Dynamic Echocardiographic Changes Induced by Exercise in Healthy, Young Individuals with Early Repolarization Pattern
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Echocardiographic Imaging and Analysis
2.3. Treadmill Exercise Testing
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. ECG Characteristics
3.3. Treadmill Exercise Test Results
3.4. Echocardiographic Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antzelevitch, C.; Dendramis, G. Genetics, Molecular Biology, and Emerging Concepts of Early Repolarization Syndrome. In Cardiac Repolarization: Basic Science and Clinical Management, 1st ed.; El-Sherif, N., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 255–262. [Google Scholar]
- Zeppenfeld, K.; Tfelt-Hansen, J.; De Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; De Chillou, C.; et al. 2022 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Elenizi, K. Prevalence and Clinical Significance of Early Repolarization in Athletes: A Systematic Review. Ann. Noninvasive Electrocardiol. 2025, 30, e70032. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-Y.; Hu, N.; Liu, R.; Zhou, H.-R.; Gao, W.-L.; Quan, X.-Q. Worldwide Prevalence of Early Repolarization Pattern in General Population and Physically Active Individuals: A Meta-Analysis. Medicine 2021, 100, e25978. [Google Scholar] [CrossRef] [PubMed]
- Haïssaguerre, M.; Nademanee, K.; Hocini, M.; Cheniti, G.; Duchateau, J.; Frontera, A.; Sacher, F.; Derval, N.; Denis, A.; Pambrun, T.; et al. Depolarization versus Repolarization Abnormality Underlying Inferolateral J-Wave Syndromes: New Concepts in Sudden Cardiac Death with Apparently Normal Hearts. Heart Rhythm 2019, 16, 781–790. [Google Scholar] [CrossRef]
- Badura, K.; Buławska, D.; Dąbek, B.; Witkowska, A.; Lisińska, W.; Radzioch, E.; Skwira, S.; Młynarska, E.; Rysz, J.; Franczyk, B. Primary Electrical Heart Disease—Principles of Pathophysiology and Genetics. Int. J. Mol. Sci. 2024, 25, 1826. [Google Scholar] [CrossRef]
