Multilayered Insights into Poorly Differentiated, BRAFV600E-Positive, Thyroid Carcinoma in a Rapidly Developing Goiter with Retrosternal Extension: From En “Y” Cervicotomy to SPECT/CT-Positive Lung Metastases
Abstract
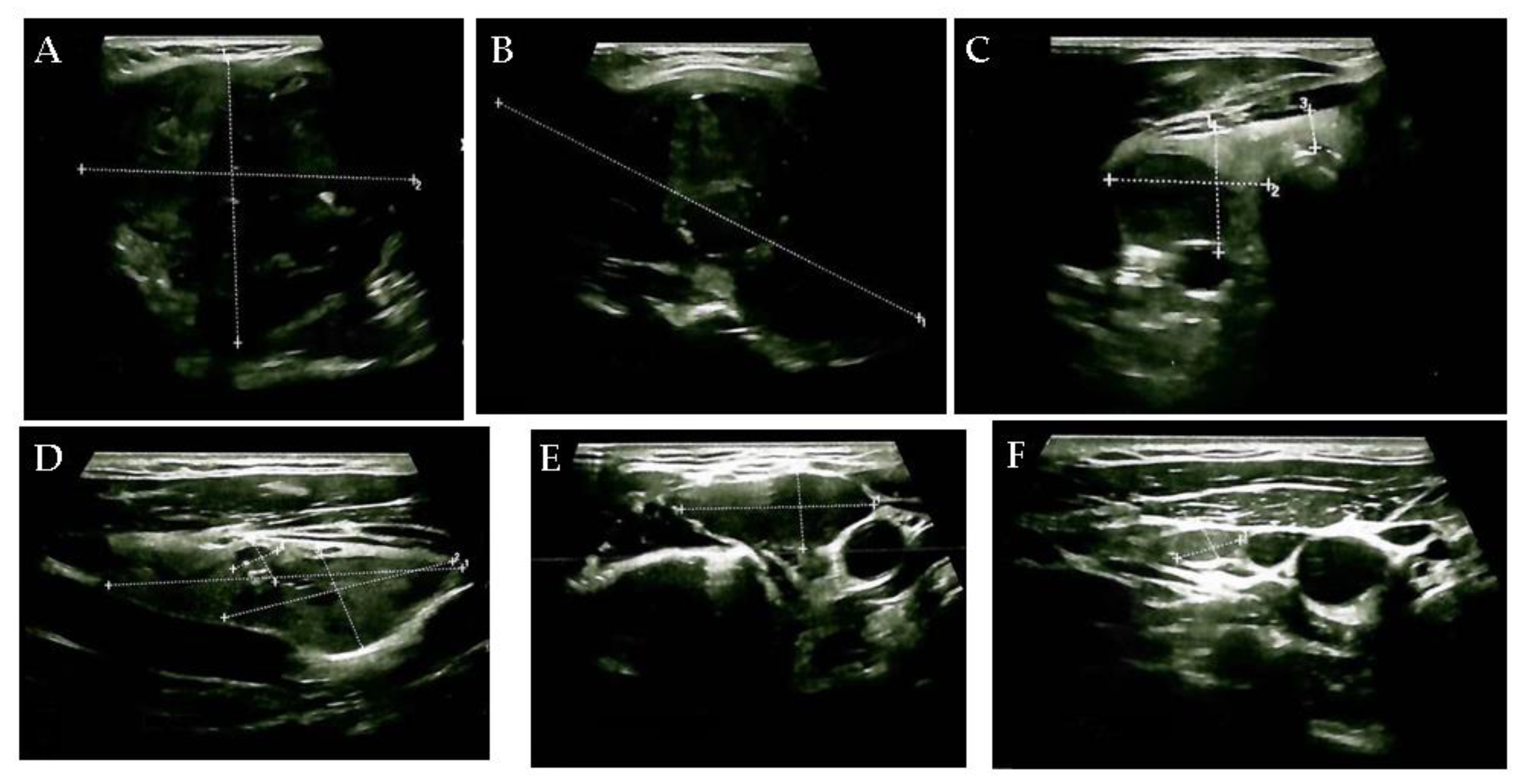
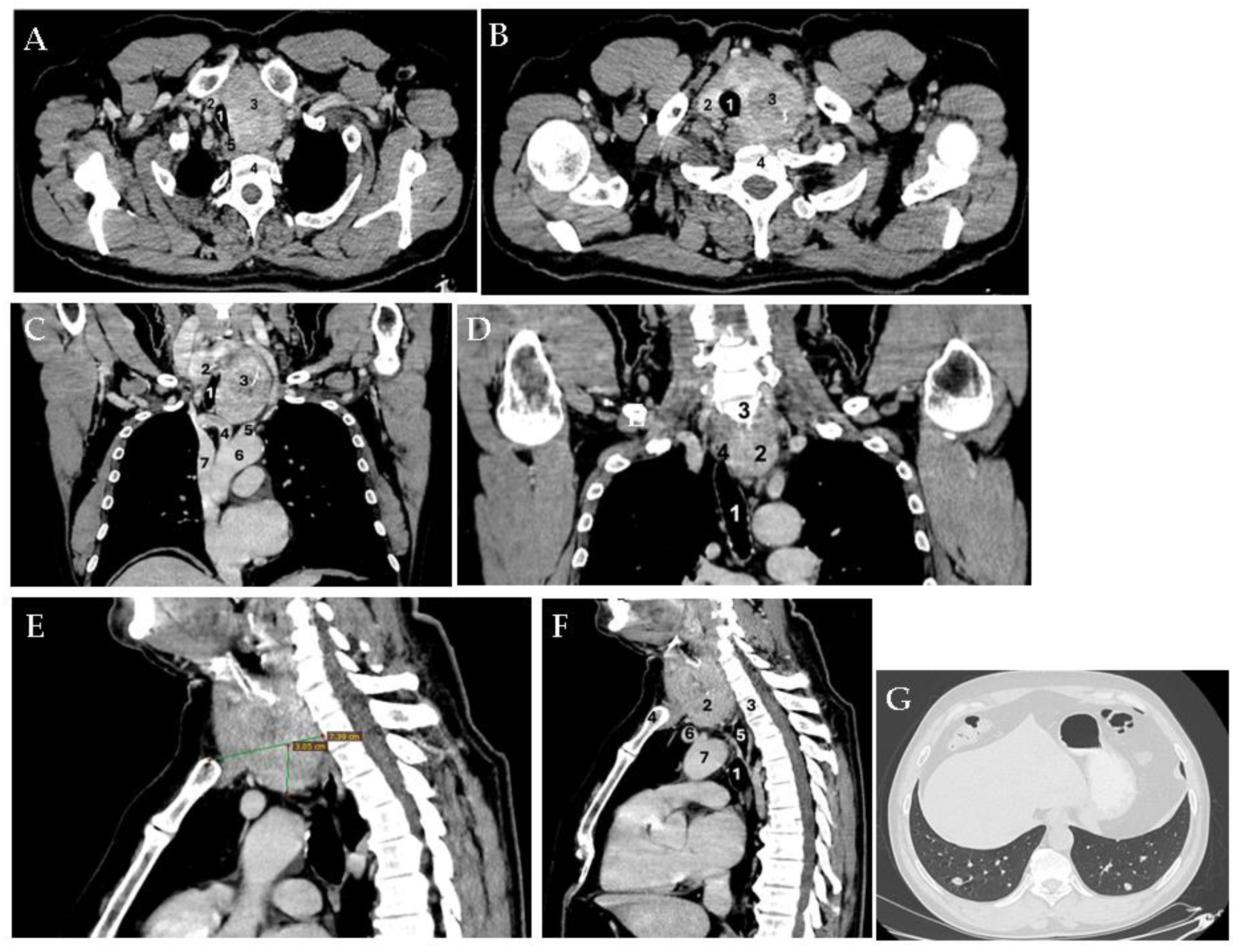
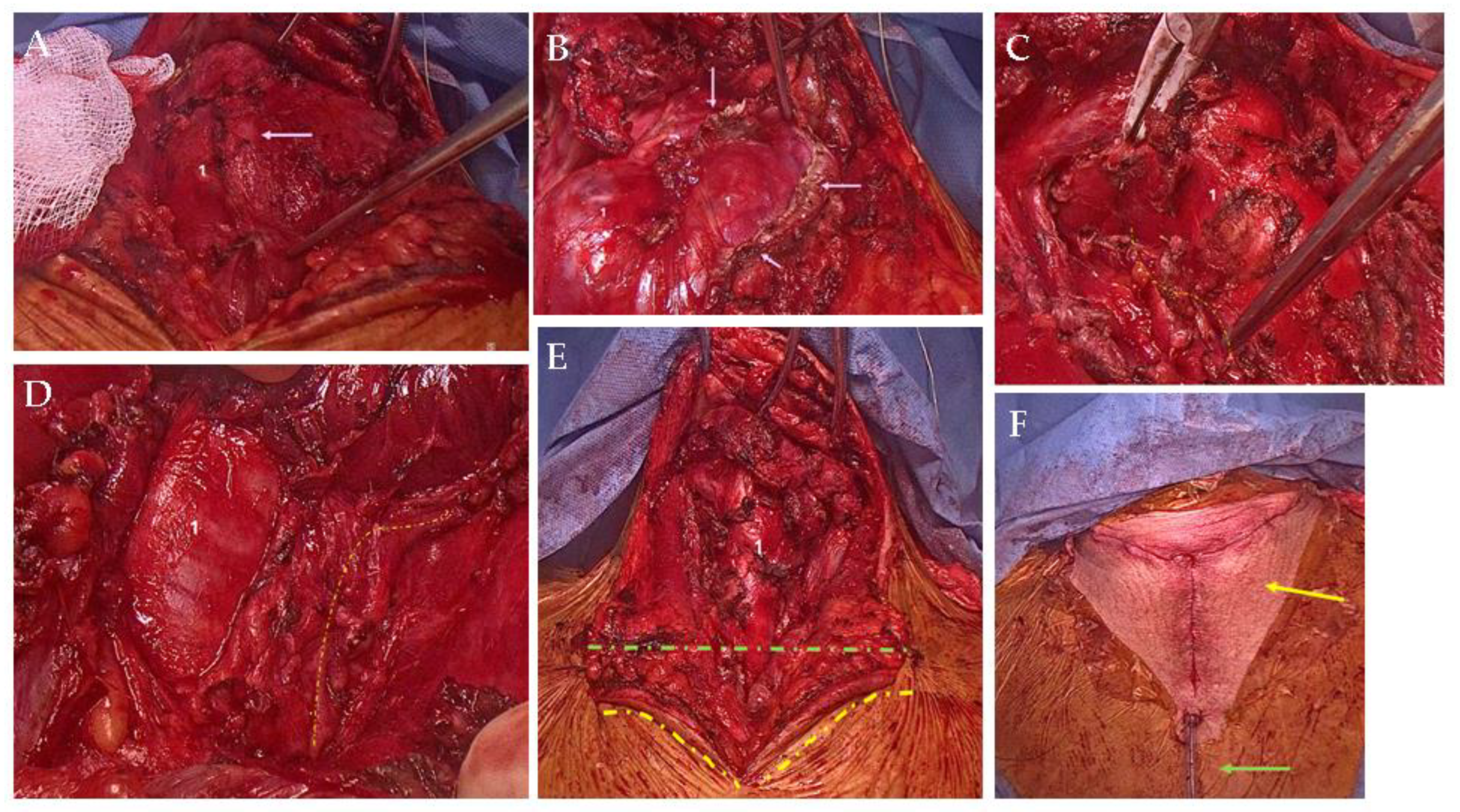

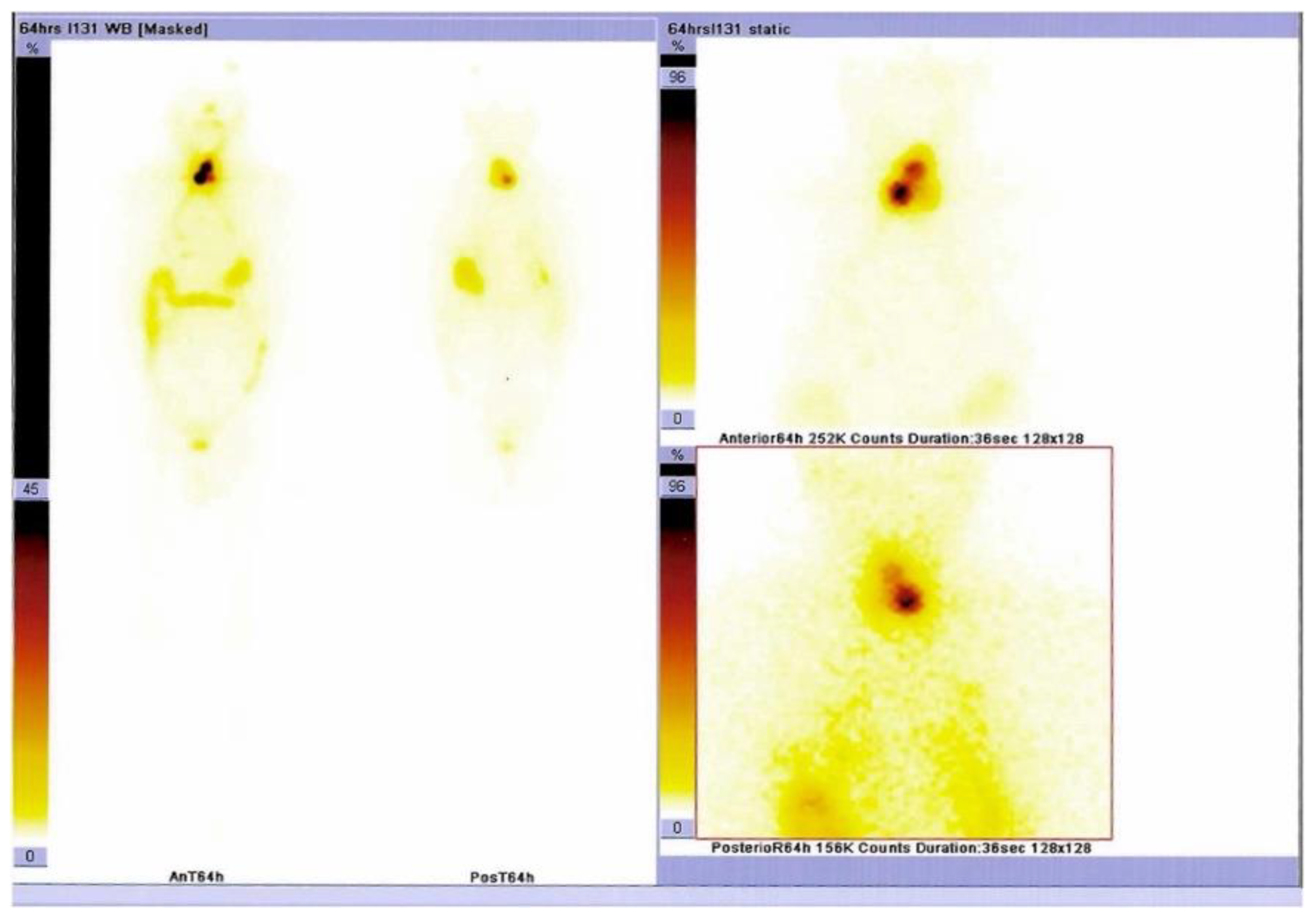
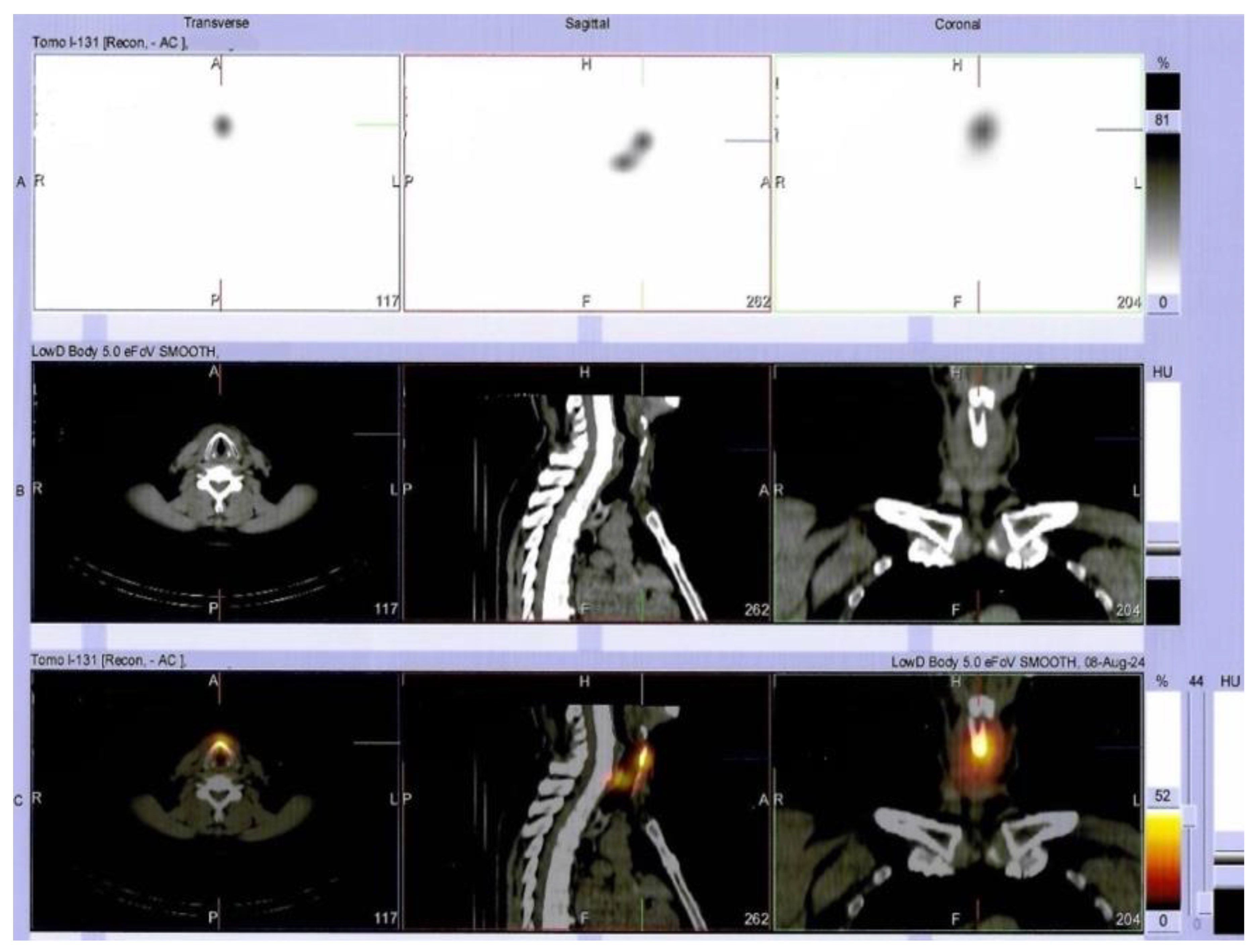

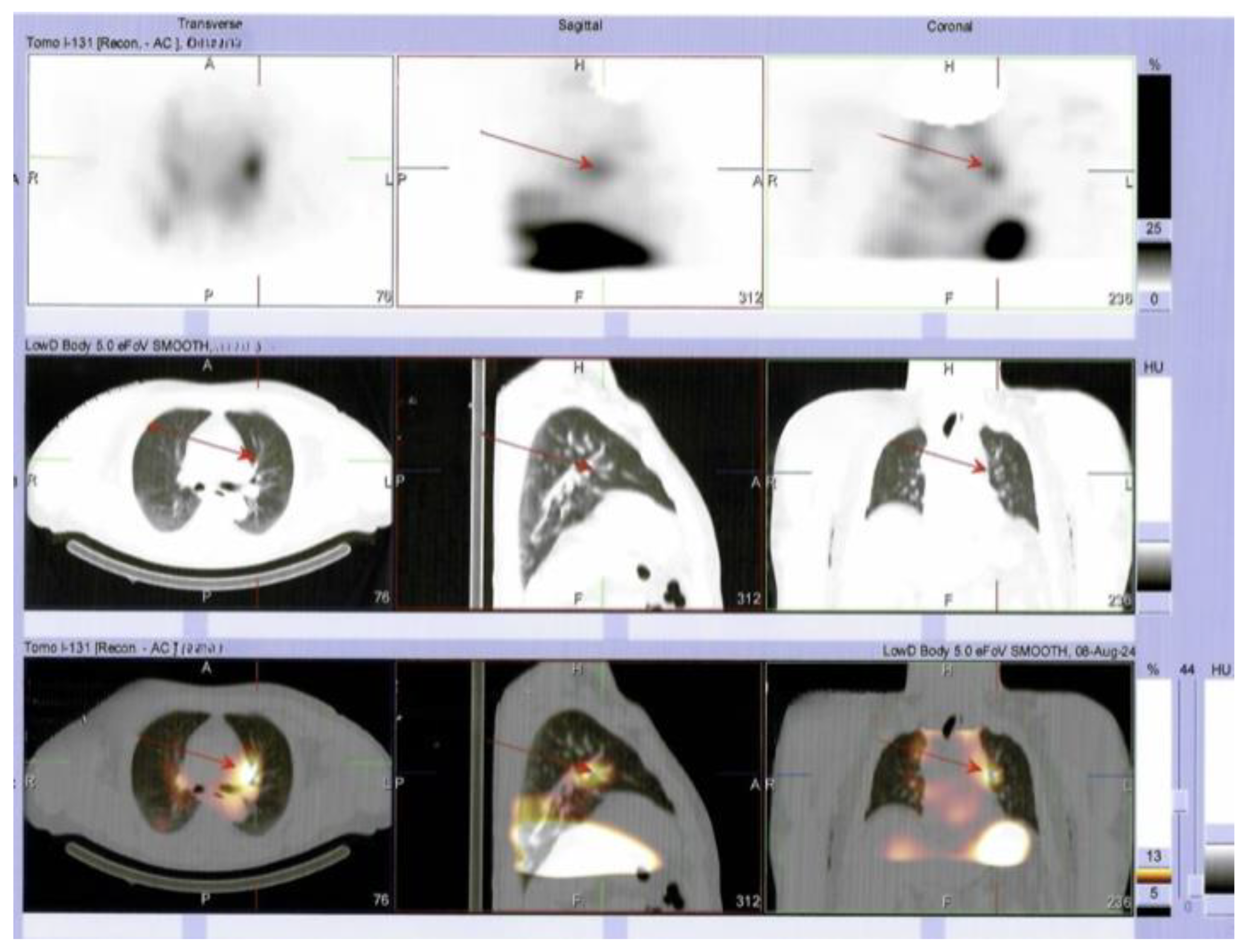
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | computed tomography |
| FT4 | free levothyroxine |
| PTH | parathyroid hormone |
| P1NP | procollagen type 1 N-terminal propetide |
| TSH | Thyroid-stimulating hormone |
| TgAb | anti-thyroglobulin antibodies |
| TPOAbs | anti-thyroperoxidase antibodies |
| TRAb | TSH-receptor antibodies |
| T4 | thyroxine |
