Discovery of ETS1 as a New Gene Predisposing to Dilated Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Recruitment and Basic Clinical Characterization of Study Participants
2.3. Genetic Analysis of Research Participants
2.4. Recombination of Gene Expression Plasmids
2.5. Cell Transfection and Dual Reporter Gene Analysis
2.6. Statistical Analysis
3. Results
3.1. Demographic and Phenotypic Characteristics of the Recruited Family Affected with DCM
3.2. Discovery of a New DCM-Causative Mutation in ETS1
3.3. Functional Loss of Tyr149*-Mutant ETS1 in Transactivating CLDN5
3.4. Inability of Tyr149*-Mutant ETS1 to Transcriptionally Activate ALK1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, N.A.; Burke, M.A. Dilated Cardiomyopathy: A Genetic Journey from Past to Future. Int. J. Mol. Sci. 2024, 25, 11460. [Google Scholar] [CrossRef]
- Eldemire, R.; Mestroni, L.; Taylor, M.R.G. Genetics of Dilated Cardiomyopathy. Annu. Rev. Med. 2024, 75, 417–426. [Google Scholar] [CrossRef] [PubMed]
- McGurk, K.A.; Zhang, X.; Theotokis, P.; Thomson, K.; Harper, A.; Buchan, R.J.; Mazaika, E.; Ormondroyd, E.; Wright, W.T.; Macaya, D.; et al. The Penetrance of Rare Variants in Cardiomyopathy-Associated Genes: A Cross-sectional Approach to Estimating Penetrance for Secondary Findings. Am. J. Hum. Genet. 2023, 110, 1482–1495. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, E.; Martínez-Legazpi, P.; González-Mansilla, A.; Espinosa, M.Á.; Mombiela, T.; Guzmán De-Villoria, J.A.; Borja, M.G.; Díaz-Otero, F.; Gómez de Antonio, R.; Fernández-García, P.; et al. Cardiac stasis imaging, stroke, and silent brain infarcts in patients with nonischemic dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2024, 327, H446–H453. [Google Scholar] [CrossRef] [PubMed]
- Ródenas-Alesina, E.; Lozano-Torres, J.; Tobías-Castillo, P.E.; Badia-Molins, C.; Calvo-Barceló, M.; Vila-Olives, R.; Casas-Masnou, G.; San Emeterio, A.O.; Soriano-Colomé, T.; Fernández-Galera, R.; et al. Risk of Stroke and Incident Atrial Fibrillation in Patients in Sinus Rhythm with Nonischemic Dilated Cardiomyopathy. Am. J. Cardiol. 2024, 233, 11–18. [Google Scholar] [CrossRef]
- Fan, Z.; Wu, C.; Wang, C.; Liu, C.; Fang, L.; Ma, L.; Zou, W.; Yuan, B.; Ji, Z.; Cai, B.; et al. Impact of Concurrent Ischaemic Stroke on Unfavourable Outcomes in Men and Women with Dilated Cardiomyopathy. Rev. Cardiovasc. Med. 2024, 25, 215. [Google Scholar] [CrossRef]
- Lakdawala, N.K.; Givertz, M.M. Dilated cardiomyopathy with conduction disease and arrhythmia. Circulation 2010, 122, 527–534. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, K.; Liu, Q.; Song, W.; Jin, D.; Zhao, S. Nonspecific intraventricular conduction delay predicts the prognosis of dilated cardiomyopathy. BMC Cardiovasc. Disord. 2023, 23, 409. [Google Scholar] [CrossRef]
- Jiménez-Blanco Bravo, M.; Alonso Salinas, G.L.; Parra Esteban, C.; Toquero Ramos, J.; Amores Luque, M.; Zamorano Gómez, J.L.; García-Izquierdo, E.; Álvarez-García, J.; Fernández Lozano, I.; Castro Urda, V. Right Bundle Branch Block Predicts Appropriate Implantable Cardioverter Defibrillator Therapies in Patients with Non-Ischemic Dilated Cardiomyopathy and a Prophylactic Implantable Cardioverter Defibrillator. Diagnostics 2024, 14, 1173. [Google Scholar] [CrossRef]
- Raafs, A.G.; Vos, J.L.; Henkens, M.T.H.M.; Verdonschot, J.A.J.; Sikking, M.; Stroeks, S.; Gerretsen, S.; Hazebroek, M.R.; Knackstedt, C.; Nijveldt, R.; et al. Left Atrial Strain Is an Independent Predictor of New-Onset Atrial Fibrillation in Dilated Cardiomyopathy. JACC Cardiovasc. Imaging 2023, 16, 991–992. [Google Scholar] [CrossRef]
- Iovănescu, M.L.; Hădăreanu, D.R.; Toader, D.M.; Florescu, C.; Istrătoaie, O.; Donoiu, I.; Militaru, C. The Impact of Atrial Fibrillation on All Heart Chambers Remodeling and Function in Patients with Dilated Cardiomyopathy-A Two- and Three-Dimensional Echocardiography Study. Life 2023, 13, 1421. [Google Scholar] [CrossRef]
- Siow, Y.K.; Lin, C.Y.; Chung, F.P.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Liao, J.N.; Chang, T.Y.; Tuan, T.C.; et al. Catheter ablation in patients with atrial fibrillation and dilated cardiomyopathy. Front. Cardiovasc. Med. 2024, 11, 1305485. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Peng, F.; Li, J.; Gong, H. Exploring novel biomarkers in dilated cardiomyopathy-induced heart failure by integrated analysis and in vitro experiments. Exp. Ther. Med. 2023, 26, 325. [Google Scholar] [CrossRef] [PubMed]