- Morita, H.; Asada, S.; Ueoka, A.; Mizuno, T.; Masuda, T.; Miyamoto, M.; Kawada, S.; Nakagawa, K.; Nishii, N.; Yuasa, S. Risk Stratification for the Occurrence of Ventricular Fibrillation in Patients with Early Repolarization Syndrome. Heart Rhythm 2024, 21, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hocini, M.; Strom, M.; Cuculich, P.S.; Cooper, D.H.; Sacher, F.; Haïssaguerre, M.; Rudy, Y. The Electrophysiological Substrate of Early Repolarization Syndrome. JACC Clin. Electrophysiol. 2017, 3, 894–904. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Yan, G.; Ackerman, M.J.; Borggrefe, M.; Corrado, D.; Guo, J.; Gussak, I.; Hasdemir, C.; Horie, M.; Huikuri, H.; et al. J-Wave Syndromes Expert Consensus Conference Report: Emerging Concepts and Gaps in Knowledge. J. Arrhythmia 2016, 32, 315–339. [Google Scholar] [CrossRef]
- Oliva, A.; Grassi, S.; Pinchi, V.; Cazzato, F.; Coll, M.; Alcalde, M.; Vallverdú-Prats, M.; Perez-Serra, A.; Martínez-Barrios, E.; Cesar, S.; et al. Structural Heart Alterations in Brugada Syndrome: Is It Really a Channelopathy? A Systematic Review. J. Clin. Med. 2022, 11, 4406. [Google Scholar] [CrossRef]
- De Raffele, M.; Di Domenico, A.; Balla, C.; Vitali, F.; Boccadoro, A.; Pavasini, R.; Micillo, M.; Cocco, M.; Campo, G.; Bertini, M.; et al. Structural Abnormalities in Brugada Syndrome and Non-Invasive Cardiac Imaging: A Systematic Review. Biology 2023, 12, 606. [Google Scholar] [CrossRef]
- Boukens, B.J.; Potse, M.; Coronel, R. Fibrosis and Conduction Abnormalities as Basis for Overlap of Brugada Syndrome and Early Repolarization Syndrome. Int. J. Mol. Sci. 2021, 22, 1570. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, P.W.; Antzelevitch, C.; Haissaguerre, M.; Huikuri, H.V.; Potse, M.; Rosso, R.; Sacher, F.; Tikkanen, J.T.; Wellens, H.; Yan, G.-X. The Early Repolarization Pattern. J. Am. Coll. Cardiol. 2015, 66, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Frigy, A.; Gábor-Kelemen, H.; László, S.A.; Szabó, I.A.; Kocsis, L. Electrocardiographic Changes Associated with Early Repolarization Pattern in Healthy Young Males. Medicina 2022, 58, 1048. [Google Scholar] [CrossRef] [PubMed]
- Szabó, I.A.; Kocsis, L.; László, S.; Fehérvári, L.; Fárr, A.-M.; Frigy, A. Korai Repolarizációs Mintázatot Mutató Fiatal Férfiak Echokardiográfiás Jellemzőinek Összehasonlító Vizsgálata. Orvosi Hetil. 2021, 162, 741–745. [Google Scholar] [CrossRef]
