Uncovering Sternoclavicular Arthritis, Suspected Pseudogout, in a Fever of Unknown Origin by Whole-Body MRI
Abstract
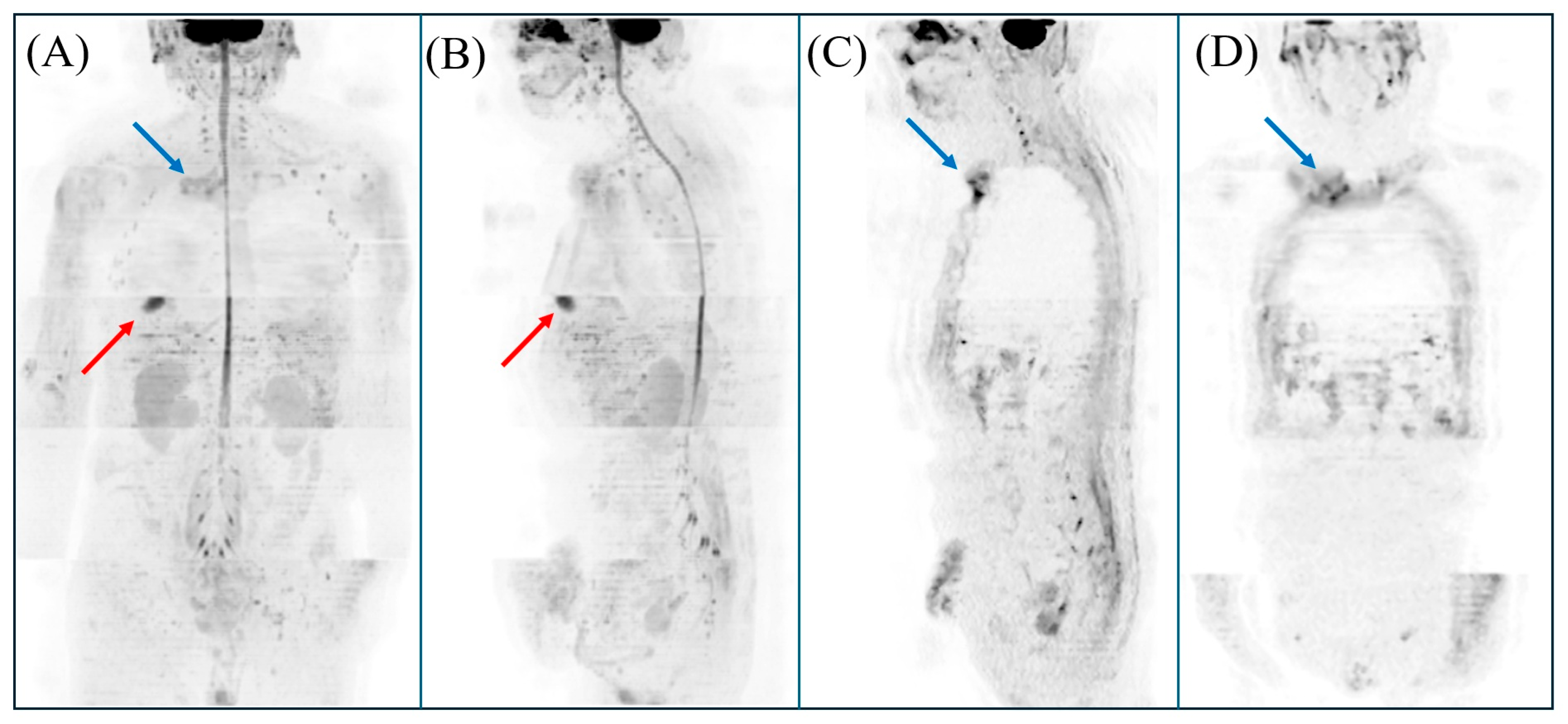

Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| DWIBS | Diffusion-weighted whole-body imaging with background body signal suppression |
| FUO | Fever of unknown origin |
References
- Takahara, T.; Imai, Y.; Yamashita, T.; Yasuda, S.; Nasu, S.; Van Cauteren, M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): Technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004, 22, 275–282. [Google Scholar] [PubMed]
- Nakata, M.; Yokota, N.; Kenzaka, T. Diffusion-weighted whole-body magnetic resonance imaging with background body signal suppression was useful in a patient with isolated myocardial abscess confined to the right atrial wall: A case report. BMC Cardiovasc. Disord. 2023, 23, 341. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Hayashi, K.; Sato, M.; Nakaya, Y.; Miura, T.; Takaku, N.; Iwasaki, T.; Kobayashi, Y. Visualizing Aortic Inflammation by Diffusion-Weighted Whole-Body Imaging with Background Body Signal Suppression (DWIBS). Diagnostics 2025, 15, 1151. [Google Scholar] [CrossRef] [PubMed]
- Nakata, M.; Wakugawa, T.; Uehara, H.; Kenzaka, T. Comparison of diffusion-weighted whole-body magnetic resonance imaging and abdominal ultrasonography versus contrast-enhanced computed tomography in diagnosing acute focal bacterial nephritis: A retrospective cohort study. Quant. Imaging Med. Surg. 2025, 15, 3298–3307. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Shinozaki, F.; Tanaka, S.; Sunaoshi, T.; Kano, D.; Sugiyama, E.; Shite, M.; Haga, R.; Fukamizu, Y.; Fujita, T.; et al. Diagnosis of complications associated with acute cholecystitis using computed tomography and diffusion-weighted imaging with background body signal suppression/T2 image fusion. Exp. Ther. Med. 2017, 14, 743–747. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kimura, K.; Hayashi, K.; Suzuki, A.; Sato, M.; Nakaya, Y.; Takaku, N.; Miura, T.; Kobayashi, Y. A case of synovitis acne pustulosis hyperostosis osteitis (SAPHO) syndrome with myeloperoxidase anti-neutrophil cytoplasmic antibody: Exploring an association or coincidence. Cureus 2025, 17, e83866. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Quinlan, J.D. Fever of unknown origin in adults. Am. Fam. Physician 2022, 105, 137–143. [Google Scholar] [PubMed]
- Wright, W.F.; Stelmash, L.; Betrains, A.; Mulders-Manders, C.M.; Rovers, C.P.; Vanderschueren, S.; Auwaerter, P.G.; for the International Fever and Inflammation of Unknown Origin Research Working Group. Recommendations for Updating Fever and Inflammation of Unknown Origin from a Modified Delphi Consensus Panel. Open Forum Infect. Dis. 2024, 11, ofae298. [Google Scholar] [CrossRef] [PubMed]
