Novel Structure–Function Models for Estimating Retinal Ganglion Cell Count Using Pattern Electroretinography in Glaucoma Suspects
Abstract
1. Introduction
2. Materials and Methods
2.1. SD-OCT Testing
2.2. ssPERG Testing
2.3. Humphrey Field Analyzer Testing
2.4. RGC Count Estimation with the CSFI (eRGCCCCSFI)
2.5. Statistics
3. Results
3.1. Subject Characteristics
3.2. Consistency Across eRGCC Models and Correlation with OCT Parameters
3.2.1. Correlation Analysis Between eRGCCCSFI, eRGCCMag, and eRGCCMagD
3.2.2. Correlation Analysis Between eRGCCCSFI, eRGCCMag, and eRGCCMagD, with AvGCL-IPLT and AvRNFLT Measurements
3.3. Predictive Ability of eRGCC Models
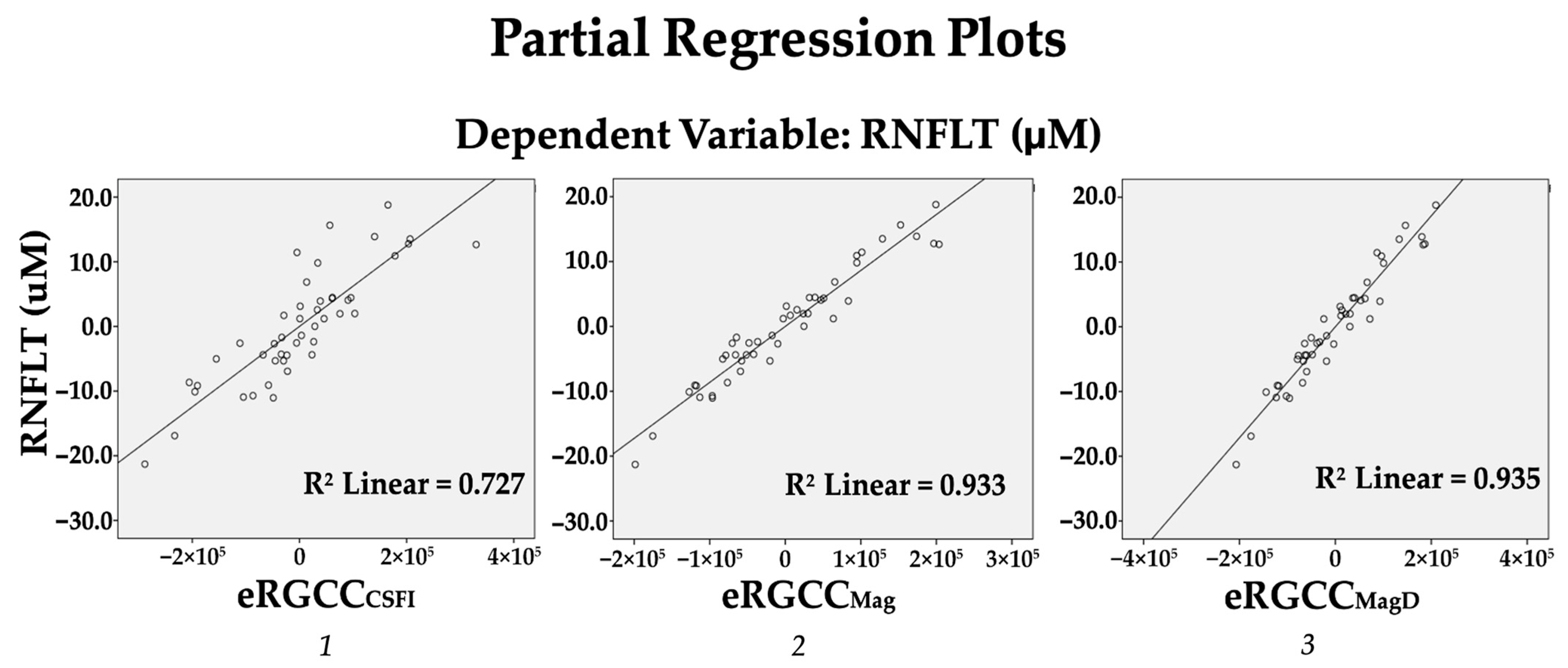
3.3.1. Strength of Association Between eRGCC Models and AvRNFLT
3.3.2. Strength of Association Between eRGCC Models and AvGCL-IPLT
3.3.3. Strength of Association Between eRGCC Models and Rim Area
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RGC | Retinal ganglion cell |
| GS | Glaucoma suspects |
| CSFI | Combined structure–function index |
| OCT | Optical coherence tomography |
| ssPERG | Steady-state pattern electroretinogram |
| eRGCC | Estimated RGC count |
| eRGCCMag | ssPERG Magnitude-based eRGCC |
| eRGCCMagD | ssPERG MagnitudeD-based eRGCC |
| eRGCCCSFI | CSFI-based eRGCC |
| AvRNFLT | Average retinal nerve fiber layer thickness |
| AvGCL-IPLT | Average ganglion cell layer–inner plexiform layer thickness |
| VF | Visual field |
| SAP | Standard automated perimetry |
| IOP | Intraocular pressure |
| RNFLT | Retinal nerve fiber layer thickness |
| RNFL | Retinal nerve fiber layer |
| PERG | Pattern electroretinogram |
| RGCC | Retinal ganglion cell counts |
| GCL-IPLT | Ganglion cell layer–inner plexiform layer thickness |
| GCL-IPL | Ganglion cell layer–inner plexiform layer |
| SD-OCT | Spectral-domain optical coherence tomography |
| ISCEV | International Society for Clinical Electrophysiology of Vision |
| PERGLA | Pattern electroretinogram optimized for glaucoma screening |
| RGCCSAP | Standard automated perimetry-derived retinal ganglion cell count |
| RGCCOCT | Optical coherence tomography-derived retinal ganglion cell count |
| CCT | Central corneal thickness |
| GSS 2 | Glaucoma Staging System |
| SE | Standard error |
| HFA | Humphrey Field Analyzer |
| MD | Mean deviation |
References
- Quigley, H.A. Glaucoma. Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Dunkelberger, G.R.; Green, W.R. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am. J. Ophthalmol. 1989, 107, 453–464. [Google Scholar] [CrossRef]