- Kocsis, L.; Pap, Z.; Frigy, A. Cardiac Morphofunctional Characteristics of Individuals with Early Repolarization Pattern: A Literature Review. J. Cardiovasc. Dev. Dis. 2022, 10, 4. [Google Scholar] [CrossRef]
- Scheirlynck, E.; Motoc, A.; De Asmundis, C.; Sieira, J.; Koulalis, J.; Van Malderen, S.; Chierchia, G.; Pappaert, G.; Haugaa, K.; Lie, Ø.; et al. Long-Term Evolution of Echocardiography Parameters in Brugada Syndrome Patients. Eur. Heart J. Cardiovasc. Imaging 2021, 22 (Suppl. S1), jeaa356.018. [Google Scholar] [CrossRef]
- Scheirlynck, E.; Van Malderen, S.; Motoc, A.; Lie, Ø.H.; De Asmundis, C.; Sieira, J.; Chierchia, G.-B.; Brugada, P.; Cosyns, B.; Droogmans, S. Contraction Alterations in Brugada Syndrome; Association with Life-Threatening Ventricular Arrhythmias. Int. J. Cardiol. 2020, 299, 147–152. [Google Scholar] [CrossRef]
- Wuestenfeld, J.C.; Baersch, F.; Ruedrich, P.; Paech, C.; Wolfarth, B. Blood Pressure Response to Dynamic Exercise Testing in Adolescent Elite Athletes, What Is Normal? Front. Pediatr. 2022, 10, 974926. [Google Scholar] [CrossRef]
- Trenkwalder, T.; Rübsamen, N.; Schmitt, V.H.; Arnold, N.; Kaess, B.M.; Sinning, C.R.; Zeller, T.; Beutel, M.E.; Schmidtmann, I.; Nickels, S.; et al. Left Ventricular Geometry and Function in Early Repolarization: Results from the Population-Based Gutenberg Health Study. Clin. Res. Cardiol. 2019, 108, 1107–1116. [Google Scholar] [CrossRef]
- Quattrini, F.M.; Pelliccia, A.; Assorgi, R.; DiPaolo, F.M.; Squeo, M.R.; Culasso, F.; Castelli, V.; Link, M.S.; Maron, B.J. Benign Clinical Significance of J-Wave Pattern (Early Repolarization) in Highly Trained Athletes. Heart Rhythm 2014, 11, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Miragoli, M.; Goldoni, M.; Demola, P.; Paterlini, A.; Li Calzi, M.; Gioia, M.I.; Visioli, F.; Rossi, S.; Pelà, G. Left Ventricular Geometry Correlates with Early Repolarization Pattern in Adolescent Athletes. Scand. J. Med. Sci. Sports 2019, 29, 1727–1735. [Google Scholar] [CrossRef]