- Andrej Tavakoli, A.; Reichert, M.; Blank, T.; Dinter, D.; Weckbach, S.; Buchheidt, D.; Schoenberg, O.S.; Attenberger, U. Findings in whole body MRI and conventional imaging in patients with fever of unknown origin—A retrospective study. BMC Med. Imaging 2020, 20, 94. [Google Scholar] [CrossRef]
- Giani, T.; Bernardini, A.; Basile, M.; Di Maurizo, M.; Perrone, A.; Renzo, S.; Filistrucchi, V.; Cimaz, R.; Lionetti, P. Usefulness of magnetic resonance enterography in detecting signs of sacroiliitis in young patients with inflammatory bowel disease. Pediatr. Rheumatol. Online J. 2020, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Davidovic, L.; Tsay, V. Potential application of diffusion-weighted whole-body imaging with background body signal suppression for disease activity assessment in Takayasu arteritis—In search of the “golden mean”: Case report. Ann. Vasc. Surg. 2019, 61, e9–e468. [Google Scholar] [CrossRef] [PubMed]
- Oguro, E.; Ohshima, S.; Kikuchi-Taura, A.; Murata, A.; Kuzuya, K.; Okita, Y.; Matsuoka, H.; Teshigawara, S.; Yoshimura, M.; Yoshida, Y.; et al. Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS) as a novel imaging modality for disease activity assessment in Takayasu’s arteritis. Intern. Med. 2019, 58, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Hanai, S.; Sato, T.; Nagatani, K.; Minota, S. Pseudogout of the sternoclavicular joints. Intern. Med. 2014, 53, 521–522. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, W.; Doherty, M.; Bardin, T.; Barskova, V.; Guerne, P.A.; Jansen, T.L.; Leeb, B.F.; Perez-Ruiz, F.; Pimentao, J.; Punzi, L.; et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: Terminology and diagnosis. Ann. Rheum. Dis. 2011, 70, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Rho, Y.H.; Zhu, Y.; Zhang, Y.; Reginato, A.M.; Choi, H.K. Risk factors for pseudogout in the general population. Rheumatology 2012, 51, 2070–2074. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, M.; Hayashi, K.; Sato, M.; Iwasaki, T.; Kobayashi, Y. Uncovering Sternoclavicular Arthritis, Suspected Pseudogout, in a Fever of Unknown Origin by Whole-Body MRI. Diagnostics 2025, 15, 2032. https://doi.org/10.3390/diagnostics15162032
Hayashi M, Hayashi K, Sato M, Iwasaki T, Kobayashi Y. Uncovering Sternoclavicular Arthritis, Suspected Pseudogout, in a Fever of Unknown Origin by Whole-Body MRI. Diagnostics. 2025; 15(16):2032. https://doi.org/10.3390/diagnostics15162032
Chicago/Turabian StyleHayashi, Maho, Koji Hayashi, Mamiko Sato, Toshiko Iwasaki, and Yasutaka Kobayashi. 2025. "Uncovering Sternoclavicular Arthritis, Suspected Pseudogout, in a Fever of Unknown Origin by Whole-Body MRI" Diagnostics 15, no. 16: 2032. https://doi.org/10.3390/diagnostics15162032
APA StyleHayashi, M., Hayashi, K., Sato, M., Iwasaki, T., & Kobayashi, Y. (2025). Uncovering Sternoclavicular Arthritis, Suspected Pseudogout, in a Fever of Unknown Origin by Whole-Body MRI. Diagnostics, 15(16), 2032. https://doi.org/10.3390/diagnostics15162032