- Masè, M.; Rossi, M.; Setti, M.; Barbati, G.; Teso, M.V.; Ribichini, F.L.; Koni, M.; Stolfo, D.; Merlo, M.; Sinagra, G. Applicability and performance of heart failure prognostic scores in dilated cardiomyopathy: The real-world experience of an Italian referral center for cardiomyopathies. Int. J. Cardiol. 2024, 396, 131562. [Google Scholar] [CrossRef] [PubMed]
- Hammersley, D.J.; Mukhopadhyay, S.; Chen, X.; Jones, R.E.; Ragavan, A.; Javed, S.; Rajabali, H.; Androulakis, E.; Curran, L.; Mach, L.; et al. Precision prediction of heart failure events in patients with dilated cardiomyopathy and mildly reduced ejection fraction using multi-parametric cardiovascular magnetic resonance. Eur. J. Heart Fail. 2024, 26, 2553–2562. [Google Scholar] [CrossRef]
- Tsabedze, N.; du Plessis, A.; Mpanya, D.; Vorster, A.; Wells, Q.; Scholtz, L.; Manga, P. Cardiovascular Magnetic Resonance Imaging Findings in Africans with Idiopathic Dilated Cardiomyopathy. Diagnostics 2023, 13, 617. [Google Scholar] [CrossRef]
- Antoniou, N.; Kalaitzoglou, M.; Tsigkriki, L.; Baroutidou, A.; Tsaousidis, A.; Koulaouzidis, G.; Giannakoulas, G.; Charisopoulou, D. Speckle Tracking Echocardiography in Patients with Non-Ischemic Dilated Cardiomyopathy Who Undergo Cardiac Resynchronization Therapy: A Narrative Review. Diagnostics 2024, 14, 1178. [Google Scholar] [CrossRef]
- Winiarczyk, M.; Dziewięcka, E.; Wiśniowska-Śmiałek, S.; Stępień, A.; Graczyk, K.; Leśniak-Sobelga, A.; Hlawaty, M.; Woźniak, J.; Savitskaya, M.; Holcman, K.; et al. Left ventricular diastolic dysfunction worsens prognosis in patients with heart failure due to dilated cardiomyopathy. ESC Heart Fail. 2025, 12, 1183–1193. [Google Scholar] [CrossRef]
- Kimura, Y.; Beukers, H.K.C.; Rademaker, R.; Chen, H.S.; Ebert, M.; Jensen, T.; Piers, S.R.; Wijnmaalen, A.P.; de Riva, M.; Dekkers, O.M.; et al. Volume-Weighted Unipolar Voltage Predicts Heart Failure Mortality in Patients with Dilated Cardiomyopathy and Ventricular Arrhythmias. JACC Clin. Electrophysiol. 2023, 9, 965–975. [Google Scholar] [CrossRef]
- Ma, H.Y.; Xie, G.Y.; Tao, J.; Li, Z.Z.; Liu, P.; Zheng, X.J.; Wang, R.P. Identification of patients with nonischemic dilated cardiomyopathy at risk of malignant ventricular arrhythmias: Insights from cardiac magnetic resonance feature tracking. BMC Cardiovasc. Disord. 2024, 24, 29. [Google Scholar] [CrossRef]
- Amyar, A.; Al-Deiri, D.; Sroubek, J.; Kiang, A.; Ghanbari, F.; Nakamori, S.; Rodriguez, J.; Kramer, D.B.; Manning, W.J.; Kwon, D.; et al. Radiomic Cardiac MRI Signatures for Predicting Ventricular Arrhythmias in Patients with Nonischemic Dilated Cardiomyopathy. JACC Adv. 2025, 4, 101684. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Y.; Li, W.; Guo, J.; Wan, K.; Wang, J.; Xu, Z.; Han, Y.; Sun, J.; Chen, Y. Cardiac MRI to Predict Sudden Cardiac Death Risk in Dilated Cardiomyopathy. Radiology 2023, 307, e222552. [Google Scholar] [CrossRef] [PubMed]
- Kantor, P.F.; Shi, L.; Colan, S.D.; Orav, E.J.; Wilkinson, J.D.; Hamza, T.H.; Webber, S.A.; Canter, C.E.; Towbin, J.A.; Everitt, M.D.; et al. Progressive Left Ventricular Remodeling for Predicting Mortality in Children with Dilated Cardiomyopathy: The Pediatric Cardiomyopathy Registry. J. Am. Heart Assoc. 2024, 13, e022557. [Google Scholar] [CrossRef] [PubMed]
- Street-de Palma, C.; Lim, Z.; Field, E.; Kaski, J.P.; Norrish, G. Imaging based risk factors for heart failure death in childhood dilated cardiomyopathy: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2025, 12, 1568494. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; He, J.; Liu, J.; Guo, X.; Chu, H.; Xu, H.; Wang, Y. Comprehensive review on gene mutations contributing to dilated cardiomyopathy. Front. Cardiovasc. Med. 2023, 10, 1296389. [Google Scholar] [CrossRef]
- Alexander, P.M.; Daubeney, P.E.; Nugent, A.W.; Lee, K.J.; Turner, C.; Colan, S.D.; Robertson, T.; Davis, A.M.; Ramsay, J.; Justo, R.; et al. Long-term outcomes of dilated cardiomyopathy diagnosed during childhood: Results from a national population-based study of childhood cardiomyopathy. Circulation 2013, 128, 2039–2046. [Google Scholar] [CrossRef]
- Hammersley, D.J.; Jones, R.E.; Owen, R.; Mach, L.; Lota, A.S.; Khalique, Z.; De Marvao, A.; Androulakis, E.; Hatipoglu, S.; Gulati, A.; et al. Phenotype, Outcomes and Natural History of Early-Stage Non-Ischaemic Cardiomyopathy. Eur. J. Heart Fail. 2023, 25, 2050–2059. [Google Scholar] [CrossRef]
- Merlo, M.; Cannatà, A.; Pio Loco, C.; Stolfo, D.; Barbati, G.; Artico, J.; Gentile, P.; De Paris, V.; Ramani, F.; Zecchin, M.; et al. Contemporary Survival Trends and Etiological Characterization in Non-Ischemic Dilated Cardiomyopathy. Eur. J. Heart Fail. 2020, 22, 1111–1121. [Google Scholar] [CrossRef]
