Retinal Vessel Diameter Reductions Are Associated with Retinal Ganglion Cell Dysfunction, Thinning of the Ganglion Cell and Inner Plexiform Layers, and Decreased Visual Field Global Indices in Glaucoma Suspects †
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. OCT Measurements
2.3. Optical Coherence Tomography Angiography (OCTA) Measurements of Vessel Diameter
2.4. Steady State Pattern Electroretinography
| Results | Mag | MagD | MagD/Mag ratio |
| Within reference range | >1.0 | >0.752 | >0.752 |
| Borderline reference range | 0.7–1.0 | 0.45–0.752 | 0.642–0.752 |
| Abnormal | <0.7 | <0.45 | <0.642 |
| Mag—Magnitude, MagD—MagnitudeD, MagD/Mag ratio—MagnitudeD over Magnitude ratio. | |||
2.5. Statistical Analysis
3. Results
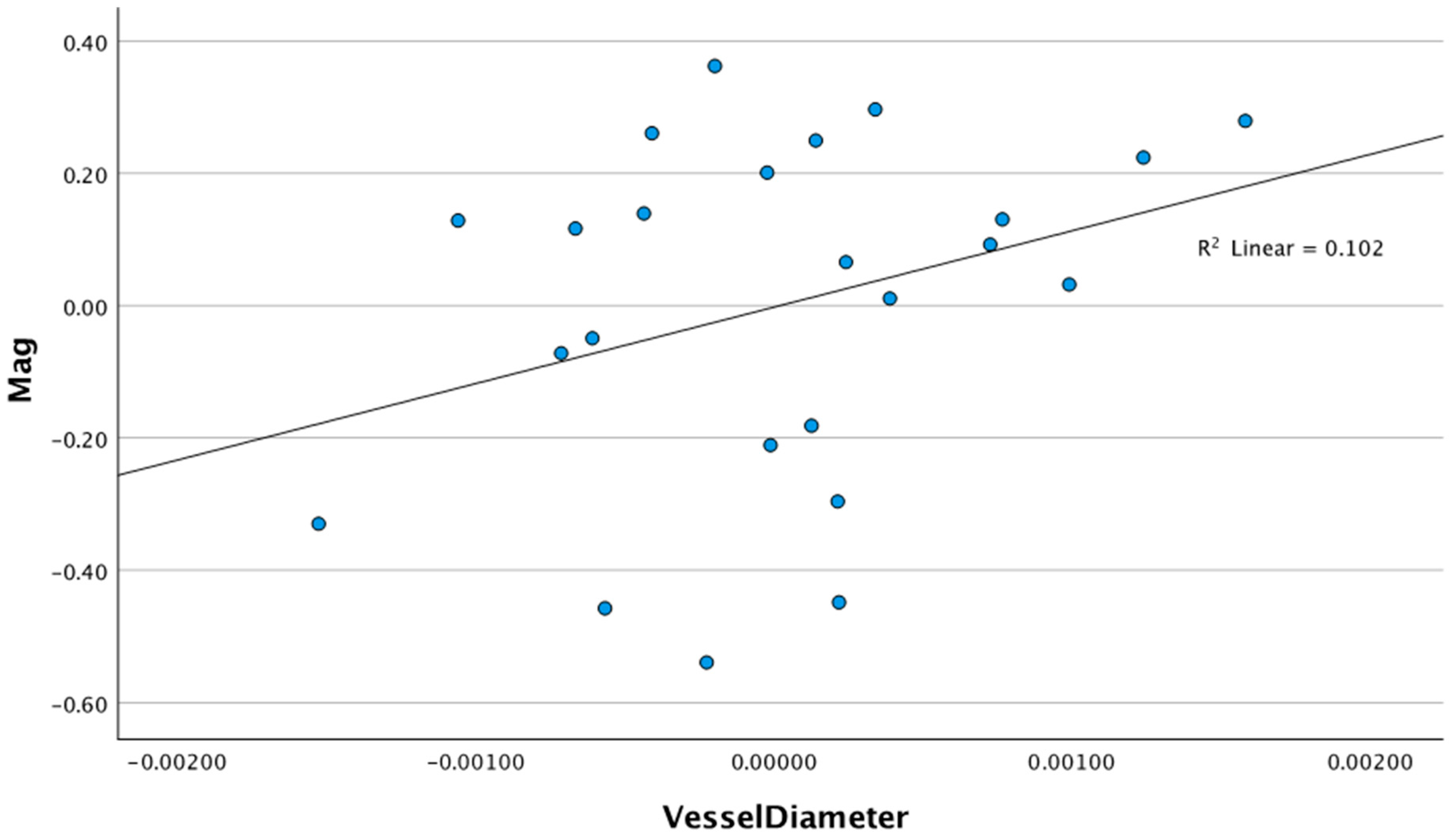
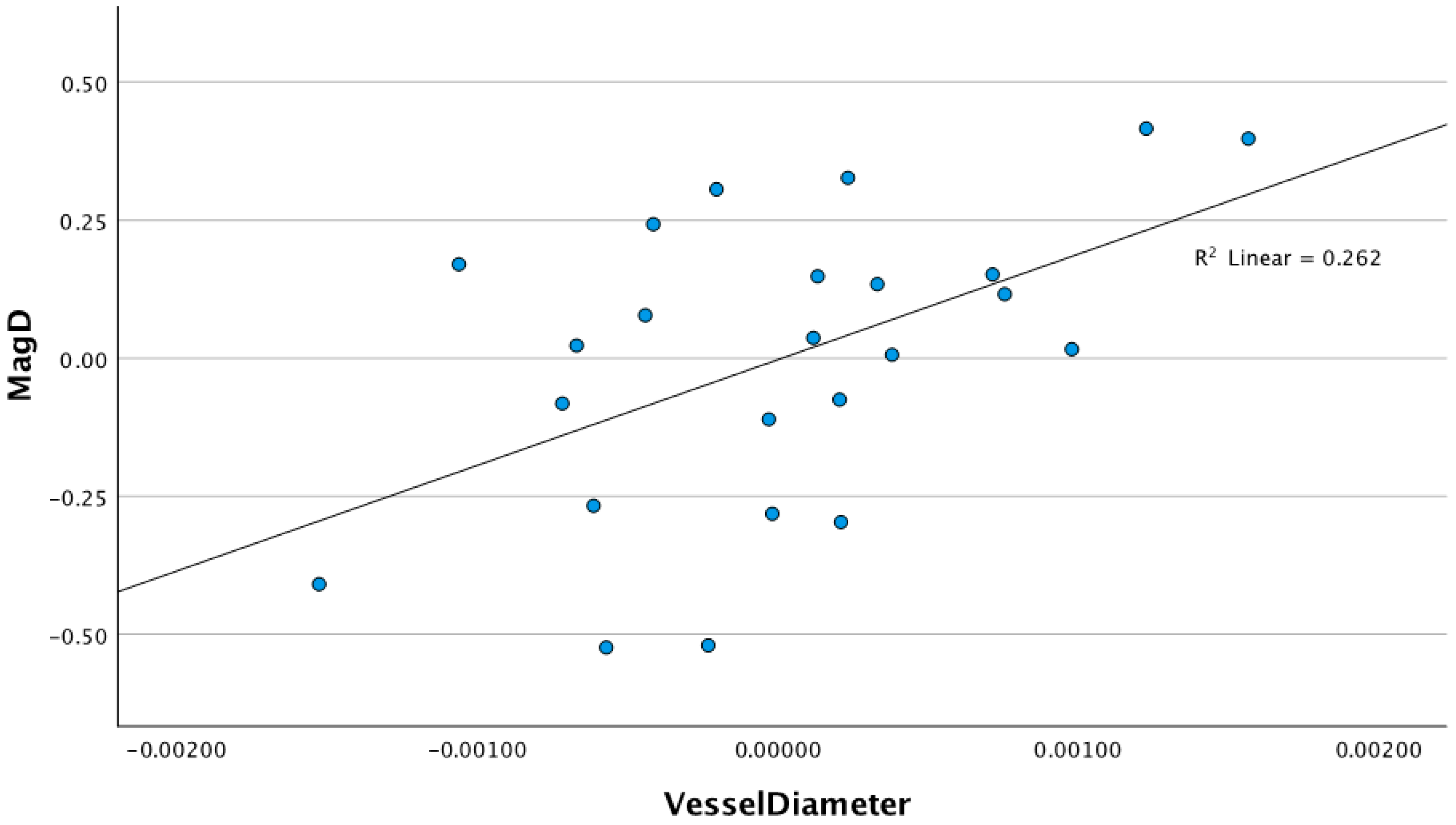
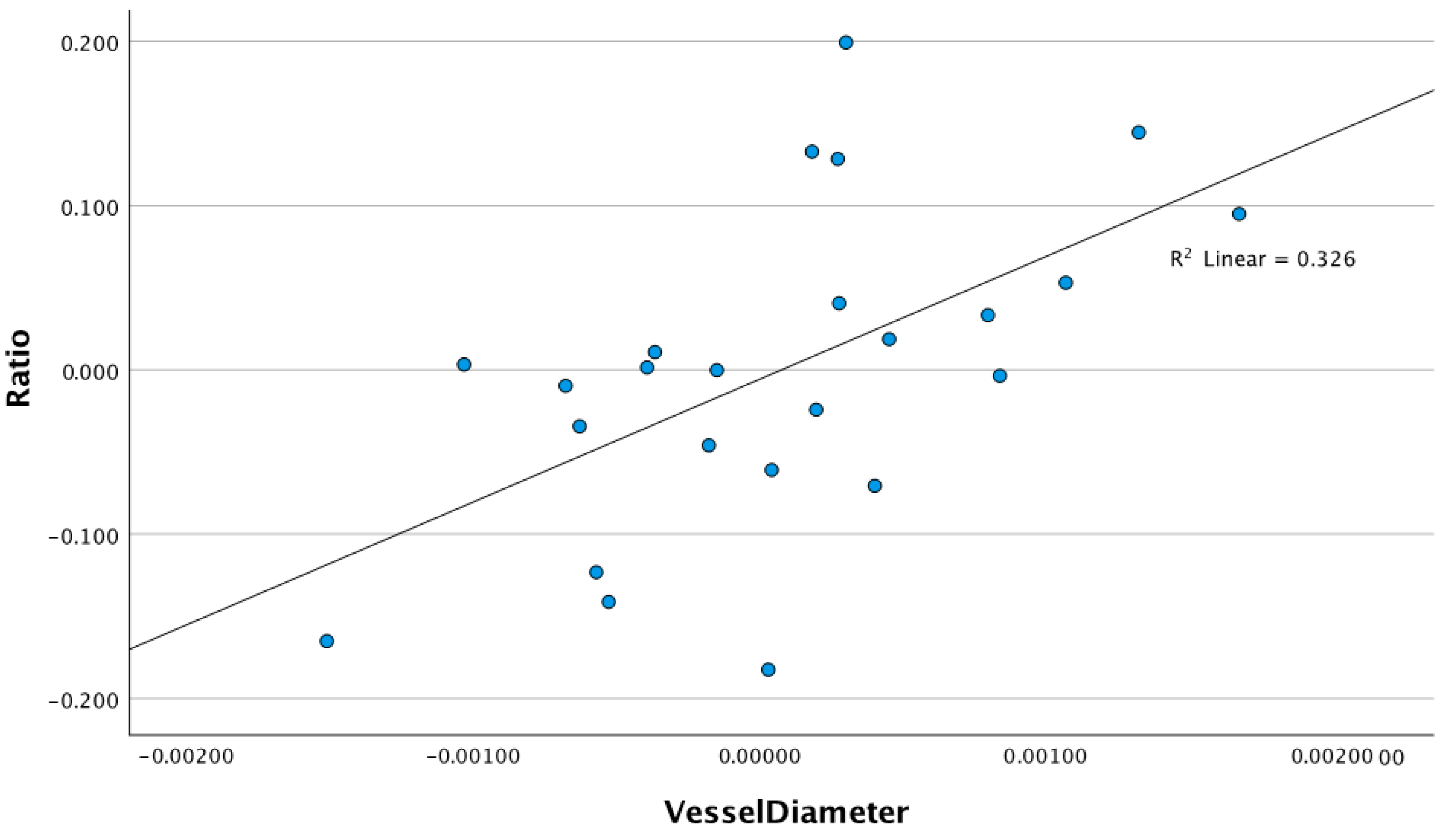
3.1. Relationship Between Retinal Vessel Diameter and ssPERG Parameters
3.2. Relationship Between Retinal Vessel Diameter and Ganglion Cell Layer-Inner Plexiform Layer Measurements
3.3. Relationship Between Retinal Vessel Diameter and Humphrey Field Analyzer Global Indices
3.4. Relationship Between ssPERG Parameters and Ganglion Cell Layer-Inner Plexiform Layer Thickness Measurements (Table 5)
|
Step 1 (Age, Signal Strength, and CCT) | Step 2 (Magnitude) | |||||
|---|---|---|---|---|---|---|
| GCL-IPLT Sectors | ΔR2 | B (95% CI) | ΔR2 | B (95% CI) | R2 | SE |
| Average | 0.521 ** | 112.5 (71.5–153.4) | 0.071 | 4.9 (−0.8–10.7) | 0.521 | 4.841 |
| Minimum | 0.445 ** | 97.5 (51.9–143.2) | 0.093 | 5.9 (−0.4–12.2) | 0.538 | 5.050 |
| Superior | 0.522 ** | 125.2 (82.4–167.9) | 0.165 ** | 7.9 (2.7–13.1) | 0.687 | 4.197 |
| Superonasal | 0.605 ** | 125.1 (83.2–167.0) | 0.076 * | 5.8 (0.1–11.5) | 0.681 | 4.574 |
| Superotemporal | 0.432 ** | 108.6 (64.6–152.6) | 0.170 ** | 7.6 (2.0–13.1) | 0.601 | 4.472 |
| Inferior | 0.801 ** | 134.9 (106.6–163.2) | 0.013 | 2.3 (−1.9–6.4) | 0.814 | 3.324 |
| Inferonasal | 0.668 ** | 131.4 (92.6–170.3) | 0.084 * | 6.1 (1.1–11.2) | 0.752 | 4.078 |
| Inferotemporal | 0.669 ** | 105.6 (74.3–136.8) | 0.020 | 2.4 (−2.1–7.0) | 0.689 | 3.670 |
4. Discussion
4.1. Retinal Vessel Diameters and Their Associations with ssPERG Parameters
4.2. Retinal Vessel Diameter and Its Association with Sectoral GCL-IPL Thickness Measurements and HVF 24-2 Global Indices
4.3. Associations Between SSPERG Parameters and GCL-IPL Thickness Measurements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stein, J.D.; Khawaja, A.P.; Weizer, J.S. Glaucoma in Adults-Screening, Diagnosis, and Management: A Review. JAMA 2021, 325, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Bourne, R.R.A.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.; Limburg, H.; et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e888–e897. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Fechtner, R.D.; Weinreb, R.N. Mechanisms of optic nerve damage in primary open angle glaucoma. Surv. Ophthalmol. 1994, 39, 23–42. [Google Scholar] [CrossRef]
- McMonnies, C.W. The interaction between intracranial pressure, intraocular pressure and lamina cribrosal compression in glaucoma. Clin. Exp. Optom. 2016, 99, 219–226. [Google Scholar] [CrossRef]
- Tielsch, J.M.; Katz, J.; Singh, K.; Quigley, H.A.; Gottsch, J.D.; Javitt, J.; Sommer, A. A population-based evaluation of glaucoma screening: The Baltimore Eye Survey. Am. J. Epidemiol. 1991, 134, 1102–1110. [Google Scholar] [CrossRef]