- Harwerth, R.S.; Wheat, J.L.; Fredette, M.J.; Anderson, D.R. Linking structure and function in glaucoma. Prog. Retin. Eye Res. 2010, 29, 249–271. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan-Baumrind, L.A.; Quigley, H.A.; Pease, M.E.; Kerrigan, D.F.; Mitchell, R.S. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Investig. Ophthalmol. Vis. Sci. 2000, 41, 741–748. [Google Scholar]
- Harwerth, R.S.; Quigley, H.A. Visual field defects and retinal ganglion cell losses in patients with glaucoma. Arch. Ophthalmol. Chic. III 2006, 124, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S. Glaucoma suspects: A practical approach. Taiwan J. Ophthalmol. 2018, 8, 74–81. [Google Scholar] [CrossRef]
- Sommer, A. Clinically Detectable Nerve Fiber Atrophy Precedes the Onset of Glaucomatous Field Loss. Arch. Ophthalmol. 1991, 109, 77. [Google Scholar] [CrossRef]
- Lieberman, M.F.; Drake, M.V.; Stamper, R.L. Becker-Shaffer’s Diagnosis and Therapy of the Glaucomas; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Sarkar, K.C.; Das, P.; Pal, R.; Shaw, C. Optical coherence tomographic assessment of retinal nerve fiber layer thickness changes before and after glaucoma filtration surgery. Oman J. Ophthalmol. 2014, 7, 3–8. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Lisboa, R.; Weinreb, R.N.; Girkin, C.A.; Liebmann, J.M.; Zangwill, L.M. A combined index of structure and function for staging glaucomatous damage. Arch. Ophthalmol. Chic. III 2012, 130, 1107–1116. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Zangwill, L.M.; Bowd, C.; Mansouri, K.; Weinreb, R.N. The structure and function relationship in glaucoma: Implications for detection of progression and measurement of rates of change. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6939–6946. [Google Scholar] [CrossRef]
- Orshan, D.; Tirsi, A.; Sheha, H.; Gliagias, V.; Tsai, J.; Park, S.C.; Obstbaum, S.A.; Tello, C. Structure-function models for estimating retinal ganglion cell count using steady-state pattern electroretinography and optical coherence tomography in glaucoma suspects and preperimetric glaucoma: An electrophysiological pilot study. Doc. Ophthalmol. Adv. Ophthalmol. 2022, 145, 221–235. [Google Scholar] [CrossRef]
- Shaikh, Y.; Yu, F.; Coleman, A.L. Burden of undetected and untreated glaucoma in the United States. Am. J. Ophthalmol. 2014, 158, 1121–1129.e1. [Google Scholar] [CrossRef] [PubMed]
- Zrenner, E. Chapter 12 The physiological basis of the pattern electroretinogram. Prog. Retin. Res. 1990, 9, 427–464. [Google Scholar] [CrossRef]
- Takao, M.; Miyoshi, T.; Watanabe, M.; Fukuda, Y. Changes in Visual Response Properties of Cat Retinal Ganglion Cells within Two Weeks after Axotomy. Exp. Neurol. 2002, 177, 171–182. [Google Scholar] [CrossRef]