- Kadhi, A.; Mohammed, F.; Nemer, G. The Genetic Pathways Underlying Immunotherapy in Dilated Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 613295. [Google Scholar] [CrossRef]
- Lv, Y.; Gao, R.F.; Yang, C.X.; Xu, Y.J.; Yang, Y.Q. Increased gestational palmitic acid predisposes offspring to congenital heart disease. Cell Rep. Med. 2023, 4, 100984. [Google Scholar] [CrossRef]
- Peters, S.A.; Wright, L.; Yao, J.; McCall, L.; Thompson, T.; Thompson, B.; Johnson, R.; Huynh, Q.; Santiago, C.F.; Trainer, A.; et al. Environmental Risk Factors Are Associated with the Natural History of Familial Dilated Cardiomyopathy. J. Am. Heart Assoc. 2025, 14, e037311. [Google Scholar] [CrossRef]
- Tsabedze, N.; Ramsay, M.; Krause, A.; Wells, Q.; Mpanya, D.; Manga, P. The genetic basis for adult-onset idiopathic dilated cardiomyopathy in people of African descent. Heart Fail. Rev. 2023, 28, 879–892. [Google Scholar] [CrossRef]
- Chun, Y.W.; Miyamoto, M.; Williams, C.H.; Neitzel, L.R.; Silver-Isenstadt, M.; Cadar, A.G.; Fuller, D.T.; Fong, D.C.; Liu, H.; Lease, R.; et al. Impaired Reorganization of Centrosome Structure Underlies Human Infantile Dilated Cardiomyopathy. Circulation 2023, 147, 1291–1303. [Google Scholar] [CrossRef]
- Hofmeyer, M.; Haas, G.J.; Jordan, E.; Cao, J.; Kransdorf, E.; Ewald, G.A.; Morris, A.A.; Owens, A.; Lowes, B.; Stoller, D.; et al. Rare Variant Genetics and Dilated Cardiomyopathy Severity: The DCM Precision Medicine Study. Circulation 2023, 148, 872–881. [Google Scholar] [CrossRef]
- Stroeks, S.L.V.M.; Lunde, I.G.; Hellebrekers, D.M.E.I.; Claes, G.R.F.; Wakimoto, H.; Gorham, J.; Krapels, I.P.C.; Vanhoutte, E.K.; van den Wijngaard, A.; Henkens, M.T.H.M.; et al. Prevalence and Clinical Consequences of Multiple Pathogenic Variants in Dilated Cardiomyopathy. Circ. Genom. Precis. Med. 2023, 16, e003788. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Otway, R.; Chin, E.; Horvat, C.; Ohanian, M.; Wilcox, J.A.L.; Su, Z.; Prestes, P.; Smolnikov, A.; Soka, M.; et al. DMD-Associated Dilated Cardiomyopathy: Genotypes, Phenotypes, and Phenocopies. Circ. Genom. Precis. Med. 2023, 16, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.N.; Yang, C.X.; Ding, Y.Y.; Qiao, Q.; Di, R.M.; Sun, Y.M.; Wang, J.; Yang, L.; Xu, Y.J.; Yang, Y.Q. Identification of BMP10 as a Novel Gene Contributing to Dilated Cardiomyopathy. Diagnostics 2023, 13, 242. [Google Scholar] [CrossRef]
- Bertero, E.; Fracasso, G.; Eustachi, V.; Coviello, D.; Cecconi, M.; Giovinazzo, S.; Toma, M.; Merlo, M.; Sinagra, G.; Porto, I.; et al. Diagnostic yield and predictive value on left ventricular remodelling of genetic testing in dilated cardiomyopathy. ESC Heart Fail. 2023, 10, 2745–2750. [Google Scholar] [CrossRef]
- Sono, R.; Larrinaga, T.M.; Huang, A.; Makhlouf, F.; Kang, X.; Su, J.; Lau, R.; Arboleda, V.A.; Biniwale, R.; Fishbein, G.A.; et al. Whole-Exome Sequencing Identifies Homozygote Nonsense Variants in LMOD2 Gene Causing Infantile Dilated Cardiomyopathy. Cells 2023, 12, 1455. [Google Scholar] [CrossRef]
- Lian, H.; Song, S.; Chen, W.; Shi, A.; Jiang, H.; Hu, S. Genetic characterization of dilated cardiomyopathy patients undergoing heart transplantation in the Chinese population by whole-exome sequencing. J. Transl. Med. 2023, 21, 476. [Google Scholar] [CrossRef]
- Yu, T.; Yan, F.; Xu, Y.; Hunag, Y.; Gong, H.; Zhao, P.; Sun, D.; Zhang, Y.; Zhang, F.; He, X. Identification of a novel TNNI3 synonymous variant causing intron retention in autosomal recessive dilated cardiomyopathy. Gene 2023, 856, 147102. [Google Scholar] [CrossRef]
- Shi, H.Y.; Xie, M.S.; Guo, Y.H.; Yang, C.X.; Gu, J.N.; Qiao, Q.; Di, R.M.; Qiu, X.B.; Xu, Y.J.; Yang, Y.Q. VEZF1 loss-of-function mutation underlying familial dilated cardiomyopathy. Eur. J. Med. Genet. 2023, 66, 104705. [Google Scholar] [CrossRef] [PubMed]
- Majdalani, P.; Levitas, A.; Krymko, H.; Slanovic, L.; Braiman, A.; Hadad, U.; Dabsan, S.; Horev, A.; Zarivach, R.; Parvari, R. A Missense Variation in PHACTR2 Associates with Impaired Actin Dynamics, Dilated Cardiomyopathy, and Left Ventricular Non-Compaction in Humans. Int. J. Mol. Sci. 2023, 24, 1388. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.L.; Henry, A.; Cannie, D.; Lee, M.; Miller, D.; McGurk, K.A.; Bond, I.; Xu, X.; Issa, H.; Francis, C.; et al. Genome-wide association analysis provides insights into the molecular etiology of dilated cardiomyopathy. Nat. Genet. 2024, 56, 2646–2658. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, S.J.; Rämö, J.T.; Kramarenko, D.R.; Wijdeveld, L.F.J.M.; Haas, J.; Chaffin, M.D.; Garnier, S.; Gaziano, L.; Weng, L.C.; Lipov, A.; et al. Genome-wide association study reveals mechanisms underlying dilated cardiomyopathy and myocardial resilience. Nat. Genet. 2024, 56, 2636–2645. [Google Scholar] [CrossRef]