- Bonomi, L.; Marchini, G.; Marraffa, M.; Bernardi, P.; Morbio, R.; Varotto, A. Vascular risk factors for primary open angle glaucoma: The Egna-Neumarkt Study. Ophthalmology 2000, 107, 1287–1293. [Google Scholar] [CrossRef]
- Ster, A.M.; Popp, R.A.; Petrisor, F.M.; Stan, C.; Pop, V.I. The Role of Oxidative Stress and Vascular Insufficiency in Primary Open Angle Glaucoma. Med. Pharm. Rep. 2014, 87, 143–146. [Google Scholar] [CrossRef]
- Carter, C.J.; Brooks, D.E.; Doyle, D.L.; Drance, S.M. Investigations into a vascular etiology for low-tension glaucoma. Ophthalmology 1990, 97, 49–55. [Google Scholar] [CrossRef]
- Shughoury, A.; Mathew, S.; Arciero, J.; Wurster, P.; Adjei, S.; Ciulla, T.; Siesky, B.; Harris, A. Retinal oximetry in glaucoma: Investigations and findings reviewed. Acta Ophthalmol. 2020, 98, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Pakter, H.M.; Ferlin, E.; Fuchs, S.C.; Maestri, M.K.; Moraes, R.S.; Nunes, G.; Moreira, L.B.; Gus, M.; Fuchs, F.D. Measuring arteriolar-to-venous ratio in retinal photography of patients with hypertension: Development and application of a new semi-automated method. Am. J. Hypertens. 2005, 18, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Knudtson, M.D.; Lee, K.E.; Hubbard, L.D.; Wong, T.Y.; Klein, R.; Klein, B.E.K. Revised formulas for summarizing retinal vessel diameters. Curr. Eye Res. 2003, 27, 143–149. [Google Scholar] [CrossRef]
- Kim, J.M.; Sae Kim, M.; Ju Jang, H.; Ho Park, K.; Caprioli, J. The association between retinal vessel diameter and retinal nerve fiber layer thickness in asymmetric normal tension glaucoma patients. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5609–5614. [Google Scholar] [CrossRef]
- Tirsi, A.; Bruehl, H.; Sweat, V.; Tsui, W.; Reddy, S.; Javier, E.; Lee, C.; Convit, A. Retinal vessel abnormalities are associated with elevated fasting insulin levels and cerebral atrophy in nondiabetic individuals. Ophthalmology 2009, 116, 1175–1181. [Google Scholar] [CrossRef]
- Papastathopoulos, K.I.; Jonas, J.B. Focal narrowing of retinal arterioles in optic nerve atrophy. Ophthalmology 1995, 102, 1706–1711. [Google Scholar] [CrossRef]
- Lee, S.B.; Uhm, K.B.; Hong, C. Retinal vessel diameter in normal and primary open-angle glaucoma. Korean J. Ophthalmol. KJO 1998, 12, 51–59. [Google Scholar] [CrossRef]
- Liew, G.; Nguyen, H.; Ho, I.V.; White, A.J.; Burlutsky, G.; Gopinath, B.; Mitchell, P. Prevalence of Vitreoretinal Interface Disorders in an Australian Population: The Blue Mountains Eye Study. Ophthalmol. Sci. 2021, 1, 100019. [Google Scholar] [CrossRef]
- Adiarti, R.; Ekantini, R.; Agni, A.N.; Wong, T.Y.; Sasongko, M.B. Retinal Arteriolar Narrowing in Young Adults With Glaucomatous Optic Disc. J. Glaucoma 2018, 27, 699–702. [Google Scholar] [CrossRef]
- Rao, H.L.; Pradhan, Z.S.; Suh, M.H.; Moghimi, S.; Mansouri, K.; Weinreb, R.N. Optical Coherence Tomography Angiography in Glaucoma. J. Glaucoma 2020, 29, 312–321. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.; Liu, Q.; Chen, X.; Li, L. Comparisons of retinal vessel density and glaucomatous parameters in optical coherence tomography angiography. PLoS ONE 2020, 15, e0234816. [Google Scholar] [CrossRef]
