Differential Regulation of Angiogenesis, Lymphangiogenesis, and Neural Tissue in Normal and Inflamed Dental Pulp: Immunohistochemical Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Reliability
2.2. Ethical Consideration
2.3. Demographic Characteristics
2.4. Diagnostic Criteria
2.5. Immunohistochemistry
2.6. Quantification and Assessment
2.7. Statistical Analysis
3. Results
3.1. Demographic Characteristics:
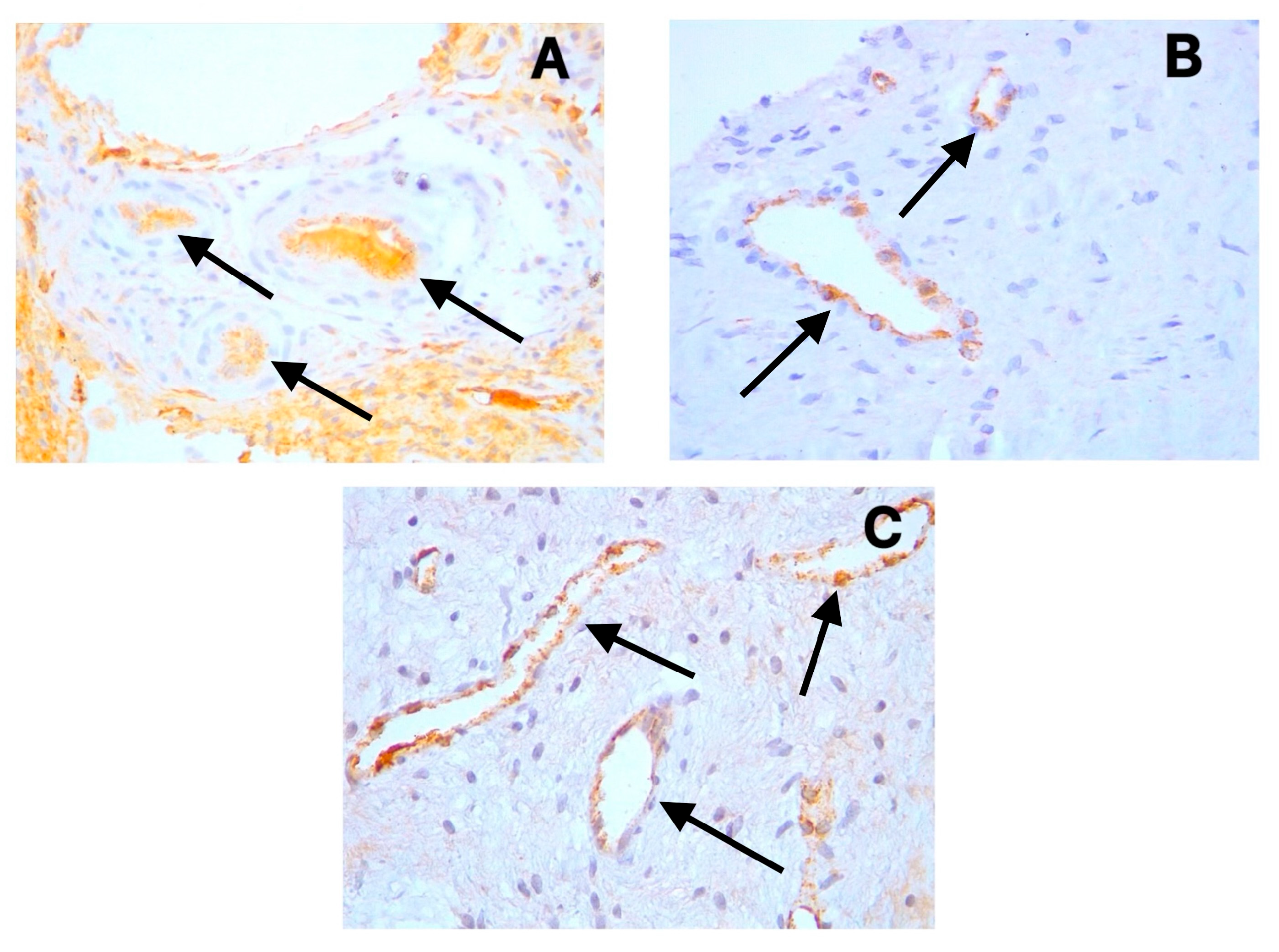
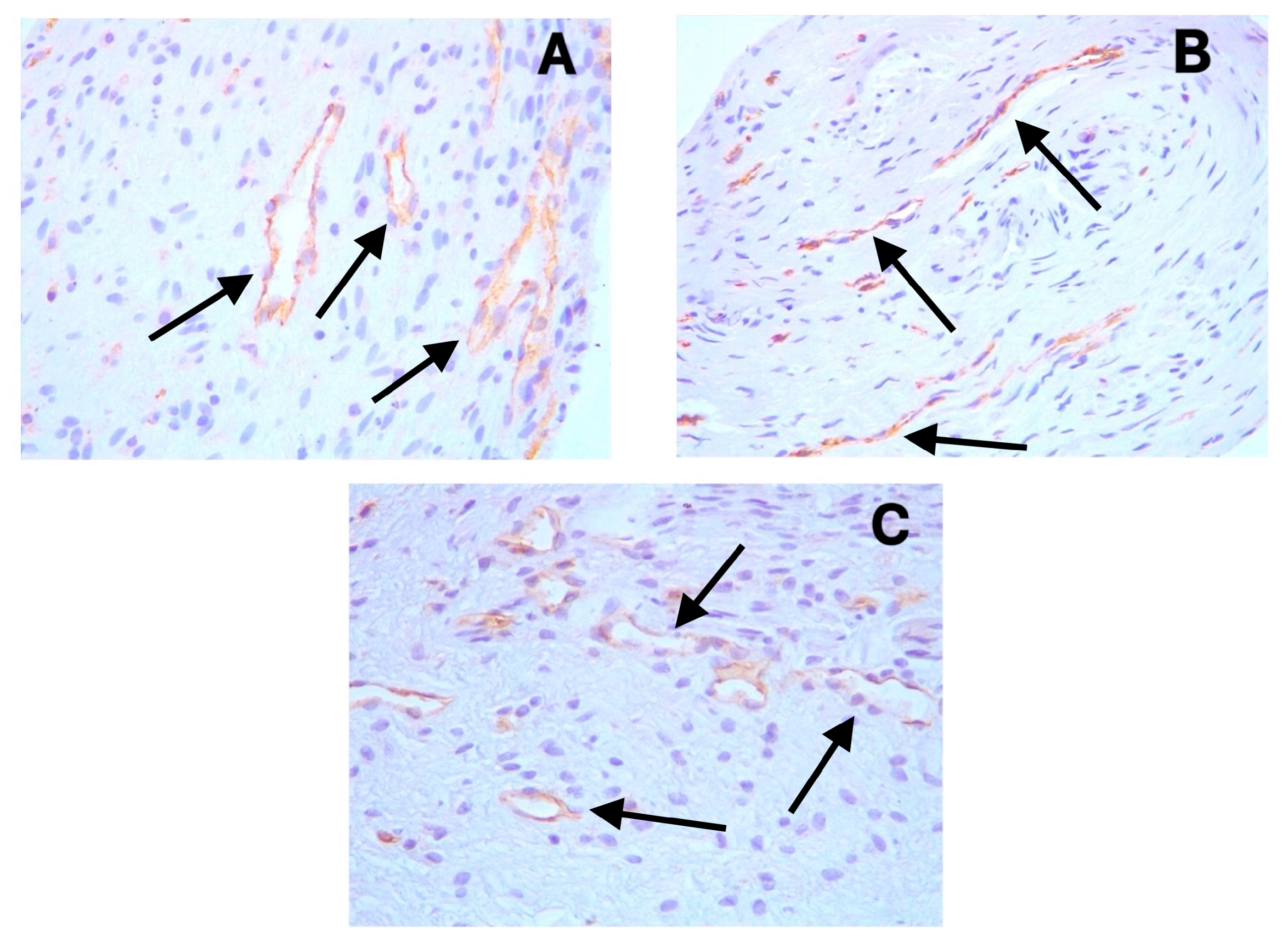
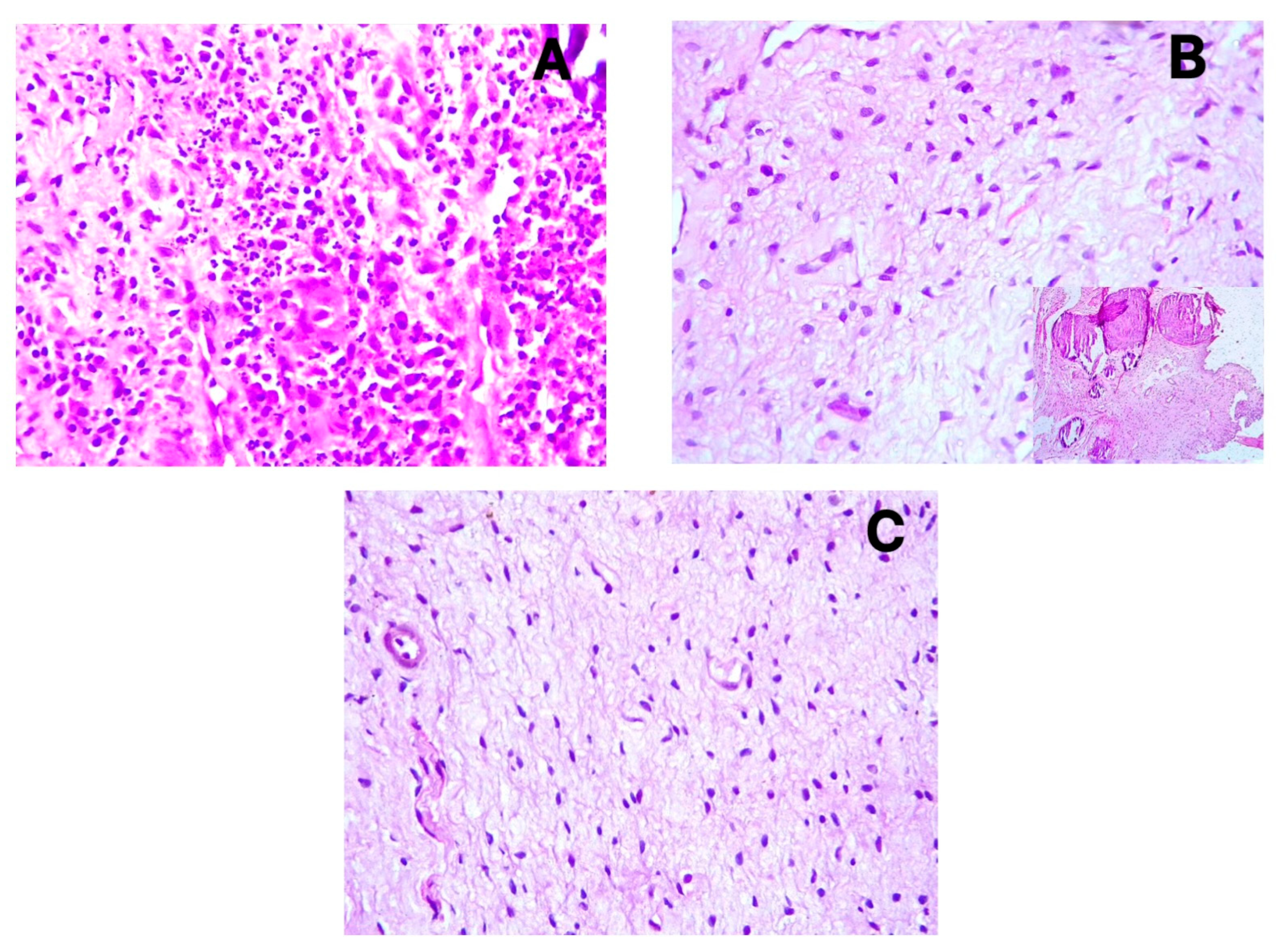
3.2. Immunohistochemical Findings
3.3. Vessel and Nerve Density Analysis
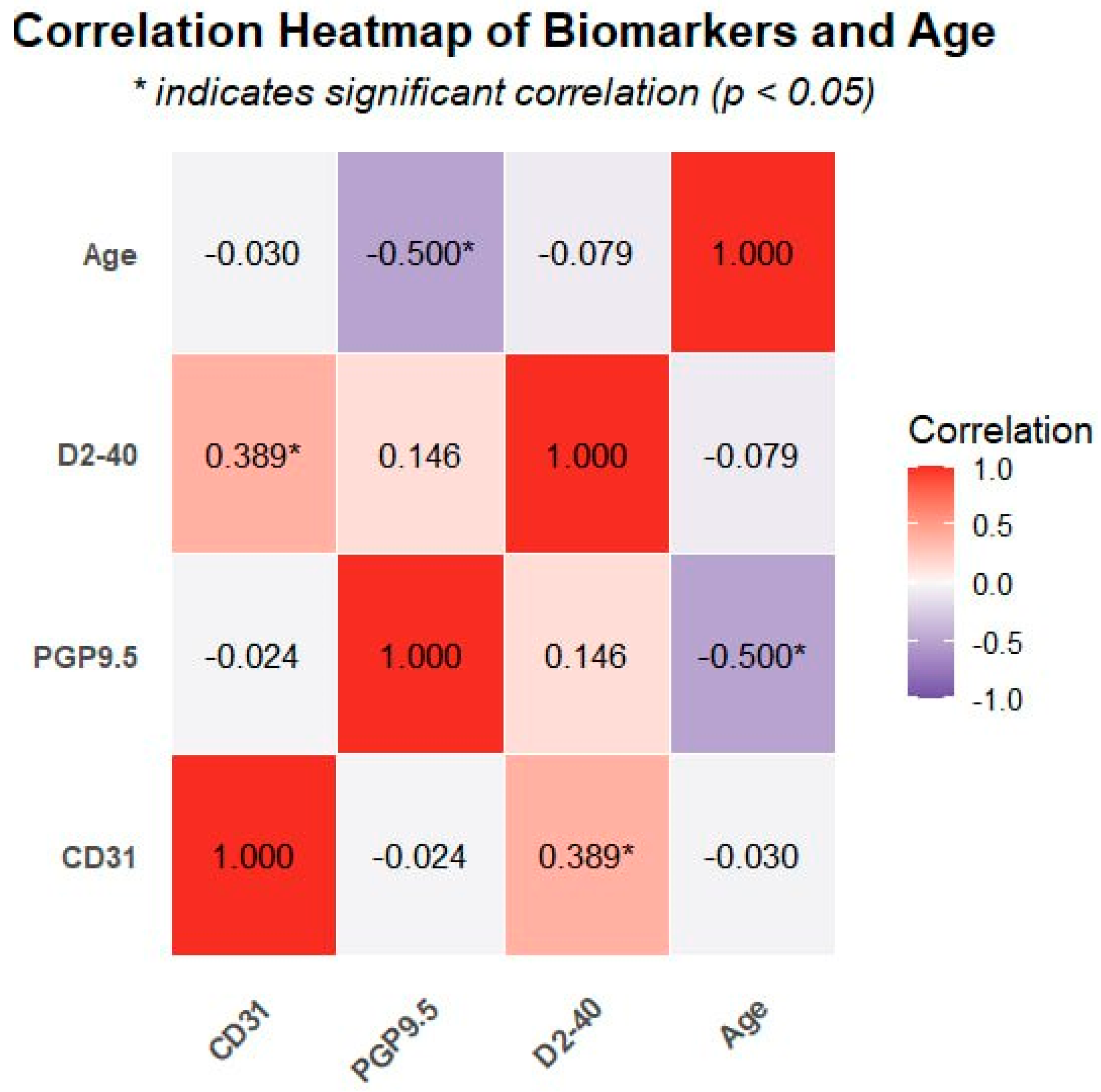
3.4. Correlation Analysis
4. Discussion
4.1. Lymphatic Vessels in Dental Pulp
4.2. Blood Vessel Changes in Pulpitis
4.3. Neural Tissue Response to Inflammation
4.4. Clinical Implications and Limitations
4.5. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghannam, M.G.; Alameddine, H.; Bordoni, B. Anatomy, Head and Neck, Pulp (Tooth); StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Chen, J.; Xu, H.; Xia, K.; Cheng, S.; Zhang, Q. Resolvin E1 accelerates pulp repair by regulating inflammation and stimulating dentin regeneration in dental pulp stem cells. Stem Cell Res. Ther. 2021, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Vaseenon, S.; Weekate, K.; Srisuwan, T.; Chattipakorn, N.; Chattipakorn, S. Observation of inflammation, oxidative stress, mitochondrial dynamics, and apoptosis in dental pulp following a diagnosis of irreversible pulpitis. Eur. Endod. J. 2023, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, M.S.; Khalaf, B.S.; Abass, S.M. Management of trauma to the anterior segment of the maxilla: Alveolar fracture and primary incisors crown and root fracture. J. Baghdad Coll. Dent. 2021, 33, 16–20. [Google Scholar] [CrossRef]