- Li, Y.; Ma, K.; Dong, Z.; Gao, S.; Zhang, J.; Huang, S.; Yang, J.; Fang, G.; Li, Y.; Li, X.; et al. Frameshift variants in C10orf71 cause dilated cardiomyopathy in human, mouse, and organoid models. J. Clin. Investig. 2024, 134, e177172. [Google Scholar] [CrossRef]
- Amor-Salamanca, A.; Santana Rodríguez, A.; Rasoul, H.; Rodríguez-Palomares, J.F.; Moldovan, O.; Hey, T.M.; Delgado, M.G.; Cuenca, D.L.; de Castro Campos, D.; Basurte-Elorz, M.T.; et al. Role of TBX20 Truncating Variants in Dilated Cardiomyopathy and Left Ventricular Noncompaction. Circ. Genom. Precis. Med. 2024, 17, e004404. [Google Scholar] [CrossRef]
- Gao, X.; Pang, S.; Ding, L.; Yan, H.; Cui, Y.; Yan, B. Genetic and functional variants of the TBX20 gene promoter in dilated cardiomyopathy. Mol. Genet. Genom. Med. 2024, 12, e2355. [Google Scholar] [CrossRef]
- de Frutos, F.; Ochoa, J.P.; Webster, G.; Jansen, M.; Remior, P.; Rasmussen, T.B.; Sabater-Molina, M.; Barriales-Villa, R.; Girolami, F.; Cesar, S.; et al. Clinical Features and Outcomes of Pediatric MYH7-Related Dilated Cardiomyopathy. J. Am. Heart Assoc. 2024, 13, e036208. [Google Scholar] [CrossRef]
- Kraoua, L.; Louati, A.; Ahmed, S.B.; Abida, N.; Khemiri, M.; Menif, K.; Mrad, R.; Zaffran, S.; Jaouadi, H. Homozygous TNNI3 frameshift variant in a consanguineous family with lethal infantile dilated cardiomyopathy. Mol. Genet. Genom. Med. 2024, 12, e2486. [Google Scholar] [CrossRef]
- Ochoa, J.P.; Lalaguna, L.; Mirelis, J.G.; Dominguez, F.; Gonzalez-Lopez, E.; Salas, C.; Roustan, G.; McGurk, K.A.; Zheng, S.L.; Barton, P.J.R.; et al. Biallelic Loss of Function Variants in Myocardial Zonula Adherens Protein Gene (MYZAP) Cause a Severe Recessive Form of Dilated Cardiomyopathy. Circ. Heart Fail. 2024, 17, e011226. [Google Scholar] [CrossRef]
- Ha, C.; Kim, D.; Bak, M.; Park, J.H.; Kim, Y.G.; Jang, J.H.; Kim, J.W.; Choi, J.O.; Jang, M.A. CRYAB stop-loss variant causes rare syndromic dilated cardiomyopathy with congenital cataract: Expanding the phenotypic and mutational spectrum of alpha-B crystallinopathy. J. Hum. Genet. 2024, 69, 159–162. [Google Scholar] [CrossRef]
- Perret, C.; Proust, C.; Esslinger, U.; Ader, F.; Haas, J.; Pruny, J.F.; Isnard, R.; Richard, P.; Trégouët, D.A.; Charron, P.; et al. DNA-pools targeted-sequencing as a robust cost-effective method to detect rare variants: Application to dilated cardiomyopathy genetic diagnosis. Clin. Genet. 2024, 105, 185–189. [Google Scholar] [CrossRef]
- Tran, D.D.; Lien, N.T.K.; Tung, N.V.; Huu, N.C.; Nguyen, P.T.; Tien, D.A.; Thu, D.T.H.; Huy, B.Q.; Oanh, T.T.K.; Lien, N.T.P.; et al. Three Novel Pathogenic Variants in Unrelated Vietnamese Patients with Cardiomyopathy. Diagnostics 2024, 14, 2709. [Google Scholar] [CrossRef]
- Voinescu, O.R.; Ionescu, B.I.; Militaru, S.; Afana, A.S.; Sascau, R.; Vasiliu, L.; Onciul, S.; Dobrescu, M.A.; Cozlac, R.A.; Cozma, D.; et al. Genetic Characterization of Dilated Cardiomyopathy in Romanian Adult Patients. Int. J. Mol. Sci. 2024, 25, 2562. [Google Scholar] [CrossRef]
- d’Apolito, M.; Ranaldi, A.; Santoro, F.; Cannito, S.; Gravina, M.; Santacroce, R.; Ragnatela, I.; Margaglione, A.; D’Andrea, G.; Casavecchia, G.; et al. De Novo p.Asp3368Gly Variant of Dystrophin Gene Associated with X-Linked Dilated Cardiomyopathy and Skeletal Myopathy: Clinical Features and In Silico Analysis. Int. J. Mol. Sci. 2024, 25, 2787. [Google Scholar] [CrossRef]
- Mozafary Bazargany, M.; Esmaeili, S.; Hesami, M.; Houshmand, G.; Mahdavi, M.; Maleki, M.; Kalayinia, S. A novel likely pathogenic homozygous RBCK1 variant in dilated cardiomyopathy with muscle weakness. ESC Heart Fail. 2024, 11, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Kubanek, M.; Binova, J.; Piherova, L.; Krebsova, A.; Kotrc, M.; Hartmannova, H.; Hodanova, K.; Musalkova, D.; Stranecky, V.; Palecek, T.; et al. Genotype is associated with left ventricular reverse remodelling and early events in recent-onset dilated cardiomyopathy. ESC Heart Fail. 2024, 11, 4127–4138. [Google Scholar] [CrossRef] [PubMed]
- León, P.; Franco, P.; Hinojosa, N.; Torres, K.; Moreano, A.; Romero, V.I. TTN novel splice variant in familial dilated cardiomyopathy and splice variants review: A case report. Front. Cardiovasc. Med. 2024, 11, 1387063. [Google Scholar] [CrossRef] [PubMed]