- Nascimento ESilva, R.; Chiou, C.A.; Wang, M.; Wang, H.; Shoji, M.K.B.; Chou, J.C.; D’souza, E.E.B.; Greenstein, S.H.; Brauner, S.C.; Alves, M.R.; et al. Microvasculature of the Optic Nerve Head and Peripapillary Region in Patients With Primary Open-Angle Glaucoma. J. Glaucoma 2019, 28, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, A.; Zangwill, L.M.; Diniz-Filho, A.; Suh, M.H.; Yousefi, S.; Saunders, L.J.; Belghith, A.; Manalastas, P.I.C.; Medeiros, F.A.; Weinreb, R.N. Relationship between Optical Coherence Tomography Angiography Vessel Density and Severity of Visual Field Loss in Glaucoma. Ophthalmology 2016, 123, 2498–2508. [Google Scholar] [CrossRef] [PubMed]
- Banitt, M.R.; Ventura, L.M.; Feuer, W.J.; Savatovsky, E.; Luna, G.; Shif, O.; Bosse, B.; Porciatti, V. Progressive loss of retinal ganglion cell function precedes structural loss by several years in glaucoma suspects. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2346–2352. [Google Scholar] [CrossRef]
- Porciatti, V.; Ventura, L.M. Normative data for a user-friendly paradigm for pattern electroretinogram recording. Ophthalmology 2004, 111, 161–168. [Google Scholar] [CrossRef]
- Gillmann, K.; Mansouri, K.; Rao, H.L.; Ouabas, A.; Chaudhary, A.; Bravetti, G.E.; Mermoud, A. A Prospective Evaluation of the Repeatability and Reliability of New Steady-state Pattern Electroretinogram Parameters. J. Glaucoma 2018, 27, 1079–1085. [Google Scholar] [CrossRef]
- Ventura, L.M.; Porciatti, V. Pattern electroretinogram in glaucoma. Curr. Opin. Ophthalmol. 2006, 17, 196–202. [Google Scholar] [CrossRef]
- Gordon, P.S.; Kostic, M.; Monsalve, P.F.; Triolo, G.; Golubev, L.; Luna, G.; Ventura, L.M.; Feuer, W.J.; Porciatti, V. Long-term PERG monitoring of untreated and treated glaucoma suspects. Doc. Ophthalmol. Adv. Ophthalmol. 2020, 141, 149–156. [Google Scholar] [CrossRef]
- Mavilio, A.; Scrimieri, F.; Errico, D. Can Variability of Pattern ERG Signal Help to Detect Retinal Ganglion Cells Dysfunction in Glaucomatous Eyes? BioMed Res. Int. 2015, 2015, 571314. [Google Scholar] [CrossRef]
- Gedde, S.J.; Lind, J.T.; Wright, M.M.; Chen, P.P.; Muir, K.W.; Vinod, K.; Li, T.; Mansberger, S.L. Primary Open-Angle Glaucoma Suspect Preferred Practice Pattern®. Ophthalmology 2021, 128, P151–P192. [Google Scholar] [CrossRef]
- Brusini, P.; Filacorda, S. Enhanced Glaucoma Staging System (GSS 2) for classifying functional damage in glaucoma. J. Glaucoma 2006, 15, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Mwanza, J.C.; Oakley, J.D.; Budenz, D.L.; Anderson, D.R.; Cirrus Optical Coherence Tomography Normative Database Study Group. Ability of cirrus HD-OCT optic nerve head parameters to discriminate normal from glaucomatous eyes. Ophthalmology 2011, 118, 241–248.e1. [Google Scholar] [CrossRef] [PubMed]
- Mwanza, J.C.; Durbin, M.K.; Budenz, D.L.; Girkin, C.A.; Leung, C.K.; Liebmann, J.M.; Peace, J.H.; Werner, J.S.; Wollstein, G. Profile and predictors of normal ganglion cell-inner plexiform layer thickness measured with frequency-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7872–7879. [Google Scholar] [CrossRef] [PubMed]
- Munk, M.R.; Giannakaki-Zimmermann, H.; Berger, L.; Huf, W.; Ebneter, A.; Wolf, S.; Zinkernagel, M.S.; Stieger, K. OCT-angiography: A qualitative and quantitative comparison of 4 OCT-A devices. PLoS ONE 2017, 12, e0177059. [Google Scholar] [CrossRef]
- Vessel Analysis. ImageJ Wiki. Available online: https://imagej.github.io/plugins/vessel-analysis (accessed on 26 September 2023).
