 4.43 | 36.71 ± 7.08 | 0.873 |
| Parameter | Baseline (n = 17) | 1-Year Follow-up (n = 17) | 2-Year Follow-up (n = 15) | 3-Year Follow-up (n = 8) | |
|---|---|---|---|---|---|
| NYHA 1 | 2.76 ± 0.83 | 2.69 ± 0.87 | 2.57 ± 0.94 | 2.86 ± 1.07 | |
| Clinical improvement 2 | 0.63 ± 0.72 | 0.79 ± 0.70 | 0.71 ± 0.49 | ||
| 6MWT (m) | 330.63 ± 152.17 | 350.33 ± 155.73 | 323.92 ± 138.46 | 309.00 ± 174.48 | |
| NT-proBNP (pg/mL) | 4820.97 ± 10,288.15 | 2932.30 ± 3388.65 | 2177.07 ± 2100.31 | 1798.29 ± 1953.28 | |
| SG | PAPs (mmHg) | 88.10 ± 11.90 | 72.60 ± 20.38 | 73.31 ± 22.66 | 76.17 ± 30.76 |
| PAPd (mmHg) | 26.25 ± 9.26 | 26.40 ± 8.49 | 27.62 ± 9.47 | 29.86 ± 12.76 | |
| PAPm (mmHg) | 43.23 ± 8.55 | 41.70 ± 8.65 | 41.38 ± 9.41 | 43.00 ± 11.79 | |
| PCWP (mmHg) | 10.71 ± 3.35 | 10.93 ± 2.40 | 11.23 ± 2.45 | 10.71 ± 3.50 | |
| PVR (dyn·s·cm−5) | 675.38 ± 369.60 | 648.93 ± 374.91 | 639.00 ± 320.66 | 684.86 ± 503.41 | |
| PVRI (dyn·s·cm−5·m2) | 1275.19 ± 691.34 | 1254.07 ± 711.95 | 1204.46 ± 544.86 | 1293.14 ± 941.22 | |
| CO (L/min) | 4.44 ± 1.55 | 4.50 ± 1.35 | 4.78 ± 1.65 | 4.71 ± 1.35 | |
| CI (L/min/m2) | 2.38 ± 0.79 | 2.41 ± 1.15 | 2.41 ± 9.16 | 2.55 ± 6.49 | |
| PA-BPA | Number of involved segments | 10.88 ± 5.67 | |||
| Proportion of dilated segments relative to affected segments (%) | 43.79 ± 24.27 | 60.78 ± 19.60 | 63.31 ± 19.36 | ||
| RVP syst. (mmHg) | 89.00 ± 13.47 | 85.73 ± 12.36 | 78.30 ± 12.23 | 84.17 ± 12.00 | |
| RVP diast. (mmHg) | 31.56 ± 9.38 | 25.17 ± 7.69 | 25.40 ± 7.11 | 25.33 ± 8.19 | |
| RVP mean (mmHg) | 52.56 ± 7.69 | 46.58 ± 7.51 | 45.50 ± 7.26 | 47.50 ± 8.16 | |
| Echocardiography | RVP (mmHg) | 77.33 ± 7.69 | 77.73 ± 8.99 | 77.85 ± 7.23 | 81.00 ± 7.55 |
| TAPSE (mm) | 19.50 ± 5.15 | 20.00 ± 4.61 | 18.54 ± 4.79 | 20.43 ± 5.25 | |
| RVD1 (mm) | 41.75 ± 6.08 | 41.40 ± 6.12 | 39.62 ± 4.41 | 37.14 ± 4.13 | |
| RAA (cm2) | 25.16 ± 5.53 | 23.16 ± 8.11 | 26.48 ± 12.14 | 22.66 ± 8.40 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolodzey, G.; Péter, A.; Daragó, A.; Balogh, L.; Bereczky, Z.; Barta, J.; Csanádi, Z.; Szűk, T. Assessment of Right Ventricular Pressure in Chronic Thromboembolic Pulmonary Hypertension: Comparison of Diagnostic Modalities and Balloon Pulmonary Angioplasty Outcomes. Diagnostics 2025, 15, 2050. https://doi.org/10.3390/diagnostics15162050
Kolodzey G, Péter A, Daragó A, Balogh L, Bereczky Z, Barta J, Csanádi Z, Szűk T. Assessment of Right Ventricular Pressure in Chronic Thromboembolic Pulmonary Hypertension: Comparison of Diagnostic Modalities and Balloon Pulmonary Angioplasty Outcomes. Diagnostics. 2025; 15(16):2050. https://doi.org/10.3390/diagnostics15162050
Chicago/Turabian StyleKolodzey, Gábor, Andrea Péter, Andrea Daragó, László Balogh, Zsuzsanna Bereczky, Judit Barta, Zoltán Csanádi, and Tibor Szűk. 2025. "Assessment of Right Ventricular Pressure in Chronic Thromboembolic Pulmonary Hypertension: Comparison of Diagnostic Modalities and Balloon Pulmonary Angioplasty Outcomes" Diagnostics 15, no. 16: 2050. https://doi.org/10.3390/diagnostics15162050
APA StyleKolodzey, G., Péter, A., Daragó, A., Balogh, L., Bereczky, Z., Barta, J., Csanádi, Z., & Szűk, T. (2025). Assessment of Right Ventricular Pressure in Chronic Thromboembolic Pulmonary Hypertension: Comparison of Diagnostic Modalities and Balloon Pulmonary Angioplasty Outcomes. Diagnostics, 15(16), 2050. https://doi.org/10.3390/diagnostics15162050