- Al-Dahan, Z.A.; Khalaf, M.S.; Al-Assadi, A.H. Apexification and periapical healing of immature teeth using Mineral Trioxide Aggregate. J. Baghdad Coll. Dent. 2014, 26, 108–112. [Google Scholar] [CrossRef]
- Wiśniewska, K.; Rybak, Z.; Szymonowicz, M.; Kuropka, P.; Dobrzyński, M. Review on the lymphatic vessels in the dental pulp. Biology 2021, 10, 1257. [Google Scholar] [CrossRef] [PubMed]
- Baru, O.; Nutu, A.; Braicu, C.; Cismaru, C.A.; Berindan-Neagoe, I.; Buduru, S.; Badea, M. Angiogenesis in regenerative dentistry: Are we far enough for therapy? Int. J. Mol. Sci. 2021, 22, 929. [Google Scholar] [CrossRef] [PubMed]
- Berggreen, E.; Haug, S.R.; Mkonyi, L.E.; Bletsa, A. Characterization of the dental lymphatic system and identification of cells immunopositive to specific lymphatic markers. Eur. J. Oral Sci. 2009, 117, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, F.; Sá, A.; Gomez, R. Lymphangiogenesis in human dental pulp. Int. Endod. J. 2003, 36, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Ambe, K.; Kon, H.; Takada, S.; Ohno, T.; Watanabe, H. Immunohistochemical investigation of lymphatic vessel formation control in mouse tooth development: Lymphatic vessel-forming factors and receptors in tooth development in mice. Tissue Cell 2012, 44, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Rodd, H.D.; Boissonade, F.M. Immunocytochemical investigation of neurovascular relationships in human tooth pulp. J. Anat. 2003, 202, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Szeląg, E.; Popecki, P.; Puła, B. Expression of CD31, podoplanin and Sox18 in dental pulp. In Book of Abstracts Katowice, Proceedings of the 7th International Scientific Conference of Medical Students and Young Doctors, Katowice, Poland, 17–18 May 2012; Medical University of Silesia: Katowice, Poland, 2012; p. 28. [Google Scholar]
- Gerli, R.; Secciani, I.; Sozio, F.; Rossi, A.; Weber, E.; Lorenzini, G. Absence of lymphatic vessels in human dental pulp: A morphological study. Eur. J. Oral Sci. 2010, 118, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Gasse, H.; Staszyk, C. Absence of lymphatic vessels in the dog dental pulp: An immunohistochemical study. J. Anat. 2010, 217, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Lohrberg, M.; Wilting, J. The lymphatic vascular system of the mouse head. Cell Tissue Res. 2016, 366, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Mahdee, A.F.; Alhelal, A.G.; Whitworth, J.; Eastham, J.; Gillespie, J. Evidence for complex physiological processes in the enamel organ of the rodent mandibular incisor throughout amelogenesis. Med. J. Babylon 2017, 14, 68–82. [Google Scholar]
- Kim, M.E.; Lee, J.S. Advances in the Regulation of Inflammatory Mediators in Nitric Oxide Synthase: Implications for Disease Modulation and Therapeutic Approaches. Int. J. Mol. Sci. 2025, 26, 1204. [Google Scholar] [CrossRef] [PubMed]
- Mahdee, A.; Eastham, J.; Whitworth, J.; Gillespie, J. Evidence for changing nerve growth factor signalling mechanisms during development, maturation and ageing in the rat molar pulp. Int. Endod. J. 2019, 52, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Amita, K. Prognostic