- Serra-Grima, R.; Doñate, M.; Álvarez-García, J.; Barradas-Pires, A.; Ferrero, A.; Carballeira, L.; Puig, T.; Rodríguez, E.; Cinca, J. Long-Term Follow-up of Early Repolarization Pattern in Elite Athletes. Am. J. Med. 2015, 128, 192.e1–192.e9. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, W.; Trenkwalder, T.; Haller, B.; Meindl, C.; Schoenfeld, J.; Kaess, B.M.; Hengstenberg, C.; Schunkert, H.; Pressler, A.; Halle, M.; et al. The Early Repolarization Pattern: Echocardiographic Characteristics in Elite Athletes. Ann. Noninvasive Electrocardiol. 2019, 24, e12617. [Google Scholar] [CrossRef] [PubMed]
- McNamara, D.A.; Bennett, A.J.; Ayers, C.; De Lemos, J.A.; Berry, J.D.; Link, M.S. Early Repolarization Pattern Is Associated With Increased Left Ventricular Mass. JACC Clin. Electrophysiol. 2019, 5, 395–397. [Google Scholar] [CrossRef]
- Liu, Y.; Mi, N.; Zhou, Y.; An, P.; Bai, Y.; Guo, Y.; Hong, C.; Ji, Z.; Ye, P.; Wu, C. Transverse False Tendons in the Left Ventricular Cavity Are Associated with Early Repolarization. PLoS ONE 2015, 10, e0125173. [Google Scholar] [CrossRef][Green Version]
- Gulel, O.; Dağasan, G.; Yüksel, S.; Soylu, K.; Şahin, M. Evaluation of Left Ventricular Myocardial Deformation Parameters in Individuals with Electrocardiographic Early Repolarization Pattern. Anatol. J. Cardiol. 2016, 16, 850–854. [Google Scholar] [CrossRef]
- Çöllüoğlu, T.; Önalan, O.; Çakan, F. The Diagnostic Value of 2D-speckle Tracking Echocardiography for Identifying Subclinical Ventricular Dysfunction in Subjects with Early Repolarization Pattern. Echocardiography 2021, 38, 1141–1148. [Google Scholar] [CrossRef]

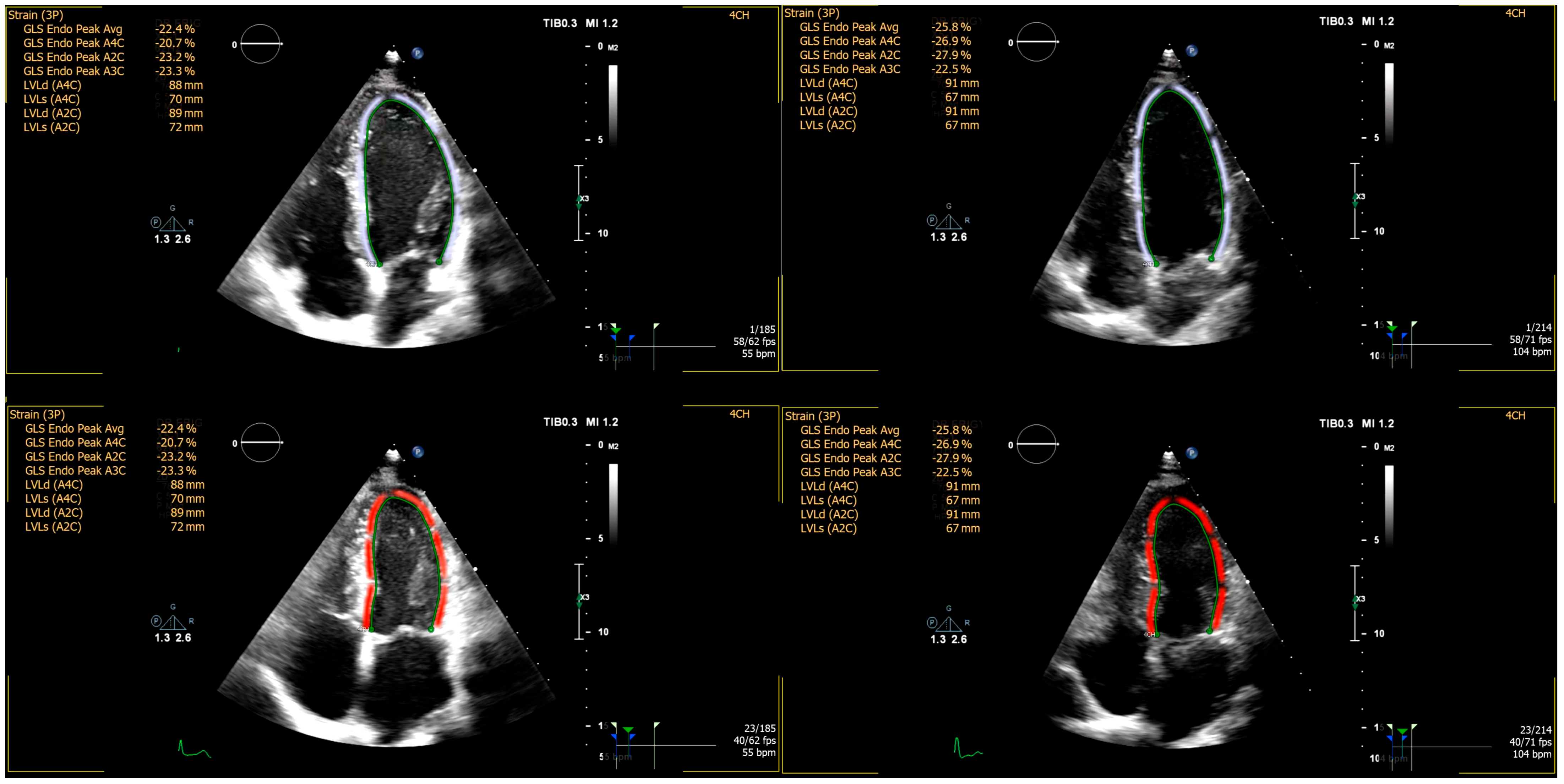
| ERP+ | ERP− | p | |
|---|---|---|---|
| Anthropometric measurements | |||
| Age, years | 22.9 ± 1.7 | 22.2 ± 1.7 | 0.171 |
| Weight, kg | 76.9 ± 9.7 | 74.4 ± 9.2 | 0.393 |
| Height, cm | 179 ± 6 | 178 ± 6 | 0.579 |
| Body surface area, m2 | 1.95 ± 0.14 | 1.92 ± 0.14 | 0.385 |
| Body mass index, kg/m2. | 23.9 ± 2.5 | 23.3 ± 2.3 | 0.449 |
| Vital signs | |||
| Systolic blood pressure, mmHg | 123 ± 13 | 123 ± 13 | 0.941 |
| Diastolic blood pressure, mmHg | 79 ± 7 | 78 ± 7 | 0.777 |