| SPECT/CT | single-photon emission computed tomography combined with CT |
References
- Schipor, S.; Publik, M.A.; Manda, D.; Ceausu, M. Aggressive Thyroid Carcinomas Clinical and Molecular Features: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 5535. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, X.; Huang, G. Giant poorly differentiated thyroid carcinoma: A case report and literature review. Asian J. Surg. 2024, 48, 2513–2514. [Google Scholar] [CrossRef] [PubMed]
- Boudina, M.; Zisimopoulou, E.; Xirou, P.; Chrisoulidou, A. Aggressive Types of Malignant Thyroid Neoplasms. J. Clin. Med. 2024, 13, 6119. [Google Scholar] [CrossRef] [PubMed]
- Alam, I.S.; Patel, K.N. Management of Poorly Differentiated Thyroid Cancer and Differentiated High-Grade Thyroid Carcinoma. Surg. Clin. N. Am. 2024, 104, 751–765. [Google Scholar] [CrossRef]
- Apelian, S.; Ismail, S.; Roumieh, D.; Saad, B.; Alshehabi, Z. Poorly differentiated thyroid carcinoma arising from substernal goiter: A case report. Ann. Med. Surg. 2024, 86, 3020–3024. [Google Scholar] [CrossRef]
- Kim, J.Y.; Myung, J.K.; Kim, S.; Tae, K.; Choi, Y.Y.; Lee, S.J. Prognosis of Poorly Differentiated Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Endocrinol. Metab. 2024, 39, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Cirello, V.; Gambale, C.; Nikitski, A.V.; Masaki, C.; Roque, J.; Colombo, C. Poorly differentiated thyroid carcinoma: Molecular, clinico-pathological hallmarks and therapeutic perspectives. Panminerva Med. 2024, 66, 155–173. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mair, M.; Shaha, A.R.; Paleri, V.; Sawhney, S.; Mishra, A.; Bhandarkar, S.; D’Cruz, A.K. Current evidences in poorly differentiated thyroid carcinoma: A systematic review and subsection