- Liuhanen, S.; Sallisalmi, M.; Pettilä, V.; Oksala, N.; Tenhunen, J. Indirect measurement of the vascular endothelial glycocalyx layer thickness in human submucosal capillaries with a plug-in for ImageJ. Comput. Methods Programs Biomed. 2013, 110, 38–47. [Google Scholar] [CrossRef]
- Fredette, M.J.; Anderson, D.R.; Porciatti, V.; Feuer, W. Reproducibility of pattern electroretinogram in glaucoma patients with a range of severity of disease with the new glaucoma paradigm. Ophthalmology 2008, 115, 957–963. [Google Scholar] [CrossRef]
- Bach, M.; Brigell, M.G.; Hawlina, M.; Holder, G.E.; Johnson, M.A.; McCulloch, D.L.; Meigen, T.; Viswanathan, S. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc. Ophthalmol. Adv. Ophthalmol. 2013, 126, 1–7. [Google Scholar] [CrossRef]
- Tirsi, A.; Wong, A.; Zhu, D.; Stoffels, G.; Derr, P.; Tello Md, C. Pattern Electroretinogram Parameters and their Associations with Optical Coherence Tomography in Glaucoma Suspects. J. Curr. Glaucoma Pract. 2022, 16, 96–104. [Google Scholar] [CrossRef]
- Tirsi, A.; Orshan, D.; Wong, B.; Gliagias, V.; Tsai, J.; Obstbaum, S.A.; Tello, C. Associations between steady-state pattern electroretinography and estimated retinal ganglion cell count in glaucoma suspects. Doc. Ophthalmol. Adv. Ophthalmol. 2022, 145, 11–25. [Google Scholar] [CrossRef]
- Tirsi, A.; Shah, P.P.; Gliagias, V.; Barmas-Alamdari, D.; Orshan, D.; Tsai, J.; Tello, C. Posterior Pole Asymmetry Analysis as a Diagnostic Tool in Glaucoma Suspects: An Electrophysiological Approach. Clin. Ophthalmol. 2023, 17, 1777–1787. [Google Scholar] [CrossRef]
- Tirsi, A.; Gliagias, V.; Moehringer, J.; Orshan, D.; Tello, S.; Derr, P.; Park, S.C.; Obstbaum, S.A.; Tello, C.; Monteiro, M. Pattern Electroretinogram Parameters Are Associated with Optic Nerve Morphology in Preperimetric Glaucoma after Adjusting for Disc Area. J. Ophthalmol. 2021, 2021, 8025337. [Google Scholar] [CrossRef] [PubMed]
- Orshan, D.; Tirsi, A.; Sheha, H.; Gliagias, V.; Tsai, J.; Park, S.C.; Obstbaum, S.A.; Tello, C. Structure-function models for estimating retinal ganglion cell count using steady-state pattern electroretinography and optical coherence tomography in glaucoma suspects and preperimetric glaucoma: An electrophysiological pilot study. Doc. Ophthalmol. Adv. Ophthalmol. 2022, 145, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Ventura, L.M.; Porciatti, V.; Ishida, K.; Feuer, W.J.; Parrish, R.K. Pattern electroretinogram abnormality and glaucoma. Ophthalmology 2005, 112, 10–19. [Google Scholar] [CrossRef]
- Oner, A.; Gumus, K.; Arda, H.; Karakucuk, S.; Mirza, E. Pattern electroretinographic recordings in eyes with myopia. Eye Contact Lens 2009, 35, 238–241. [Google Scholar] [CrossRef]
- Sng, C.C.A.; Ang, M.; Barton, K. Central corneal thickness in glaucoma. Curr. Opin. Ophthalmol. 2017, 28, 120–126. [Google Scholar] [CrossRef]
- Ooto, S.; Hangai, M.; Tomidokoro, A.; Saito, H.; Araie, M.; Otani, T.; Kishi, S.; Matsushita, K.; Maeda, N.; Shirakashi, M.; et al. Effects of age, sex, and axial length on the three-dimensional profile of normal macular layer structures. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8769–8779. [Google Scholar] [CrossRef]