| Heart rate, bpm | 68 ± 9 | 72 ± 9 | 0.227 |
| ERP+ | ERP− | p | |
|---|---|---|---|
| RR interval, ms | 902 ± 140 | 864 ± 140 | 0.410 |
| P wave duration, ms | 108 ± 10 | 102 ± 10 | 0.0810 |
| PQ(PR) interval, ms | 158 ± 16 | 166 ± 16 | 0.215 |
| P wave amplitude, mm | 1.2 ± 0.4 | 1.3 ± 0.4 | 0.527 |
| QRS axis, grade | 60 ± 24 | 64 ± 24 | 0.578 |
| QRS duration, ms | 91 ± 7 | 97 ± 7 | 0.017 |
| QT interval corrected, ms | 385 ± 26 | 381 ± 27 | 0.543 |
| Significant ST segment modification, % | 0 | 0 | - |
| Atrial premature beats, % | 0 | 0 | - |
| Ventricular premature beats, % | 0 | 0 | - |
| ERP+ | ERP− | p | |
|---|---|---|---|
| METs | 14.8 ± 2.6 | 16.9 ± 2.6 | 0.110 |
| Duration of exercise, min | 11.6 ± 2.4 | 12.4 ± 2.4 | 0.465 |
| Heart rate at peak, bpm | 188 ± 8 | 187 ± 8 | 0.620 |
| Systolic blood pressure at peak, mmHg | 164 ± 18 | 160 ± 18 | 0.515 |
| Diastolic blood pressure at peak, mmHg | 90 ± 9 | 86 ± 9 | 0.137 |
| ERP+ | ERP− | p | |
|---|---|---|---|
| Left ventricular morphological parameters | |||
| LVEDD, mm | 45.9 ± 3.7 | 46.8 ± 3.7 | 0.287 |
| LVESD, mm | 30.7 ± 3.8 | 31.7 ± 3.9 | 0.333 |
| IVST, mm | 9.3 ± 0.7 | 9.1 ± 0.7 | 0.376 |
| PWT, mm | 9.3 ± 0.6 | 9.4 ± 0.6 | 0.724 |
| RWT, % | 41.0 ± 4.3 | 40.2 ± 4.4 | 0.505 |
| LVEDV, mL | 118.1 ± 15.9 | 119.4 ± 14.3 | 0.783 |
| LVESV, mL | 45.4 ± 7.8 | 46.2 ± 7.3 | 0.728 |
| LVM, g | 144.7 ± 24.5 | 148.3 ± 24.6 | 0.601 |
| Left ventricular systolic and diastolic parameters | |||
| EF, % | 61.5 ± 3.3 | 61.3 ± 3.3 | 0.853 |
| SF, % | 33.1 ± 5.9 | 32.1 ± 6.0 | 0.633 |
| GLS | −23.0 ± 1.3 | −22.5 ± 1.3 | 0.177 |
| SV, mL | 72.4 ± 9.5 | 73.5 ± 9.5 | 0.691 |
| E, cm/s | 87.2 ± 16.4 | 88.0 ± 16.7 | 0.876 |
| A, cm/s | 52.2 ± 10.6 | 50.6 ± 10.9 | 0.616 |
| E/A | 1.7 ± 0.4 | 1.8 ± 0.4 | 0.815 |
| Other morphological parameters | |||
| LAD, mm | 31.4 ± 4.9 | 30.7 ± 4.8 | 0.590 |
| AoR, mm | 21.2 ± 1.3 | 21.7 ± 1.4 | 0.187 |
| RVD, mm | 36.0 ± 3.7 | 34.8 ± 3.7 | 0.277 |
| ERP+ | ERP− | p | |
|---|---|---|---|
| Left ventricular morphological parameters | |||
| LVEDD, mm | 46.2 ± 3.9 | 47.2 ± 3.9 | 0.352 |
| LVESD, mm | 26.1 ± 3.8 | 27.1 ± 3.8 | 0.322 |