meta-analysis for clinical decision making. Endocrine 2024, 85, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Coca-Pelaz, A.; Rodrigo, J.P.; Agaimy, A.; Williams, M.D.; Saba, N.F.; Nuyts, S.; Randolph, G.W.; López, F.; Vander Poorten, V.; Kowalski, L.P.; et al. Poorly differentiated thyroid carcinomas: Conceptual controversy and clinical impact. Virchows Arch. 2024, 484, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Boucai, L.; Zafereo, M.; Cabanillas, M.E. Thyroid Cancer: A Review. JAMA 2024, 331, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.; Ciuche, A.; Motaş, C.; Motaş, N.; Bluoss, C.; Pantile, D.; Davidescu, M.; Horvat, T. Cervico-mediastinal thyroid masses-our experience. Chirurgia 2014, 109, 34–43. [Google Scholar] [PubMed]
- Li, W.; Li, H.; Zhang, S.; Tao, Y.; Wang, X.; Cheng, J. To explore the risk factors and preventive measures affecting the treatment of retrosternal goiter: An observational study. Medicine 2020, 99, e23003. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Renton, B. A not-so-incidental retrosternal goitre. Br. J. Hosp. Med. 2019, 80, 736. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Shaha, A.R.; Wu, J.X. Surgical approach to the substernal goiter. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101312. [Google Scholar] [CrossRef] [PubMed]
- Aeschbacher, P.; Huguenin-Dezot, M.A.; Nesti, C.; Borbély, Y.M.; Kaderli, R.M. Minimal Invasive Resection of Large Retrosternal Thyroid Goiter. J. Vis. Exp. 2024, 211, e67308. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.V.; Banks, K.C.; Hsu, D.S.; Debbaneh, P.M.; Wang, K.H.; Velotta, J.B. Incidental paratracheal lymph node lung adenocarcinoma in a patient with goiter: A case report. AME Case