- Carigi, S.; Olivucci, G.; Cristalli, C.P.; Marzo, F.; Isidori, F.; Palmieri, S.; Schiavo, M.A.; Gualandi, F.; Amati, S.; Rocchetti, L.M.; et al. A single RBM20 missense variant is a potential contributor to dilated cardiomyopathy and/or isolated left ventricular dilatation in the Emilia Romagna region of Italy. Int. J. Cardiol. 2025, 423, 132999. [Google Scholar] [CrossRef]
- Jadidi, M.; Babaali, V.; InanlooRahatloo, K.; Salehi, N.; Mollazadeh, R. Identification of a rare variant in TNNT3 responsible for familial dilated cardiomyopathy through whole-exome sequencing and in silico analysis. Eur. J. Med. Res. 2025, 30, 424. [Google Scholar] [CrossRef]
- Jiang, W.F.; Sun, Y.M.; Qiu, X.B.; Wu, S.H.; Ding, Y.Y.; Li, N.; Yang, C.X.; Xu, Y.J.; Jiang, T.B.; Yang, Y.Q. Identification and Functional Investigation of SOX4 as a Novel Gene Underpinning Familial Atrial Fibrillation. Diagnostics 2024, 14, 2376. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Wang, J.; Ye, W.G.; Liu, X.Y.; Li, L.; Qiu, X.B.; Chen, H.; Xu, Y.J.; Yang, Y.Q.; Bai, D.; et al. Discovery of GJC1 (Cx45) as a New Gene Underlying Congenital Heart Disease and Arrhythmias. Biology 2023, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Y.J.; Guo, X.J.; Wu, S.H.; Jiang, W.F.; Zhang, D.L.; Wang, K.W.; Li, L.; Sun, Y.M.; Xu, Y.J.; et al. Discovery of TBX20 as a Novel Gene Underlying Atrial Fibrillation. Biology 2023, 12, 1186. [Google Scholar] [CrossRef]
- Cho, J.; Kim, J.; Song, J.S.; Uh, Y.; Lee, J.H.; Lee, H.S. Whole-Exome Sequencing and Analysis of the T Cell Receptor β and γ Repertoires in Rheumatoid Arthritis. Diagnostics 2024, 14, 529. [Google Scholar] [CrossRef]
- Fang, H.H.; Lee, C.L.; Chen, H.J.; Chuang, C.K.; Chiu, H.C.; Chang, Y.H.; Tu, Y.R.; Lo, Y.T.; Lin, H.Y.; Lin, S.P. Whole Exome Sequencing Facilitates Early Diagnosis of Lesch-Nyhan Syndrome: A Case Series. Diagnostics 2024, 14, 2809. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.Y.; Yang, C.X.; Zhou, H.M.; Li, Y.J.; Qiu, X.B.; Huang, R.T.; Cen, S.S.; Wang, Y.; Xu, Y.J.; et al. Discovery and functional investigation of BMP4 as a new causative gene for human congenital heart disease. Am. J. Transl. Res. 2024, 16, 2034–2048. [Google Scholar] [CrossRef]
- Abhinav, P.; Li, Y.J.; Huang, R.T.; Liu, X.Y.; Gu, J.N.; Yang, C.X.; Xu, Y.J.; Wang, J.; Yang, Y.Q. Somatic GATA4 mutation contributes to tetralogy of Fallot. Exp. Ther. Med. 2024, 27, 91. [Google Scholar] [CrossRef]
- Li, Y.J.; Zou, S.; Bian, Y.Z.; Liu, X.Y.; Yang, C.X.; Li, L.; Qiu, X.B.; Xu, Y.J.; Yang, Y.Q.; Huang, R.T. Chromosomal Location and Identification of TBX20 as a New Gene Responsible for Familial Bicuspid Aortic Valve. Diagnostics 2025, 15, 600. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.B.; Li, Y.J.; Liu, X.Y.; Huang, R.T.; Yang, C.X.; Xu, Y.J.; Lv, H.T.; Yang, Y.Q. Discovery of BMP10 as a new gene underpinning congenital heart defects. Am. J. Transl. Res. 2024, 16, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Dong, B.B.; Li, Y.J.; Yang, C.X.; Xu, Y.J.; Huang, R.T.; Liu, X.Y.; Yang, Y.Q. Identification and Functional Characterization of a Novel SOX4 Mutation Predisposing to Coffin-Siris Syndromic Congenital Heart Disease. Children 2025, 12, 608. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, S.; Hou, Y.; Lu, C.; Yang, W.; Ji, T.; Yang, Y.; Yu, Z.; Jin, Z. Cardiac-specific overexpression of Claudin-5 exerts protection against myocardial ischemia and reperfusion injury. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166535. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Wang, M.; Liu, H.; Chen, M.; Zhu, J.; Zhang, Y.; Wang, X.; Wu, Y.; Liu, D.; et al. A novel function of claudin-5 in maintaining the structural integrity of the heart and its implications in cardiac pathology. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167274. [Google Scholar] [CrossRef] [PubMed]
- Burek, M.; Arias-Loza, P.A.; Roewer, N.; Förster, C.Y. Claudin-5 as a novel estrogen target in vascular endothelium. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 298–304. [Google Scholar] [CrossRef]
- Sanford, J.L.; Edwards, J.D.; Mays, T.A.; Gong, B.; Merriam, A.P.; Rafael-Fortney, J.A. Claudin-5 localizes to the lateral membranes of cardiomyocytes and is altered in utrophin/dystrophin-deficient cardiomyopathic mice. J. Mol. Cell. Cardiol. 2005, 38, 323–332. [Google Scholar] [CrossRef]