Significance of Lymphatic Vessel Density by D2-40 Immune Marker and Mast Cell Density in Invasive Breast Cancer: A Cross Sectional Study at Tertiary Care Hospital in South India. Online J. Health Allied Sci. 2022, 21, 5. [Google Scholar]
- Vyberg, M.; Nielsen, S.; Bzorek, M.; Røge, R. External quality assessments of CD31 immunoassays-The NordiQC experience. Vasc. Cell 2020, 12, 4. [Google Scholar] [CrossRef]
- Abdulrahman, A.-q.G.; Endytiastuti, E.; Ardhani, R.; Sudarso, I.S.R.; Pidhatika, B.; Fauzi, M.B.; Susilowati, H.; Kristanti, Y.; Handajani, J. Evaluating the Efficacy of Gelatin-Chitosan-Tetraethyl Orthosilicate Calcium Hydroxide Composite as a Dental Pulp Medicament on COX-2, PGP 9.5, TNF-α Expression and Neutrophil number. F1000Research 2025, 13, 1258. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, J.M.; Miskowiak, B.; Matthews-Brzozowska, T.; Wierzbicki, P. Characteristics of dental pulp in human upper first premolar teeth based on immunohistochemical and morphometric examinations. Folia Histochem. Cytobiol. 2013, 51, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Hadaegh, Y.; Niknam, M.; Attar, A.; Maharlooei, M.K.; Tavangar, M.S.; Aarabi, A.M.; Monabati, A. Characterization of stem cells from the pulp of unerupted third molar tooth. Indian J. Dent. Res. 2014, 25, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.G.; Law, A.S.; Holland, G.; Abbott, P.V.; Roda, R.S. Identify and define all diagnostic terms for pulpal health and disease states. J. Endod. 