- Vizzeri, G.; Bowd, C.; Medeiros, F.A.; Weinreb, R.N.; Zangwill, L.M. Effect of signal strength and improper alignment on the variability of stratus optical coherence tomography retinal nerve fiber layer thickness measurements. Am. J. Ophthalmol. 2009, 148, 249–255.e1. [Google Scholar] [CrossRef]
- Papadia, M.; Sofianos, C.; Iester, M.; Bricola, G.; Mete, M.; Traverso, C.E. Corneal thickness and visual field damage in glaucoma patients. Eye 2007, 21, 943–947. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Wang, Y.; Wang, Y.; Jonas, J.B. Retinal vessel diameter in normal and glaucomatous eyes: The Beijing eye study. Clin. Experiment Ophthalmol. 2007, 35, 800–807. [Google Scholar] [CrossRef]
- Lee, T.H.; Lim, H.B.; Nam, K.Y.; Kim, K.; Kim, J.Y. Factors Affecting Repeatability of Assessment of the Retinal Microvasculature Using Optical Coherence Tomography Angiography in Healthy Subjects. Sci. Rep. 2019, 9, 16291. [Google Scholar] [CrossRef]
- Hall, J.K.; Andrews, A.P.; Walker, R.; Piltz-Seymour, J.R. Association of retinal vessel caliber and visual field defects in glaucoma. Am. J. Ophthalmol. 2001, 132, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, J.; Coleman, A.L.; Blood Flow in Glaucoma Discussion. Blood pressure, perfusion pressure, and glaucoma. Am. J. Ophthalmol. 2010, 149, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Siesky, B.A.; Harris, A.; Amireskandari, A.; Marek, B. Glaucoma and ocular blood flow: An anatomical perspective. Expert. Rev. Ophthalmol. 2012, 7, 325–340. [Google Scholar] [CrossRef]
- Shen, W.C.; Huang, B.Q.; Yang, J. Regulatory mechanisms of retinal ganglion cell death in normal tension glaucoma and potential therapies. Neural Regen. Res. 2022, 18, 87–93. [Google Scholar] [CrossRef]
- Porciatti, V.; Ventura, L.M. Retinal Ganglion Cell Functional Plasticity and Optic Neuropathy: A Comprehensive Model. J. Neuro-Ophthalmol. Off. J. N. Am. Neuro-Ophthalmol. Soc. 2012, 32, 354–358. [Google Scholar] [CrossRef]
- Doganay, S.; Evereklioglu, C.; Turkoz, Y.; Er, H. Decreased nitric oxide production in primary open-angle glaucoma. Eur. J. Ophthalmol. 2002, 12, 44–48. [Google Scholar] [CrossRef]
- Resch, H.; Garhofer, G.; Fuchsjäger-Mayrl, G.; Hommer, A.; Schmetterer, L. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009, 87, 4–12. [Google Scholar] [CrossRef]
- Emre, M.; Orgül, S.; Haufschild, T.; Shaw, S.G.; Flammer, J. Increased plasma endothelin-1 levels in patients with progressive open angle glaucoma. Br. J. Ophthalmol. 2005, 89, 60–63. [Google Scholar] [CrossRef]
- Tham, Y.C.; Cheng, C.Y.; Zheng, Y.; Aung, T.; Wong, T.Y.; Cheung, C.Y. Relationship between retinal vascular geometry with retinal nerve fiber layer and ganglion cell-inner plexiform layer in nonglaucomatous eyes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7309–7316. [Google Scholar] [CrossRef]
- Wu, R.; Cheung, C.Y.L.; Saw, S.M.; Mitchell, P.; Aung, T.; Wong, T.Y. Retinal vascular geometry and glaucoma: The Singapore Malay Eye Study. Ophthalmology 2013, 120, 77–83. [Google Scholar] [CrossRef]
- Porciatti, V.; Ventura, L.M. Physiologic significance of steady-state pattern electroretinogram losses in glaucoma: Clues from simulation of abnormalities in normal subjects. J. Glaucoma 2009, 18, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Crowston, J.G.; Fahy, E.T.; Fry, L.; Trounce, I.A.; van Wijngaarden, P.; Petrou, S.; Chrysostomou, V. Targeting retinal ganglion cell recovery. Eye 2017, 31, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef] [PubMed]