- Maffei, L.; Fiorentini, A.; Bisti, S.; Holländer, H. Pattern ERG in the monkey after section of the optic nerve. Exp. Brain Res. 1985, 59, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Porciatti, V. Electrophysiological assessment of retinal ganglion cell function. Exp. Eye Res. 2015, 141, 164–170. [Google Scholar] [CrossRef]
- Holder, G.E. Pattern Electroretinography (PERG) and an Integrated Approach to Visual Pathway Diagnosis. Prog. Retin. Eye Res. 2001, 20, 531–561. [Google Scholar] [CrossRef]
- Banitt, M.R.; Ventura, L.M.; Feuer, W.J.; Savatovsky, E.; Luna, G.; Shif, O.; Bosse, B.; Porciatti, V. Progressive loss of retinal ganglion cell function precedes structural loss by several years in glaucoma suspects. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2346–2352. [Google Scholar] [CrossRef]
- Tirsi, A.; Gliagias, V.; Zhu, D.; Wong, B.; Gupta, R.; Park, S.C.; Obstbaum, S.; Tello, C.; Patel, S. Correlations between Steady-State Pattern Electroretinogram and Humphrey Visual Field Analyzer Global Indices and Their Associations with Retinal Ganglion Cell Layer-Inner Plexiform Layer Thickness in Glaucoma Suspects. J. Ophthalmol. 2024, 2024, 2443887. [Google Scholar] [CrossRef]
- Tirsi, A.; Orshan, D.; Wong, B.; Gliagias, V.; Tsai, J.; Obstbaum, S.A.; Tello, C. Associations between steady-state pattern electroretinography and estimated retinal ganglion cell count in glaucoma suspects. Doc. Ophthalmol. Adv. Ophthalmol. 2022, 145, 11–25. [Google Scholar] [CrossRef]
- Porciatti, V.; Ventura, L.M. Normative data for a user-friendly paradigm for pattern electroretinogram recording. Ophthalmology 2004, 111, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Budenz, D.L.; Anderson, D.R.; Varma, R.; Schuman, J.; Cantor, L.; Savell, J.; Greenfield, D.S.; Patella, V.M.; Quigley, H.A.; Tielsch, J. Determinants of Normal Retinal Nerve Fiber Layer Thickness Measured by StratusOCT. Ophthalmology 2007, 114, 1046–1052. [Google Scholar] [CrossRef]
- Schuman, J.S.; Pedut-Kloizman, T.; Hertzmark, E.; Hee, M.R.; Wilkins, J.R.; Coker, J.G.; Puliafito, C.A.; Fujimoto, J.G.; Swanson, E.A. Reproducibility of nerve fiber layer thickness measurements using optical coherence tomography. Ophthalmology 1996, 103, 1889–1898. [Google Scholar] [CrossRef]
- Tirsi, A.; Gliagias, V.; Moehringer, J.; Orshan, D.; Tello, S.; Derr, P.; Park, S.C.; A Obstbaum, S.; Tello, C.; Monteiro, M. Pattern Electroretinogram Parameters Are Associated with Optic Nerve Morphology in Preperimetric Glaucoma after Adjusting for Disc Area. J. Ophthalmol. 2021, 2021, 8025337. [Google Scholar] [CrossRef]