- Milani-Nejad, N.; Schultz, E.J.; Slabaugh, J.L.; Janssen, P.M.; Rafael-Fortney, J.A. Myocardial Contractile Dysfunction Is Present without Histopathology in a Mouse Model of Limb-Girdle Muscular Dystrophy-2F and Is Prevented after Claudin-5 Virotherapy. Front. Physiol. 2016, 7, 539. [Google Scholar] [CrossRef]
- Delfín, D.A.; Xu, Y.; Schill, K.E.; Mays, T.A.; Canan, B.D.; Zang, K.E.; Barnum, J.A.; Janssen, P.M.; Rafael-Fortney, J.A. Sustaining cardiac claudin-5 levels prevents functional hallmarks of cardiomyopathy in a muscular dystrophy mouse model. Mol. Ther. 2012, 20, 1378–1383. [Google Scholar] [CrossRef]
- Luo, T.; Liu, H.; Chen, B.; Liu, H.; Abdel-Latif, A.; Kitakaze, M.; Wang, X.; Wu, Y.; Chou, D.; Kim, J.K. A Novel Role of Claudin-5 in Prevention of Mitochondrial Fission Against Ischemic/Hypoxic Stress in Cardiomyocytes. Can. J. Cardiol. 2021, 37, 1593–1606. [Google Scholar] [CrossRef]
- Mays, T.A.; Binkley, P.F.; Lesinski, A.; Doshi, A.A.; Quaile, M.P.; Margulies, K.B.; Janssen, P.M.; Rafael-Fortney, J.A. Claudin-5 levels are reduced in human end-stage cardiomyopathy. J. Mol. Cell. Cardiol. 2008, 45, 81–87. [Google Scholar] [CrossRef]
- Swager, S.A.; Delfín, D.A.; Rastogi, N.; Wang, H.; Canan, B.D.; Fedorov, V.V.; Mohler, P.J.; Kilic, A.; Higgins, R.S.D.; Ziolo, M.T.; et al. Claudin-5 levels are reduced from multiple cell types in human failing hearts and are associated with mislocalization of ephrin-B1. Cardiovasc. Pathol. 2015, 24, 160–167. [Google Scholar] [CrossRef]
- Capasso, T.L.; Li, B.; Volek, H.J.; Khalid, W.; Rochon, E.R.; Anbalagan, A.; Herdman, C.; Yost, H.J.; Villanueva, F.S.; Kim, K.; et al. BMP10-mediated ALK1 signaling is continuously required for vascular development and maintenance. Angiogenesis 2020, 23, 203–220. [Google Scholar] [CrossRef]
- Bhave, S.; Swain, L.; Qiao, X.; Martin, G.; Aryaputra, T.; Everett, K.; Kapur, N.K. ALK1 Deficiency Impairs the Wound-Healing Process and Increases Mortality in Murine Model of Myocardial Infarction. J. Cardiovasc. Transl. Res. 2024, 17, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Garrett-Sinha, L.A. Review of Ets1 structure, function, and roles in immunity. Cell. Mol. Life Sci. 2013, 70, 3375–3390. [Google Scholar] [CrossRef] [PubMed]
- Shiu, Y.T.; Jaimes, E.A. Transcription Factor ETS-1 and Reactive Oxygen Species: Role in Vascular and Renal Injury. Antioxidants 2018, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Han, X.; Sun, L.; Shao, F.; Yin, Y.; Zhang, W. ETS Transcription Factors in Immune Cells and Immune-Related Diseases. Int. J. Mol. Sci. 2024, 25, 10004. [Google Scholar] [CrossRef]
- Wang, S.; Wan, L.; Zhang, X.; Fang, H.; Zhang, M.; Li, F.; Yan, D. ETS-1 in tumor immunology: Implications for novel anti-cancer strategies. Front. Immunol. 2025, 16, 1526368. [Google Scholar] [CrossRef]
- Szabo, L.; Morey, R.; Palpant, N.J.; Wang, P.L.; Afari, N.; Jiang, C.; Parast, M.M.; Murry, C.E.; Laurent, L.C.; Salzman, J. Statistically based splicing detection reveals neural enrichment and tissue-specific induction of circular RNA during human fetal development. Genome Biol. 2015, 16, 126. [Google Scholar] [CrossRef]
- Duff, M.O.; Olson, S.; Wei, X.; Garrett, S.C.; Osman, A.; Bolisetty, M.; Plocik, A.; Celniker, S.E.; Graveley, B.R. Genome-wide identification of zero nucleotide recursive splicing in Drosophila. Nature 2015, 521, 376–379. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Gao, Z.; Kim, G.H.; Mackinnon, A.C.; Flagg, A.E.; Bassett, B.; Earley, J.U.; Svensson, E.C. Ets1 is required for proper migration and differentiation of the cardiac neural crest. Development 2010, 137, 1543–1551. [Google Scholar] [CrossRef]
- Ye, M.; Coldren, C.; Benson, W.; Goldmuntz, E.; Ostrowski, M.; Watson, D.; Perryman, B.; Grossfeld, P. Deletion of ETS-1, a gene in the Jacobsen syndrome critical region, causes ventricular septal defects and abnormal ventricular morphology in mice. Hum. Mol. Genet. 2010, 19, 648–656. [Google Scholar] [CrossRef]