- Della Santina, L.; Inman, D.M.; Lupien, C.B.; Horner, P.J.; Wong, R.O.L. Differential progression of structural and functional alterations in distinct retinal ganglion cell types in a mouse model of glaucoma. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 17444–17457. [Google Scholar] [CrossRef]
- Zhong, Z.; Petrig, B.L.; Qi, X.; Burns, S.A. In vivo measurement of erythrocyte velocity and retinal blood flow using adaptive optics scanning laser ophthalmoscopy. Opt. Express 2008, 16, 12746–12756. [Google Scholar] [CrossRef]
- Warner, R.L.; de Castro, A.; Sawides, L.; Gast, T.; Sapoznik, K.; Luo, T.; Burns, S.A. Full-field flicker evoked changes in parafoveal retinal blood flow. Sci. Rep. 2020, 10, 16051. [Google Scholar] [CrossRef]
- Shin, J.W.; Sung, K.R.; Song, M.K. Ganglion Cell-Inner Plexiform Layer and Retinal Nerve Fiber Layer Changes in Glaucoma Suspects Enable Prediction of Glaucoma Development. Am. J. Ophthalmol. 2020, 210, 26–34. [Google Scholar] [CrossRef]
- De Leon, J.M.S.; Cheung, C.Y.; Wong, T.Y.; Li, X.; Hamzah, H.; Aung, T.; Su, D.H.-W. Retinal vascular caliber between eyes with asymmetric glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 583–589. [Google Scholar] [CrossRef]
- Lam, A.; Bunya, V.; Piltz-Seymour, J.R. Visual field loss in patients with glaucoma who have asymmetric peripapillary focal arteriolar narrowing. Arch. Ophthalmol. 2002, 120, 1494–1497. [Google Scholar] [CrossRef]
- Rao, A.; Agarwal, K.; Mudunuri, H.; Padhy, D.; Roy, A.K.; Mukherjee, S. Vessel Caliber in Normal Tension and Primary Open Angle Glaucoma Eyes With Hemifield Damage. J. Glaucoma 2017, 26, 46–53. [Google Scholar] [CrossRef]
- Chiquet, C.; Gavard, O.; Arnould, L.; Mautuit, T.; Macgillivray, T.J.; Bron, A.M.; Semecas, R.; Trucco, E.; Florent, A. Retinal vessel phenotype in patients with primary open-angle glaucoma. Acta Ophthalmol. 2020, 98, e88–e93. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, M.; Dayanir, V.; Zlatanovic, M.; Zlatanovic, G.; Jaksic, V.; Jovanovic, P.; Radenkovic, M.; Djordjevic-Jocic, J.; Stankovic-Babic, G.; Jovanovic, S. Ganglion Cell-Inner Plexiform Layer Thickness in Different Glaucoma Stages Measured by Optical Coherence Tomography. Ophthalmic Res. 2018, 59, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Sabel, B.A. Vascular dysregulation in glaucoma: Retinal vasoconstriction and normal neurovascular coupling in altitudinal visual field defects. EPMA J. 2023, 14, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.C.; Konduru, R.; Zhang, X.; Tan, O.; Francis, B.A.; Varma, R.; Sehi, M.; Greenfield, D.S.; Sadda, S.R.; Huang, D. Relationship among Visual Field, Blood Flow, and Neural Structure Measurements in Glaucoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3020–3026. [Google Scholar] [CrossRef]
- Cheng, J.; Zhao, H.; Jiang, C.; Kong, X.; Sun, X. Change of Retinal Vessels in Different Sectors of the Parapapillary Area in Primary Open-Angle Glaucoma. Front. Med. 2021, 8, 705829. [Google Scholar] [CrossRef]
- Yoo, E.; Yoo, C.; Lee Bram Lee, T.E.; Kim, Y.Y. Diagnostic Ability of Retinal Vessel Diameter Measurements in Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7915–7922. [Google Scholar] [CrossRef]
- Yoo, E.; Yoo, C.; Lee, T.E.; Kim, Y.Y. Retinal vessel diameter in bilateral glaucoma suspects: Comparison between the eye converted to glaucoma and the contralateral non-converted eye. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1599–1608. [Google Scholar] [CrossRef]