2009, 35, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.W.; Xie, Y.F.; Wang, W.N.; Liu, J.; Yang, K.G.; Chen, K.; Luo, C.H.; Fei, J.; Hu, J.M. High microvessel and lymphatic vessel density predict poor prognosis in patients with esophageal squamous cell carcinoma. PeerJ 2024, 12, e18080. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Weber, M.; Korkmaz, Y.; Widbiller, M.; Feuerer, M. Inflammatory response mechanisms of the dentine–pulp complex and the periapical tissues. Int. J. Mol. Sci. 2021, 22, 1480. [Google Scholar] [CrossRef] [PubMed]
- Singla, B.; Aithabathula, R.V.; Kiran, S.; Kapil, S.; Kumar, S.; Singh, U.P. Reactive oxygen species in regulating lymphangiogenesis and lymphatic function. Cells 2022, 11, 1750. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.S.; Jia, S.; D’Souza, R.N. Modeling hypoxia induced factors to treat pulpal inflammation and drive regeneration. J. Endod. 2020, 46, S19–S25. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Huang, M.; Yang, X.; Hou, J. Dental nerves: A neglected mediator of pulpitis. Int. Endod. J. 2021, 54, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Diogenes, A. Trigeminal sensory neurons and pulp regeneration. J. Endod. 2020, 46, S71–S80. [Google Scholar] [CrossRef] [PubMed]
- Caviedes-Bucheli, J.; Lopez-Moncayo, L.F.; Muñoz-Alvear, H.D.; Gomez-Sosa, J.F.; Diaz-Barrera, L.E.; Curtidor, H.; Munoz, H.R. Expression of substance P, calcitonin gene-related peptide and vascular endothelial growth factor in human dental pulp under different clinical stimuli. BMC Oral Health 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Aging and senescence of dental pulp and hard tissues of the tooth. Front. Cell Dev. Biol. 2020, 8, 605996. [Google Scholar] [CrossRef] [PubMed]