- Gillmann, K.; Mansouri, K.; Rao, H.L.; Ouabas, A.; Chaudhary, A.; Bravetti, G.E.; Mermoud, A. A Prospective Evaluation of the Repeatability and Reliability of New Steady-state Pattern Electroretinogram Parameters. J. Glaucoma. 2018, 27, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Ventura, L.M.; Porciatti, V.; Ishida, K.; Feuer, W.; Parrishii, R. Pattern electroretinogram abnormality and glaucoma. Ophthalmology 2005, 112, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Fredette, M.-J.; Anderson, D.R.; Porciatti, V.; Feuer, W. Reproducibility of pattern electroretinogram in glaucoma patients with a range of severity of disease with the new glaucoma paradigm. Ophthalmology 2008, 115, 957–963. [Google Scholar] [CrossRef]
- Bach, M.; Brigell, M.G.; Hawlina, M.; Holder, G.E.; Johnson, M.A.; McCulloch, D.L.; Meigen, T.; Viswanathan, S. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc. Ophthalmol. Adv. Ophthalmol. 2013, 126, 1–7. [Google Scholar] [CrossRef]
- Brusini, P.; Filacorda, S. Enhanced Glaucoma Staging System (GSS 2) for classifying functional damage in glaucoma. J. Glaucoma. 2006, 15, 40–46. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Zangwill, L.M.; Anderson, D.R.; Liebmann, J.M.; Girkin, C.A.; Harwerth, R.S.; Fredette, M.-J.; Weinreb, R.N. Estimating the rate of retinal ganglion cell loss in glaucoma. Am. J. Ophthalmol. 2012, 154, 814–824.e1. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Lisboa, R.; Weinreb, R.N.; Liebmann, J.M.; Girkin, C.; Zangwill, L.M. Retinal Ganglion Cell Count Estimates Associated with Early Development of Visual Field Defects in Glaucoma. Ophthalmology 2013, 120, 736–744. [Google Scholar] [CrossRef]
- Ventura, L.M.; Sorokac, N.; De Los Santos, R.; Feuer, W.J.; Porciatti, V. The relationship between retinal ganglion cell function and retinal nerve fiber thickness in early glaucoma. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3904–3911. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Swanson, W.H.; Garway-Heath, D.F. “Structure-function relationship” in glaucoma: Past thinking and current concepts. Clin. Exp. Ophthalmol. 2012, 40, 369–380. [Google Scholar] [CrossRef]
- Garway-Heath, D.F.; Holder, G.E.; Fitzke, F.W.; Hitchings, R.A. Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2213–2220. [Google Scholar]
- Raza, A.S.; Hood, D.C. Evaluation of a Method for Estimating Retinal Ganglion Cell Counts Using Visual Fields and Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2254–2268. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.C.; Xu, L.; Thienprasiddhi, P.; Greenstein, V.C.; Odel, J.G.; Grippo, T.M.; Liebmann, J.M.; Ritch, R. The pattern electroretinogram in glaucoma patients with confirmed visual field deficits. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.J.; Kaufman, P.L.; Hubbard, W.C. Morphology of single ganglion cells in the glaucomatous primate retina. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2304–2320. [Google Scholar]