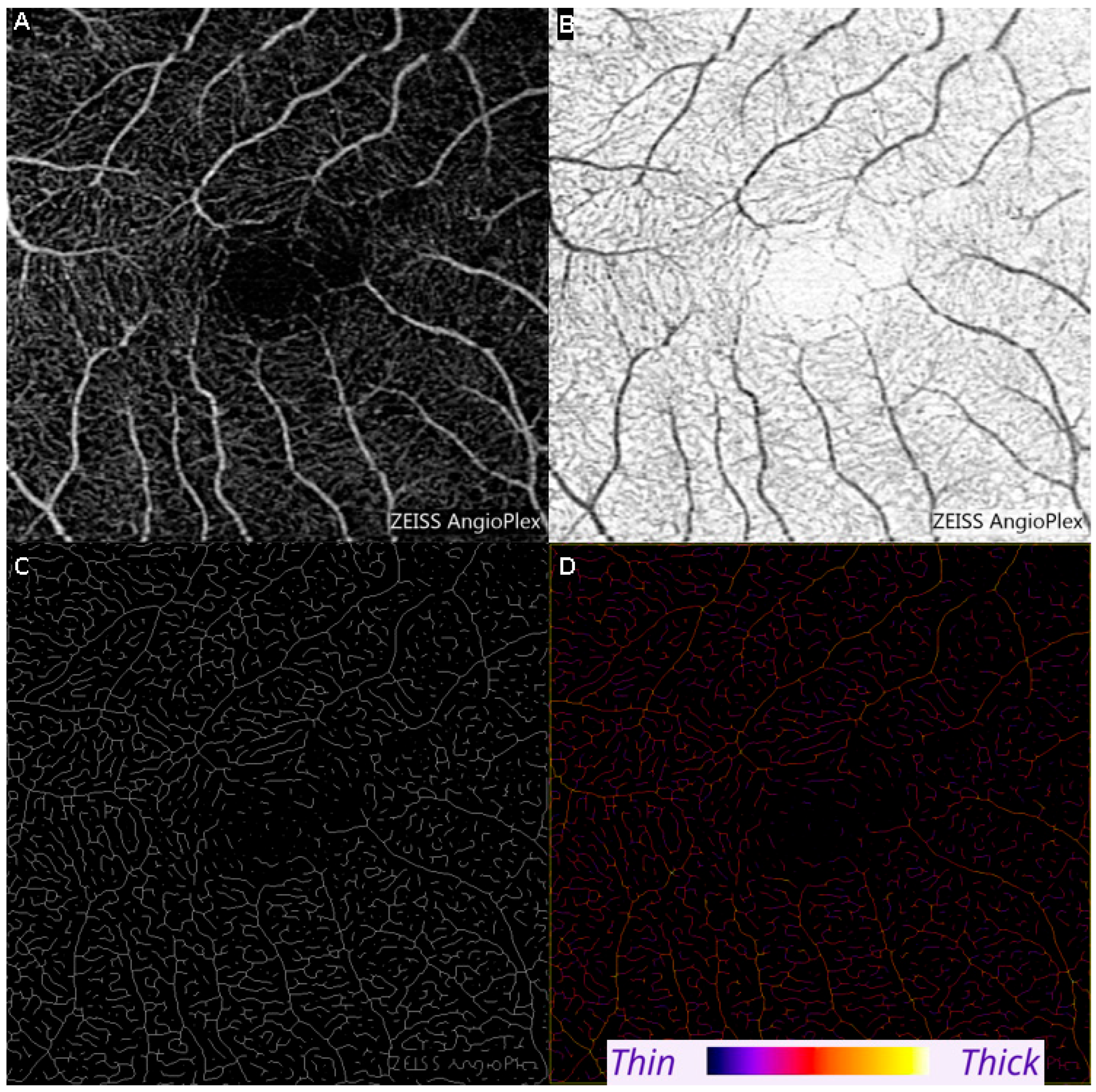
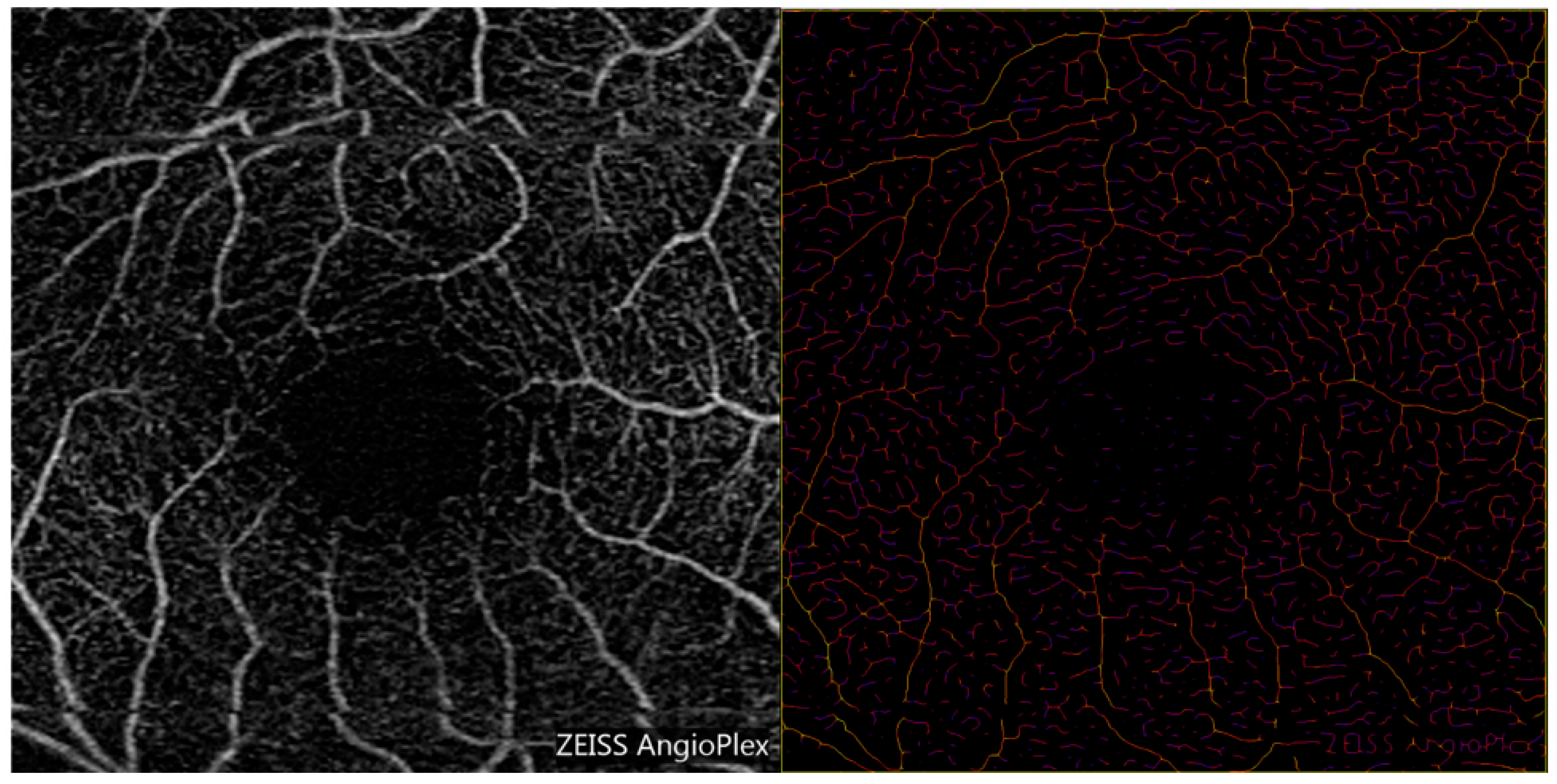
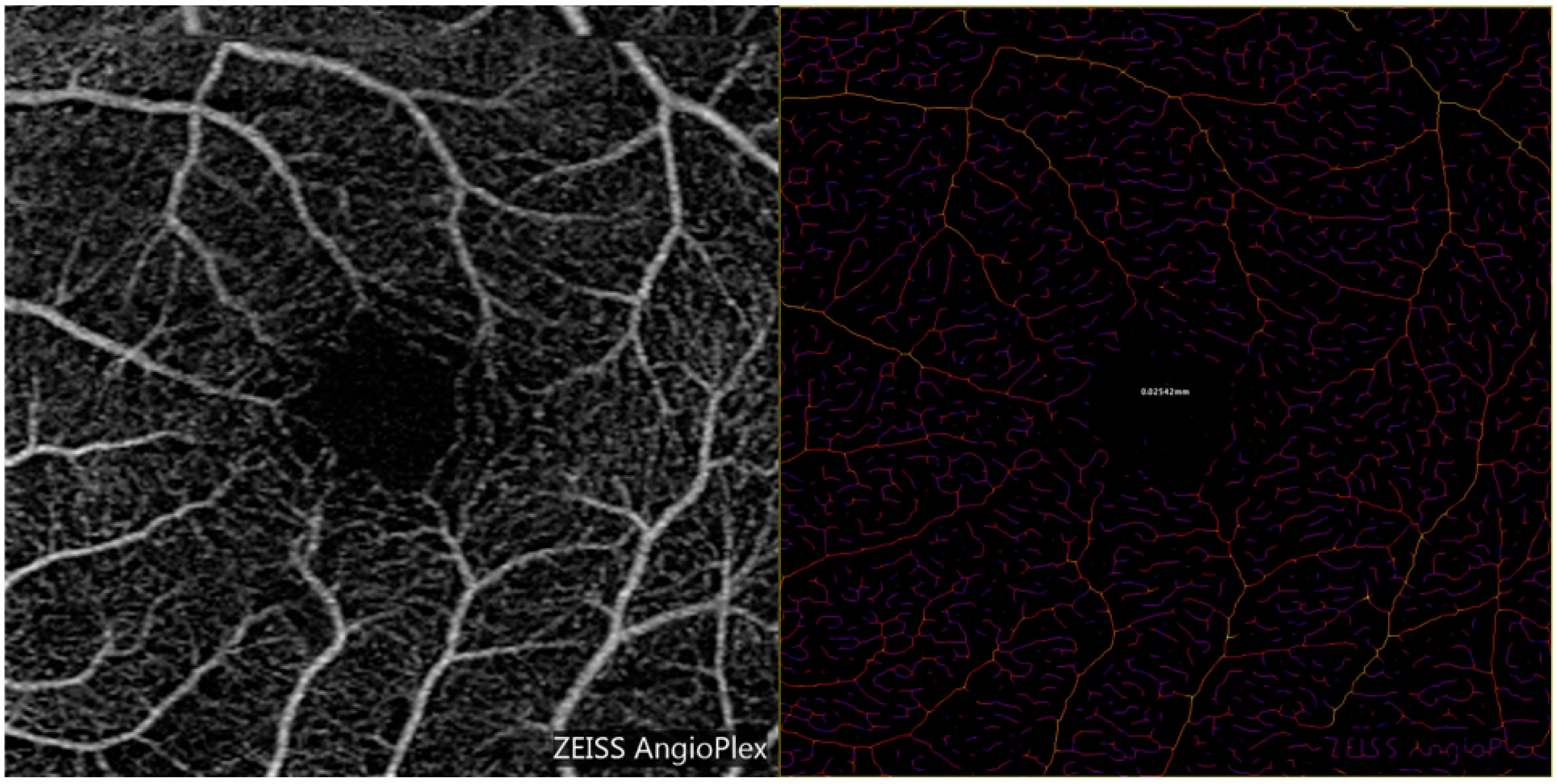
| Mean | Minimum | Maximum | |
|---|---|---|---|
| Age (years) | 59.89 ± 14.20 | 28 | 78 |
| Sex (% females) | 20 F (64.51%) | N/A | N/A |
| Race C/H/A | 25/4/2 | N/A | N/A |
| IOP (mm Hg) | 18.29 ± 4.46 | 10 | 26 |
| CCT (µm) | 551.67 ± 29.22 | 475 | 593 |
| SE (diopters) | −0.53 ± 2.34 | −5.75 | 4 |
| Humphrey Field Analyzer global indices | |||
| HFA MD 24-2 (dB) | 0.32 ± 0.94 | −2.86 | 1.60 |
| HFA PSD 24-2 (dB) | 1.52 ± 0.33 | 0.90 | 2.43 |
| HFA VFI 24-2 (%) | 99.25 ± 0.63 | 96 | 100 |
| Steady state pattern electroretinography | |||
| Magnitude (mv) | 1.62 ± 0.54 | 0.97 | 3.45 |
| MagnitudeD (mv) | 1.34 ± 0.58 | 0.50 | 2.90 |
| MagD/Mag ratio | 0.81 ± 0.14 | 0.43 | 0.87 |
| OCT-A based retinal vessel diameter | |||
| Vessel diameter (µm) | 2.42 ± 0.098 | 2.27 | 2.64 |
| Quadrants or Sectors | Mean | Minimum | Maximum |
|---|---|---|---|
| RNFL thickness measurements by quadrants | |||
| Average | 89.84 ± 9.54 | 77 | 113 |
| Superior quadrant | 105.29 ± 16.39 | 73 | 79 |
| Temporal quadrant | 65.38 ± 11.56 | 50 | 102 |
| Inferior quadrant | 117.10 ± 13.50 | 88 | 146 |
| Nasal quadrant | 69.94 ± 8.50 | 57 | 88 |
| Ganglion cell layer-inner plexiform layer thickness by sectors | |||
| Average | 78.57 ± 6.74 | 64 | 90 |
| Minimum | 76.74 ± 6.63 | 61 | 89 |
| Inferior sector | 79.41 ± 6.77 | 59 | 90 |
| Inferonasal sector | 78.67 ± 7.56 | 63 | 92 |
| Inferotemporal sector | 79.57 ± 6.54 | 65 | 91 |
| Superior sector | 77.64 ± 7.09 | 63 | 92 |
| Superonasal sector | 81.03 ± 10.13 | 64 | 92 |
| Superotemporal sector | 77.58 ± 7.39 | 64 | 94 |
| Step 1 (Age, Race, CCT, SE) | Step 2 (Vessel Diameter) | |||||
|---|---|---|---|---|---|---|
| ΔR2 | B (95% CI) | ΔR2 | B (95% CI) | R2 | SE | |
| Magnitude (mV) | 0.542 | 1.53 (−0.91–3.98) | 0.047 | 115.50 (−53.95–284.96) | 0.589 | 0.280 |
| MagnitudeD (mV) | 0.648 | 2.45 (−0.074–4.97) | 0.092 * | 190.34 (31.95–348.74) | 0.740 | 0.263 |
| MagD/Mag ratio | 0.485 | 1.80 (0.89–2.71) | 0.169 ** | 190.34 (31.95–348.74) | 0.651 | 0.090 |
|
Step 1 (Age, Signal Strength, and CCT) | Step 2 (Vessel Diameter) | |||||
|---|---|---|---|---|---|---|
| GCL-IPLT Sectors | ΔR2 | B (95% CI) | ΔR2 | B (95% CI) | R2 | SE |
| Average | 0.139 | 93.4 (38.1–160.8) | 0.286 ** | 4039.4 (1291.2–6787.6) | 0.304 | 5.440 |
| Minimum | 0.076 | 84.8 (19.0–150.6) | 0.371 ** | 4759.9 (1966.6–7553.1) | 0.447 | 5.530 |
| Superior | 0.280 | 136.6 (77.9–195.3) | 0.231 ** | 3792.0 (1139.0–6445.0) | 0.510 | 5.252 |
| Superonasal | 0.253 | 131.4 (67.0–195.9) | 0.206 * | 3865.8 (859.5–6872.2) | 0.459 | 5.952 |
| Superotemporal | 0.272 | 124.5 (68.8–180.1) | 0.110 | 2466.2 (−346.2–5278.8) | 0.382 | 5.568 |
| Inferior | 0.322 * | 137.6 (79.1–196.0) | 0.164 * | 3288.4 (487.4–6075.5) | 0.486 | 5.522 |
| Inferonasal | 0.300 | 134.4 (71.3–197.5) | 0.196 * | 3810.6 (872.3–6748.9) | 0.495 | 5.817 |
| Inferotemporal | 0.210 | 106.1 (52.1–160.0) | 0.088 | 2053.4 (−733.5–4840.4) | 0.298 | 5.517 |
|
Step 1 (Age, Signal Strength,
and CCT) | Step 2 (MagnitudeD) | |||||
|---|---|---|---|---|---|---|
| GCL-IPLT Sectors (µm) | ΔR2 | B (95% CI) | ΔR2 | B (95% CI) | R2 | SE |