| Biomarker | Symptomatic Irreversible Pulpitis | Asymptomatic Irreversible Pulpitis | Control | p-Value |
|---|---|---|---|---|
| CD31 * | 50.3 (25.5–88.2) | 39.2 (26.2–49.8) | 25.8 (17.8–49.0) | 0.001 |
| PGP9.5 * | 4.0 (2.0–4.0) | 3.0 (1.0–4.0) | 4.0 (3.0–4.0) | 0.161 |
| D2-40 † | 28.35 ± 10.6 | 24.72 ± 9.0 | 22.72 ± 6.8 | 0.299 |
| Variable | CD31 | PGP9.5 | D2-40 | Age |
|---|---|---|---|---|
| CD31 | 1.0 | −0.024 | 0.389 * | −0.03 |
| PGP9.5 | −0.024 | 1.0 | 0.146 | −0.500 ** |
| D2-40 | 0.389 * | 0.146 | 1.0 | −0.079 |
| Age | −0.03 | −0.500 ** | −0.079 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alani, N.A.; Abdullah, B.H. Differential Regulation of Angiogenesis, Lymphangiogenesis, and Neural Tissue in Normal and Inflamed Dental Pulp: Immunohistochemical Analysis. Diagnostics 2025, 15, 1819. https://doi.org/10.3390/diagnostics15141819
Alani NA, Abdullah BH. Differential Regulation of Angiogenesis, Lymphangiogenesis, and Neural Tissue in Normal and Inflamed Dental Pulp: Immunohistochemical Analysis. Diagnostics. 2025; 15(14):1819. https://doi.org/10.3390/diagnostics15141819
Chicago/Turabian StyleAlani, Nooruldeen Ammar, and Bashar Hamid Abdullah. 2025. "Differential Regulation of Angiogenesis, Lymphangiogenesis, and Neural Tissue in Normal and Inflamed Dental Pulp: Immunohistochemical Analysis" Diagnostics 15, no. 14: 1819. https://doi.org/10.3390/diagnostics15141819
APA StyleAlani, N. A., & Abdullah, B. H. (2025). Differential Regulation of Angiogenesis, Lymphangiogenesis, and Neural Tissue in Normal and Inflamed Dental Pulp: Immunohistochemical Analysis. Diagnostics, 15(14), 1819. https://doi.org/10.3390/diagnostics15141819