- Williams, P.A.; Howell, G.R.; Barbay, J.M.; Braine, C.E.; Sousa, G.; John, S.W.M.; Morgan, J.E. Retinal ganglion cell dendritic atrophy in DBA/2J glaucoma. PLoS ONE 2013, 8, e72282. [Google Scholar] [CrossRef]
- Bode, S.F.N.; Jehle, T.; Bach, M. Pattern electroretinogram in glaucoma suspects: New findings from a longitudinal study. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4300–4306. [Google Scholar] [CrossRef]
- Weber, A.J.; Harman, C.D. Structure-function relations of parasol cells in the normal and glaucomatous primate retina. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3197–3207. [Google Scholar] [CrossRef]
- Morgan, J.E. Retinal ganglion cell shrinkage in glaucoma. J. Glaucoma. 2002, 11, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Porciatti, V.; Ventura, L.M. Physiologic significance of steady-state pattern electroretinogram losses in glaucoma: Clues from simulation of abnormalities in normal subjects. J. Glaucoma 2009, 18, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.B.; Sullivan-Mee, M.; Harwerth, R.S. The relationship between retinal nerve fiber layer thickness and optic nerve head neuroretinal rim tissue in glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6802–6816. [Google Scholar] [CrossRef] [PubMed]
- Crowston, J.G.; Fahy, E.T.; Fry, L.; A Trounce, I.; van Wijngaarden, P.; Petrou, S.; Chrysostomou, V. Targeting retinal ganglion cell recovery. Eye 2017, 31, 196–198. [Google Scholar] [CrossRef]

| N = 50 Eyes (25 Subjects) | Mean ± Standard Deviation |
|---|---|
| Age (years) | 54.04 ± 15.69 |
| Sex (% females) | 9 M/16 F (64%) |
| IOP (mmHg) | 17.18 ± 3.71 |
| 24-2 MD (dB) | −0.24 ± 1.26 |
| Central corneal thickness (µm) | 546.43 ± 29.67 |
| Spherical equivalent (diopter) | −0.93 ± 2.64 |
| Disc area (mm2) | 1.94 ± 0.42 |
| Rim area (mm2) | 1.13 ± 1.63 |
| eRGCCCSFI | 969,050.14 ± 189,230.41 |
| eRGCCMag | 970,759.34 ± 181,022.09 |
| eRGCCMagD | 971,665.90 ± 180,955.78 |
| AvRNFLT | AvGCL-IPLT | Rim Area (mm2) | Rim Area (Residuals) | |
|---|---|---|---|---|
| eRGCCCSFI | 0.844 ** | 0.553 ** | 0.342 | 0.426 * |
| eRGCCMag | 0.956 ** | 0.560 ** | 0.267 | 0.365 ° |
| eRGCCMagD | 0.957 ** | 0.575 ** | 0.247 | 0.348 ° |
| Step 1 (age, sex, and CCT) | Step 2 (eRGCCCSFI) | |||||
| ΔR2 | B (95% CI) | ΔR2 | B (95% CI) | R2 | SE | |
| AvRNFLT | 0.317 * | 124.61 (70.82, 178.41) | 0.496 ** | 6.228 × 10−5 (0.00, 0.00) | 0.813 | 4.82 |
| Step 1 (age, sex, and CCT) | Step 2 (eRGCCMag) | |||||
| ΔR2 | B (95% CI) | ΔR2 | B (95% CI) | R2 | S.E. | |
| AvRNFLT | 0.317 * | 124.61 (70.82, 178.41) | 0.637 ** | 8.628 × 10−5 (0.00, 0.00) | 0.955 | 2.38 |
| Step 1 (age, sex, and CCT) | Step 2 (eRGCCMagD) | |||||