| Average | 0.521 ** | 112.5 (71.5–153.4) | 0.130 * | 5.9 (1.3–10.6) | 0.652 | 4.237 |
| Minimum | 0.445 ** | 97.5 (51.9–143.2) | 0.185 ** | 7.3 (2.3–12.3) | 0.630 | 4.522 |
| Superior | 0.522 ** | 125.2 (82.4–167.9) | 0.179 ** | 7.3 (2.8–11.8) | 0.701 | 4.103 |
| Superonasal | 0.605 ** | 125.1 (83.2–167.0) | 0.062 | 4.6 (−0.5–9.7) | 0.666 | 4.675 |
| Superotemporal | 0.432 ** | 108.6 (64.6–152.6) | 0.173 ** | 6.8 (1.9–11.7) | 0.605 | 4.452 |
| Inferior | 0.801 ** | 134.9 (106.6–163.2) | 0.014 | 2.1 (−1.6–5.7) | 0.815 | 3.316 |
| Inferonasal | 0.668 ** | 131.4 (92.6–170.3) | 0.062 * | 4.7 (0.0–9.4) | 0.731 | 4.250 |
| Inferotemporal | 0.669 ** | 105.6 (74.3–136.8) | 0.040 | 3.0 (−0.9–6.9) | 0.709 | 3.550 |
| Step 1 (Age, Signal Strength, and CCT) | Step 2 (MagD/Mag Ratio) | |||||
|---|---|---|---|---|---|---|
| GCL-IPLT Sectors | ΔR2 | B (95% CI) | ΔR2 | B (95% CI) | R2 | SE |
| Average | 0.521 ** | 112.5 (71.5–153.4) | 0.185 ** | 24.6 (9.7–39.5) | 0.706 | 3.892 |
| Minimum | 0.445 ** | 97.5 (51.9–143.2) | 0.298 ** | 32.4 (17.9–46.8) | 0.744 | 3.762 |
| Superior | 0.522 ** | 125.2 (82.4–167.9) | 0.093 * | 18.2 (0.4–36.0) | 0.615 | 4.658 |
| Superonasal | 0.605 ** | 125.1 (83.2–167.0) | 0.017 | 8.4 (−10.7–27.4) | 0.622 | 4.980 |
| Superotemporal | 0.432 ** | 108.6 (64.6–152.6) | 0.075 | 15.5 (−3.5–34.6) | 0.507 | 4.973 |
| Inferior | 0.801 ** | 134.9 (106.6–163.2) | 0.013 | 7.1 (−5.6–19.8) | 0.814 | 3.319 |
| Inferonasal | 0.668 ** | 131.4 (92.6–170.3) | 0.014 | 7.7 (−9.9–25.4) | 0.682 | 4.614 |
| Inferotemporal | 0.669 ** | 105.6 (74.3–136.8) | 0.048 | 11.5 (−1.9–24.9) | 0.717 | 3.503 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirsi, A.; Leung, N.; Gupta, R.; Hong, S.; Orshan, D.; Tsai, J.; Lacher, C.R.; Tello, I.; Potash, S.; Foster, T.; et al. Retinal Vessel Diameter Reductions Are Associated with Retinal Ganglion Cell Dysfunction, Thinning of the Ganglion Cell and Inner Plexiform Layers, and Decreased Visual Field Global Indices in Glaucoma Suspects. Diagnostics 2025, 15, 1700. https://doi.org/10.3390/diagnostics15131700
Tirsi A, Leung N, Gupta R, Hong S, Orshan D, Tsai J, Lacher CR, Tello I, Potash S, Foster T, et al. Retinal Vessel Diameter Reductions Are Associated with Retinal Ganglion Cell Dysfunction, Thinning of the Ganglion Cell and Inner Plexiform Layers, and Decreased Visual Field Global Indices in Glaucoma Suspects. Diagnostics. 2025; 15(13):1700. https://doi.org/10.3390/diagnostics15131700
Chicago/Turabian StyleTirsi, Andrew, Nicholas Leung, Rohun Gupta, Sungmin Hong, Derek Orshan, Joby Tsai, Corey Ross Lacher, Isabella Tello, Samuel Potash, Timothy Foster, and et al. 2025. "Retinal Vessel Diameter Reductions Are Associated with Retinal Ganglion Cell Dysfunction, Thinning of the Ganglion Cell and Inner Plexiform Layers, and Decreased Visual Field Global Indices in Glaucoma Suspects" Diagnostics 15, no. 13: 1700. https://doi.org/10.3390/diagnostics15131700
APA StyleTirsi, A., Leung, N., Gupta, R., Hong, S., Orshan, D., Tsai, J., Lacher, C. R., Tello, I., Potash, S., Foster, T., Kumbhani, R., & Tello, C. (2025). Retinal Vessel Diameter Reductions Are Associated with Retinal Ganglion Cell Dysfunction, Thinning of the Ganglion Cell and Inner Plexiform Layers, and Decreased Visual Field Global Indices in Glaucoma Suspects. Diagnostics, 15(13), 1700. https://doi.org/10.3390/diagnostics15131700