- Lin, L.; Pinto, A.; Wang, L.; Fukatsu, K.; Yin, Y.; Bamforth, S.D.; Bronner, M.E.; Evans, S.M.; Nie, S.; Anderson, R.H.; et al. ETS1 loss in mice impairs cardiac outflow tract septation via a cell migration defect autonomous to the neural crest. Hum. Mol. Genet. 2022, 31, 4217–4227. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, L.; Qi, H.; Chen, J.; Grossfeld, P. Endothelial Loss of ETS1 Impairs Coronary Vascular Development and Leads to Ventricular Non-Compaction. Circ. Res. 2022, 131, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Grossfeld, P. ETS1 and HLHS: Implications for the Role of the Endocardium. J. Cardiovasc. Dev. Dis. 2022, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Bronner, M.E. Dual developmental role of transcriptional regulator Ets1 in Xenopus cardiac neural crest vs. heart mesoderm. Cardiovasc. Res. 2015, 106, 67–75. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, Y.; Sethi, I.; Ye, L.; Trembley, M.A.; Cao, Y.; Akerberg, B.N.; Xiao, F.; Zhang, X.; Li, K.; et al. GATA4 Regulates Developing Endocardium Through Interaction with ETS1. Circ. Res. 2022, 131, e152–e168. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, J.H.; Xu, W.J.; Yu, H.; Wang, Q.; Zheng, H.Z.; Jiang, W.F.; Jiang, J.F.; Yang, Y.Q. A novel GATA4 loss-of-function mutation responsible for familial dilated cardiomyopathy. Int. J. Mol. Med. 2014, 33, 654–660. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.D.; Yang, Z.L.; Yuan, F.; Xu, L.; Li, R.G.; Yang, Y.Q. Prevalence and spectrum of GATA4 mutations associated with sporadic dilated cardiomyopathy. Gene 2014, 548, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.W.; Guo, G.; Wolstein, O.; Vale, M.; Castro, M.L.; Wang, L.; Otway, R.; Riek, P.; Cochrane, N.; Furtado, M.; et al. Functional characterization of a novel mutation in NKX2-5 associated with congenital heart disease and adult-onset cardiomyopathy. Circ. Cardiovasc. Genet. 2013, 6, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Qiu, X.B.; Li, R.G.; Qu, X.K.; Wang, J.; Xu, Y.J.; Liu, X.; Fang, W.Y.; Yang, Y.Q.; Liao, D.N. A novel NKX2-5 loss-of-function mutation predisposes to familial dilated cardiomyopathy and arrhythmias. Int. J. Mol. Med. 2015, 35, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Sveinbjornsson, G.; Olafsdottir, E.F.; Thorolfsdottir, R.B.; Davidsson, O.B.; Helgadottir, A.; Jonasdottir, A.; Jonasdottir, A.; Bjornsson, E.; Jensson, B.O.; Arnadottir, G.A.; et al. Variants in NKX2-5 and FLNC Cause Dilated Cardiomyopathy and Sudden Cardiac Death. Circ. Genom. Precis. Med. 2018, 11, e002151. [Google Scholar] [CrossRef]
- Wang, W.K.; Wang, B.; Cao, X.H.; Liu, Y.S. Spironolactone alleviates myocardial fibrosis via inhibition of Ets-1 in mice with experimental autoimmune myocarditis. Exp. Ther. Med. 2022, 23, 369. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, B.F.; Zhang, J.; Liu, G.; Hu, Q.; Chen, J. The histone demthylase KDM3A protects the myocardium from ischemia/reperfusion injury via promotion of ETS1 expression. Commun. Biol. 2022, 5, 270. [Google Scholar] [CrossRef]
- Tahtakran, S.A.; Selleck, M.A. Ets-1 expression is associated with cranial neural crest migration and vasculogenesis in the chick embryo. Gene Expr. Patterns 2003, 3, 455–458. [Google Scholar] [CrossRef]
- Lie-Venema, H.; Gittenberger-de Groot, A.C.; van Empel, L.J.; Boot, M.J.; Kerkdijk, H.; de Kant, E.; DeRuiter, M.C. Ets-1 and Ets-2 transcription factors are essential for normal coronary and myocardial development in chicken embryos. Circ. Res. 2003, 92, 749–756. [Google Scholar] [CrossRef]
- Takahashi, T.; Sugishita, Y.; Kinugawa, K.; Shimizu, T.; Yao, A.; Harada, K.; Matsui, H.; Nagai, R. Ets-1 is involved in transcriptional regulation of the chick inducible nitric oxide synthase gene in embryonic ventricular myocytes. Mol. Cell. Biochem. 2001, 226, 57–65. [Google Scholar] [CrossRef]
- Ruan, H.; Liao, Y.; Ren, Z.; Mao, L.; Yao, F.; Yu, P.; Ye, Y.; Zhang, Z.; Li, S.; Xu, H.; et al. Single-cell reconstruction of differentiation trajectory reveals a critical role of ETS1 in human cardiac lineage commitment. BMC Biol. 2019, 17, 89. [Google Scholar] [CrossRef]
- Conrad, S.; Demurger, F.; Moradkhani, K.; Pichon, O.; Le Caignec, C.; Pascal, C.; Thomas, C.; Bayart, S.; Perlat, A.; Dubourg, C.; et al. 11q24.2q24.3 microdeletion in two families presenting features of Jacobsen syndrome, without intellectual disability: Role of FLI1, ETS1, and SENCR long noncoding RNA. Am. J. Med. Genet. A 2019, 179, 993–1000. [Google Scholar] [CrossRef]
- Grossfeld, P.D.; Mattina, T.; Lai, Z.; Favier, R.; Jones, K.L.; Cotter, F.; Jones, C. The 11q terminal deletion disorder: A prospective study of 110 cases. Am. J. Med. Genet. A 2004, 129A, 51–61. [Google Scholar] [CrossRef]
- Glessner, J.T.; Bick, A.G.; Ito, K.; Homsy, J.; Rodriguez-Murillo, L.; Fromer, M.; Mazaika, E.; Vardarajan, B.; Italia, M.; Leipzig, J.; et al. Increased frequency of de novo copy number variants in congenital heart disease by integrative analysis of single nucleotide polymorphism array and exome sequence data. Circ. Res. 2014, 115, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, E.; Levitas, A.; Singh, S.R.; Braiman, A.; Ofir, R.; Etzion, S.; Sheffield, V.C.; Etzion, Y.; Carrier, L.; Parvari, R. PLEKHM2 mutation leads to abnormal localization of lysosomes, impaired autophagy flux and associates with recessive dilated cardiomyopathy and left ventricular noncompaction. Hum. Mol. Genet. 2015, 24, 7227–7240. [Google Scholar] [CrossRef] [PubMed]