| ΔR2 | B (95% CI) | ΔR2 | B (95% CI) | R2 | S.E. | |
| AvRNFLT | 0.317 * | 124.61 (70.82, 178.41) | 0.639 ** | 8.565 × 10−5 (0.00, 0.00) | 0.956 | 2.34 |
| Step 1 (age, sex, and CCT) | Step 2 (eRGCCCSFI) | |||||
| ΔR2 | B (95% CI) | ΔR2 | B (95% CI) | R2 | SE | |
| AvGCL_IPLT | 0.331 * | 129.45 (94.92, 164.09) | 0.189 ** | 2.502 × 10−5 (0.00, 0.00) | 0.521 | 5.03 |
| Step 1 (age, sex, and CCT) | Step 2 (eRGCCMag) | |||||
| ΔR2 | B (95% CI) | ΔR2 | B (95% CI) | R2 | SE | |
| AvGCL_IPLT | 0.331 * | 129.45 (94.82, 164.09) | 0.229 ** | 3.367 × 10−5 (0.00, 0.00) | 0.561 | 4.81 |
| Step 1 (age, sex, and CCT) | Step 2 (eRGCCMagD) | |||||
| ΔR2 | B (95% CI) | ΔR2 | B (95% CI) | R2 | SE | |
| AvGCL_IPLT | 0.331 * | 129.45 (94.82, 164.09) | 0.240 ** | 3.416 × 10−5 (0.00, 0.00) | 0.571 | 4.75 |
| Step 1 (age, sex, and CCT) | Step 2 (eRGCCCSFI) | |||||
| ΔR2 | B (95% CI) | ΔR2 | B (95% CI) | R2 | SE | |
| Rim area | 0.309 * | 0.136 (−0.118, 0.391) | 0.168 * | 6.365 × 10−7 (0.00, 0.00) | 0.477 | 1.26 |
| Step 1 (age, sex, and CCT) | Step 2 (eRGCCMag) | |||||
| ΔR2 | B (95% CI) | ΔR2 | B (95% CI) | R2 | SE | |
| Rim area | 0.309 * | 0.136 (−0.118, 0.391) | 0.158 * | 7.430 × 10−7 (0.00, 0.00) | 0.467 | 0.13 |
| Step 1 (age, sex, and CCT) | Step 2 (eRGCCMagD) | |||||
| ΔR2 | B (95% CI) | ΔR2 | B (95% CI) | R2 | SE | |
| Rim area | 0.309 * | 0.136 (−0.118, 0.391) | 0.131 * | 6.623 × 10−7 (0.00, 0.00) | 0.440 | 1.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirsi, A.; Tello, I.; Foster, T.; Kumbhani, R.; Leung, N.; Potash, S.; Orshan, D.; Tello, C. Novel Structure–Function Models for Estimating Retinal Ganglion Cell Count Using Pattern Electroretinography in Glaucoma Suspects. Diagnostics 2025, 15, 1756. https://doi.org/10.3390/diagnostics15141756
Tirsi A, Tello I, Foster T, Kumbhani R, Leung N, Potash S, Orshan D, Tello C. Novel Structure–Function Models for Estimating Retinal Ganglion Cell Count Using Pattern Electroretinography in Glaucoma Suspects. Diagnostics. 2025; 15(14):1756. https://doi.org/10.3390/diagnostics15141756
Chicago/Turabian StyleTirsi, Andrew, Isabella Tello, Timothy Foster, Rushil Kumbhani, Nicholas Leung, Samuel Potash, Derek Orshan, and Celso Tello. 2025. "Novel Structure–Function Models for Estimating Retinal Ganglion Cell Count Using Pattern Electroretinography in Glaucoma Suspects" Diagnostics 15, no. 14: 1756. https://doi.org/10.3390/diagnostics15141756
APA StyleTirsi, A., Tello, I., Foster, T., Kumbhani, R., Leung, N., Potash, S., Orshan, D., & Tello, C. (2025). Novel Structure–Function Models for Estimating Retinal Ganglion Cell Count Using Pattern Electroretinography in Glaucoma Suspects. Diagnostics, 15(14), 1756. https://doi.org/10.3390/diagnostics15141756