Rep. 2022, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Anikin, V.; Welman, K.; Asadi, N.; Dalal, P.; Reshetov, I.; Beddow, E. Retrosternal goiter in thoracic surgical practice. Khirurgiia 2021, 12, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, M.; Ito, A.; Mori, A.; Oka, M.; Omura, S.; Kida, W.; Inayoshi, Y.; Inoue, A.; Fuchigami, T. Surgical treatment of substernal goiter: An analysis of 44 cases. Auris Nasus Larynx 2017, 44, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Bastien, A.J.; Ho, A.S. Surgical Management of Substernal Thyroid Goiters. Otolaryngol. Clin. N. Am. 2024, 57, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Y.; Li, C.; Cai, Y.; He, T.; Sun, R.; Tian, W.; Tang, Z.; Sheng, J.; Liu, D.; et al. Surgery for retrosternal goiter: Cervical approach. Gland Surg. 2020, 9, 392–400. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sima, O.-C.; Cucu, A.-P.; Terzea, D.; Nistor, C.; Vasilescu, F.; Eftimie, L.-G.; Ciobica, M.-L.; Costachescu, M.; Carsote, M. Multilayered Insights into Poorly Differentiated, BRAFV600E-Positive, Thyroid Carcinoma in a Rapidly Developing Goiter with Retrosternal Extension: From En “Y” Cervicotomy to SPECT/CT-Positive Lung Metastases. Diagnostics 2025, 15, 2049. https://doi.org/10.3390/diagnostics15162049
Sima O-C, Cucu A-P, Terzea D, Nistor C, Vasilescu F, Eftimie L-G, Ciobica M-L, Costachescu M, Carsote M. Multilayered Insights into Poorly Differentiated, BRAFV600E-Positive, Thyroid Carcinoma in a Rapidly Developing Goiter with Retrosternal Extension: From En “Y” Cervicotomy to SPECT/CT-Positive Lung Metastases. Diagnostics. 2025; 15(16):2049. https://doi.org/10.3390/diagnostics15162049
Chicago/Turabian StyleSima, Oana-Claudia, Anca-Pati Cucu, Dana Terzea, Claudiu Nistor, Florina Vasilescu, Lucian-George Eftimie, Mihai-Lucian Ciobica, Mihai Costachescu, and Mara Carsote. 2025. "Multilayered Insights into Poorly Differentiated, BRAFV600E-Positive, Thyroid Carcinoma in a Rapidly Developing Goiter with Retrosternal Extension: From En “Y” Cervicotomy to SPECT/CT-Positive Lung Metastases" Diagnostics 15, no. 16: 2049. https://doi.org/10.3390/diagnostics15162049
APA StyleSima, O.-C., Cucu, A.-P., Terzea, D., Nistor, C., Vasilescu, F., Eftimie, L.-G., Ciobica, M.-L., Costachescu, M., & Carsote, M. (2025). Multilayered Insights into Poorly Differentiated, BRAFV600E-Positive, Thyroid Carcinoma in a Rapidly Developing Goiter with Retrosternal Extension: From En “Y” Cervicotomy to SPECT/CT-Positive Lung Metastases. Diagnostics, 15(16), 2049. https://doi.org/10.3390/diagnostics15162049