- Korover, N.; Etzion, S.; Cherniak, A.; Rabinski, T.; Levitas, A.; Etzion, Y.; Ofir, R.; Parvari, R.; Cohen, S. Functional defects in hiPSCs-derived cardiomyocytes from patients with a PLEKHM2-mutation associated with dilated cardiomyopathy and left ventricular non-compaction. Biol. Res. 2023, 56, 34. [Google Scholar] [CrossRef] [PubMed]
- Levitas, A.; Muhammad, E.; Zhang, Y.; Perea Gil, I.; Serrano, R.; Diaz, N.; Arafat, M.; Gavidia, A.A.; Kapiloff, M.S.; Mercola, M.; et al. A Novel Recessive Mutation in SPEG Causes Early Onset Dilated Cardiomyopathy. PLoS Genet. 2020, 16, e1009000. [Google Scholar] [CrossRef]
- Aspit, L.; Levitas, A.; Etzion, S.; Krymko, H.; Slanovic, L.; Zarivach, R.; Etzion, Y.; Parvari, R. CAP2 mutation leads to impaired actin dynamics and associates with supraventricular tachycardia and dilated cardiomyopathy. J. Med. Genet. 2019, 56, 228–235. [Google Scholar] [CrossRef]
- Parvari, R.; Levitas, A. The mutations associated with dilated cardiomyopathy. Biochem. Res. Int. 2012, 2012, 639250. [Google Scholar] [CrossRef]
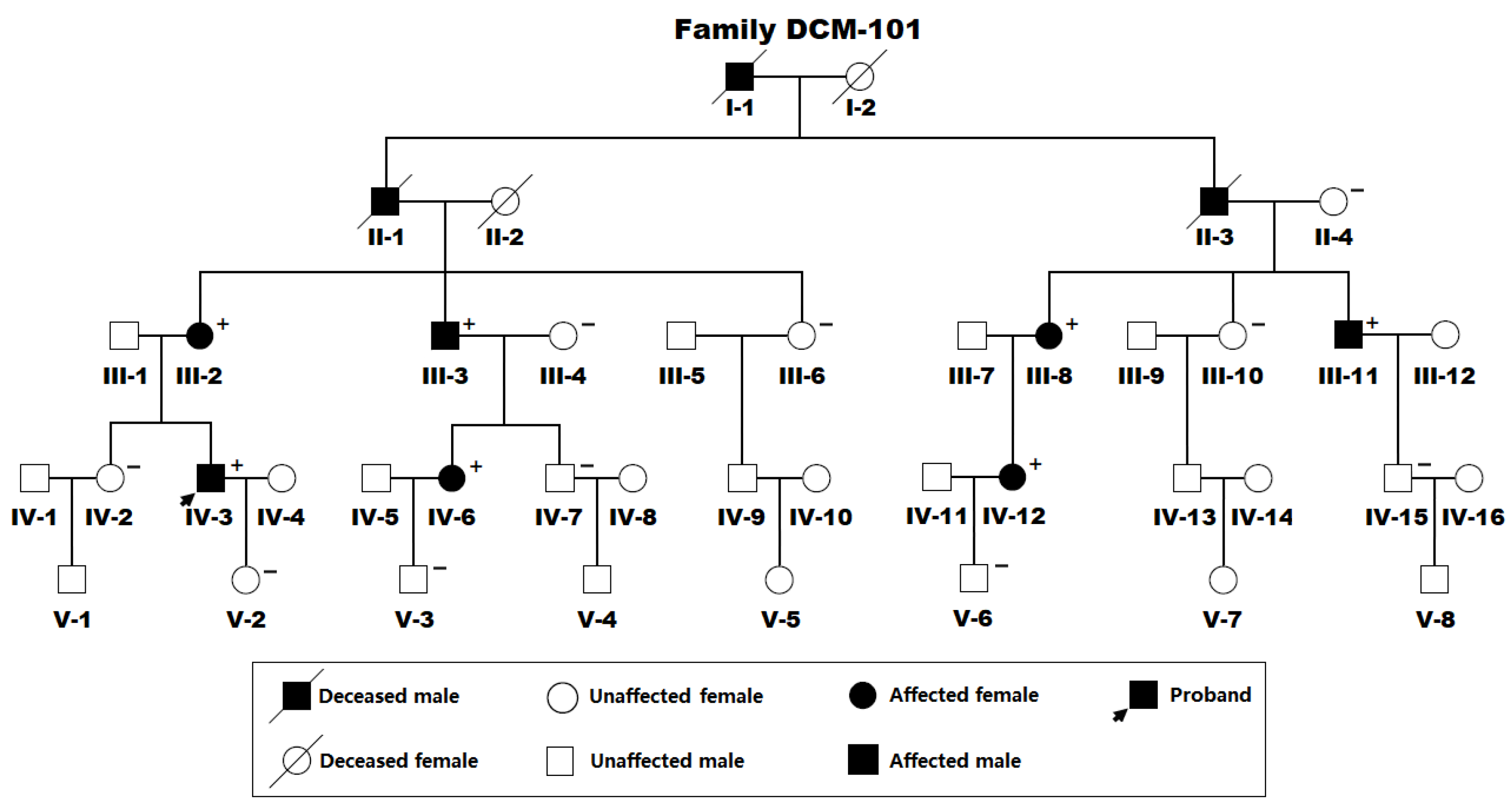
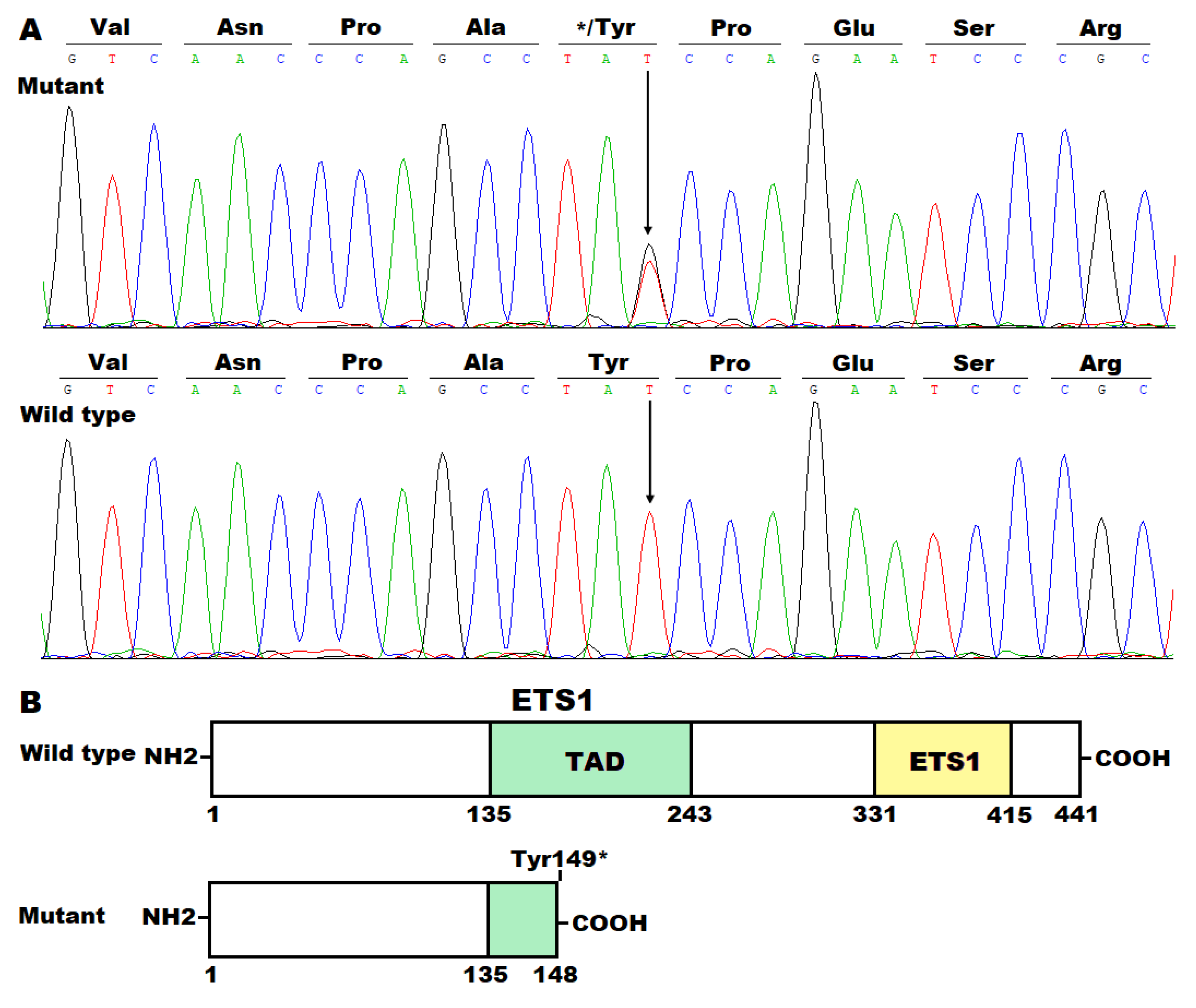
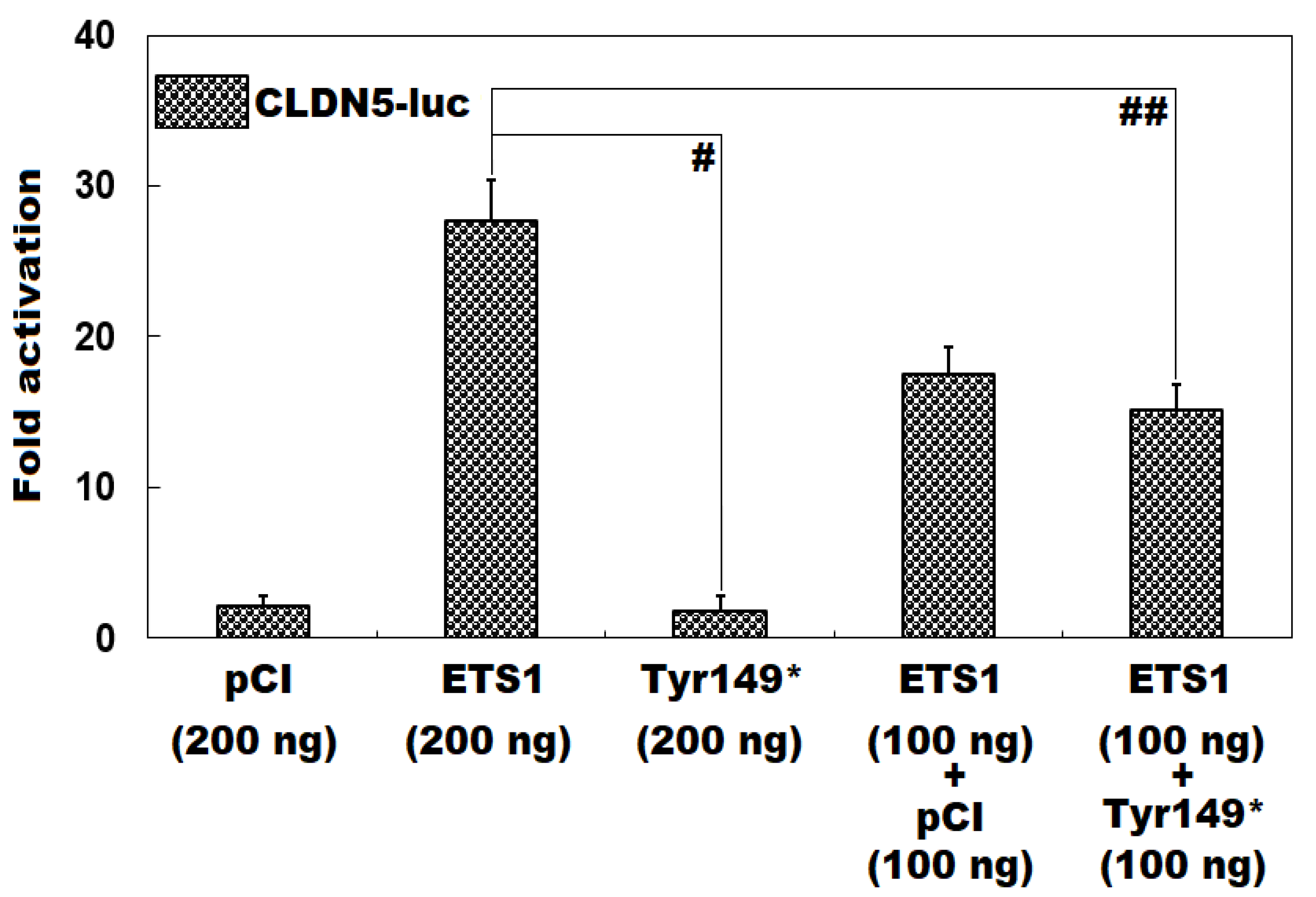
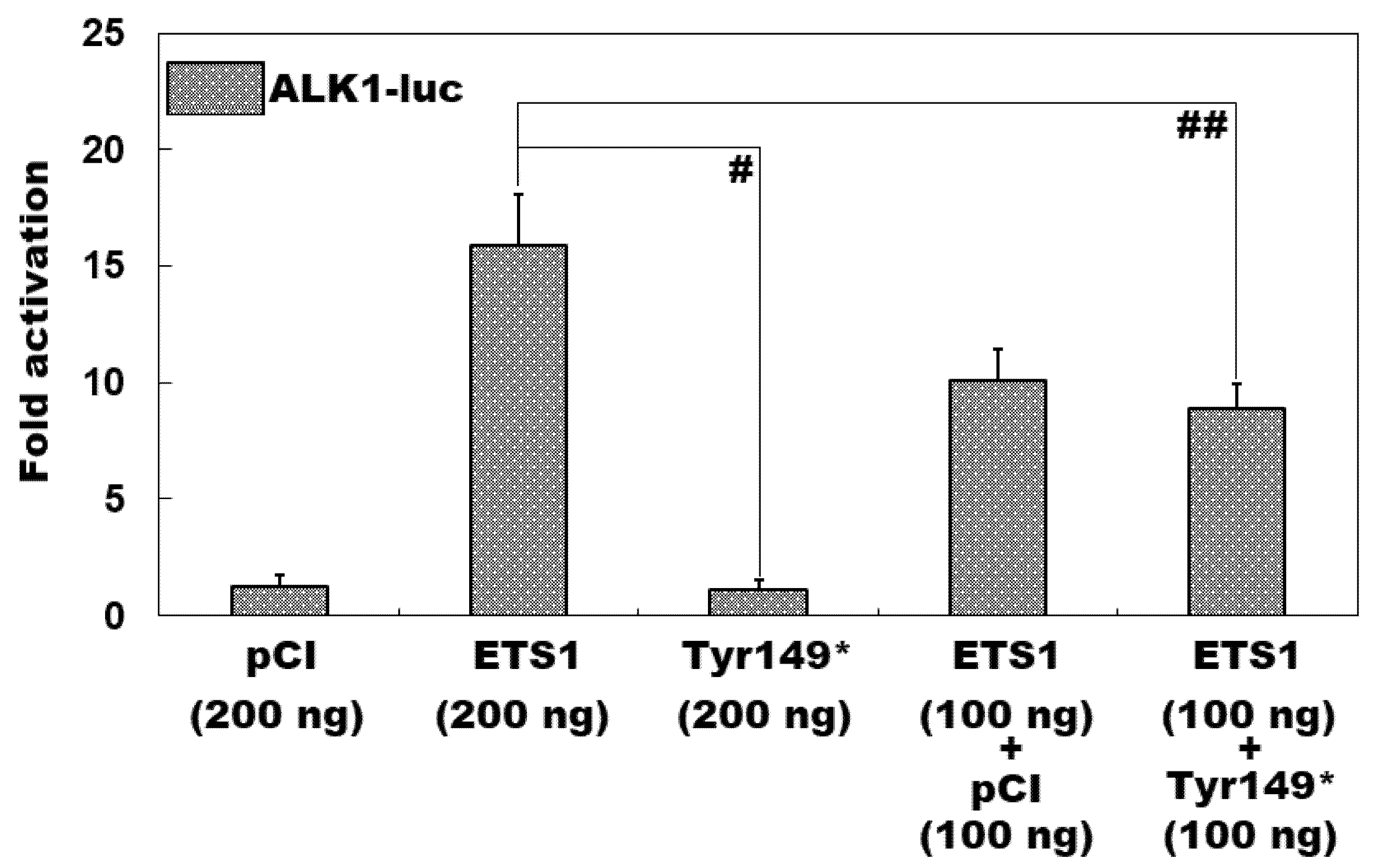
| Individuals (Family DCM-101) | Sexes | Ages (Years) | Cardiac Phenotypes | LVEDD (mm) | LVESD (mm) | LVEF (%) | LVFS (%) |
|---|---|---|---|---|---|---|---|
| III-2 | Female | 68 | DCM | 71 | 62 | 33 | 18 |
| III-3 | Male | 65 | DCM | 76 | 65 | 30 | 14 |
| III-8 | Female | 63 | DCM, AF | 70 | 60 | 27 | 13 |
| III-11 | Male | 57 | DCM, AF | 69 | 58 | 32 | 16 |
| IV-3 | Male | 45 | DCM | 58 | 48 | 36 | 18 |
| IV-6 | Female | 40 | DCM, VSD | 64 | 52 | 38 | 19 |
| IV-12 | Female | 38 | DCM, AF | 60 | 50 | 40 | 20 |
| Chr. | Position (hg19) | Ref. | Alt. | Gene | Variation |
|---|---|---|---|---|---|
| 1 | 92,184,903 | G | A | TGFBR3 | NM_003243.5: c.1532G>A; p.(Trp511*) |
| 2 | 141,777,525 | C | G | LRP1B | NM_018557.3: c.1936C>G; p.(His646Asp) |
| 3 | 71,096,210 | C | G | FOXP1 | NM_032682.6: c.547C>G; p.(Gln183Glu) |
| 4 | 157,693,868 | A | G | PDGFC | NM_016205.3: c.673A>G; p.(Lys225Glu) |
| 6 | 38,029,545 | G | C | ZFAND3 | NM_021943.3: c.289G>C; p.(Glu97Gln) |
| 7 | 133,689,816 | C | T | EXOC4 | NM_021807.4: c.2500C>T; p.(Gln834*) |
| 11 | 128,355,998 | T | G | ETS1 | NM_005238.4: c.447T>G; p.(Tyr149*) |
| 12 | 66,773,093 | G | T | GRIP1 | NM_021150.4: c.2432G>T; p.(Ser811Ile) |
| 15 | 86,266,500 | C | G | AKAP13 | NM_006738.6: c.6706C>G; p.(Leu2236Val) |
| 16 | 49,671,508 | T | C | ZNF423 | NM_015069.5: c.1555T>C; p.(Cys519Arg) |
| 18 | 34,092,506 | G | A | FHOD3 | NM_025135.5: c.511G>A; p.(Ala171Thr) |
| Coding Exons | Forward Primers (5′→3′) | Reverse Primers (5′→3′) | Amplicon Sizes (bp) |
|---|---|---|---|
| 1 | AAAGTCGGATTTCCCCCGTC | AGGGTTCTCGCTCATTTGGG | 515 |
| 2 | CTGGTGCTCTGGTGAGGATG | CTGGTGCTCTGGTGAGGATG | 486 |
| 3 | TTGGCTGGCAGTAGTGACCT | GGCACATTCCCACATACGTC | 571 |
| 4 | TCTTTAGACAAGGCCACAGTCA | TGGCCATCGTAGTGCACAGT | 568 |
| 5 | CATAGGGCCCTGTAGATGGTG | GGACTTGGCTAAAACACCACG | 514 |
| 6 | CCCAAACGCTACTCCGACAG | AAAGGGAGTCTCTCGTCGTC | 664 |
| 7 | GAAGAGGGATGTGGGAGTGC | CACAGGAGGCAGCTCTATGG | 491 |
| 8 | TTGTGACTCCCATCCTTGCC | GCTTTCCTTTCCCAACTGCG | 609 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, Z.-P.; Gu, J.-N.; Yang, C.-X.; Li, X.-L.; Zou, S.; Bian, Y.-Z.; Xu, Y.-J.; Yang, Y.-Q. Discovery of ETS1 as a New Gene Predisposing to Dilated Cardiomyopathy. Diagnostics 2025, 15, 2031. https://doi.org/10.3390/diagnostics15162031
Ke Z-P, Gu J-N, Yang C-X, Li X-L, Zou S, Bian Y-Z, Xu Y-J, Yang Y-Q. Discovery of ETS1 as a New Gene Predisposing to Dilated Cardiomyopathy. Diagnostics. 2025; 15(16):2031. https://doi.org/10.3390/diagnostics15162031
Chicago/Turabian StyleKe, Zun-Ping, Jia-Ning Gu, Chen-Xi Yang, Xue-Lin Li, Su Zou, Yi-Zhe Bian, Ying-Jia Xu, and Yi-Qing Yang. 2025. "Discovery of ETS1 as a New Gene Predisposing to Dilated Cardiomyopathy" Diagnostics 15, no. 16: 2031. https://doi.org/10.3390/diagnostics15162031
APA StyleKe, Z.-P., Gu, J.-N., Yang, C.-X., Li, X.-L., Zou, S., Bian, Y.-Z., Xu, Y.-J., & Yang, Y.-Q. (2025). Discovery of ETS1 as a New Gene Predisposing to Dilated Cardiomyopathy. Diagnostics, 15(16), 2031. https://doi.org/10.3390/diagnostics15162031