| IVST, mm | 9.1 ± 0.8 | 9.0 ± 0.8 | 0.625 |
| PWT, mm | 9.2 ± 0.9 | 9.1 ± 1.0 | 0.876 |
| RWT, % | 39.9 ± 5.4 | 38.8 ± 5.5 | 0.726 |
| LVEDV, mL | 107.7 ± 18.8 | 118.3 ± 19.1 | 0.058 |
| LVESV, mL | 38.9 ± 7.3 | 43.3 ± 7.4 | 0.067 |
| Left ventricular systolic and diastolic parameters | |||
| EF, % | 64.0 ± 3.0 | 63.4 ± 3.0 | 0.500 |
| SF, % | 43.6 ± 6.1 | 42.3 ± 6.2 | 0.503 |
| GLS | −24.8 ± 1.7 | −22.3 ± 1.7 | 0.262 |
| SV, mL | 67.5 ± 14.1 | 75.0 ± 14.4 | 0.053 |
| E, cm/s | 106.5 ± 15.7 | 110.2 ± 15.4 | 0.440 |
| A, cm/s | 69.2 ± 17.2 | 75.4 ± 17.2 | 0.201 |
| E/A | 1.6 ± 0.3 | 1.5 ± 0.3 | 0.212 |
| Other morphological parameters | |||
| LAD, mm | 31.9 ± 3.9 | 30.1 ± 3.7 | 0.106 |
| AoR, mm | 21.2 ± 1.3 | 21.7 ± 1.3 | 0.233 |
| RVD, mm | 35.9 ± 4.3 | 34.6 ± 4.2 | 0.372 |
| ERP+ | ERP− | p | |
|---|---|---|---|
| Left ventricular morphological parameters | |||
| Δ LVEDD, mm | 0.3 ± 1.7 | 0.3 ± 2.2 | 0.998 |
| Δ LVESD, mm | −4.6 ± 3.1 | −4.5 ± 4.2 | 0.939 |
| Δ IVST, mm | −0.2 ± 0.5 | −0.5 ± 0.5 | 0.528 |
| Δ PWT, mm | −0.1 ± 0.7 | −0.2 ± 0.8 | 0.710 |
| Δ RWT, % | −1.0 ± 3.7 | −1.4 ± 3.7 | 0.751 |
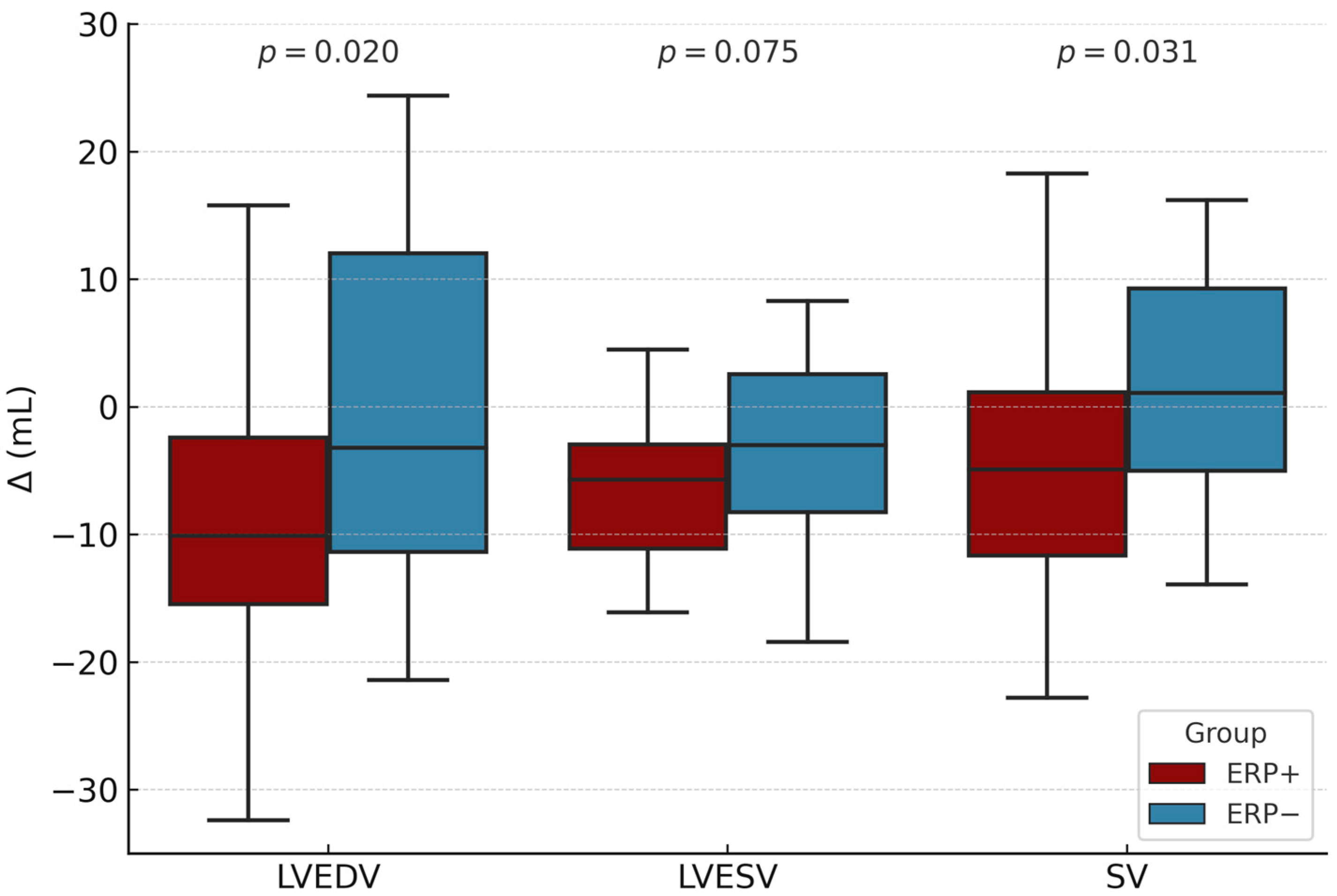
| Δ LVEDV, mL | −10.1 ± 11.8 | −1.1 ± 13.2 | 0.020 |
| Δ LVESV, mL | −6.6 ± 5.4 | −3.0 ± 5.5 | 0.075 |
| Left ventricular systolic and diastolic parameters | |||
| Δ EF, % | 2.6 ± 3.8 | 2.0 ± 3.8 | 0.635 |
| Δ SF, % | 10.5 ± 6.6 | 10.3 ± 8.2 | 0.931 |
| Δ GLS | −1.7 ± 1.5 | 0.2 ± 1.5 | 0.652 |
| Δ SV, mL | −4.8 ± 9.9 | 1.4 ± 8.9 | 0.031 |
| Δ E, cm/s | 18.5 ± 18.2 | 22.7 ± 17.0 | 0.452 |
| Δ A, cm/s | 16.2 ± 18.1 | 20.1 ± 25.6 | 0.594 |
| Δ E/A | −0.1 ± 0.4 | −0.1 ± 0.5 | 0.618 |
| Other morphological parameters | |||
| Δ LAD, mm | 0.2 ± 3.3 | −0.5 ± 3.4 | 0.483 |
| Δ AoR, mm | −0.1 ± 0.7 | 0.0 ± 0.9 | 0.487 |
| Δ RVD, mm | −0.4 ± 2.6 | 0.1 ± 1.8 | 0.534 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocsis, L.; Pap, Z.; Szabó, I.A.; Frigy, A. Dynamic Echocardiographic Changes Induced by Exercise in Healthy, Young Individuals with Early Repolarization Pattern. Diagnostics 2025, 15, 1755. https://doi.org/10.3390/diagnostics15141755
Kocsis L, Pap Z, Szabó IA, Frigy A. Dynamic Echocardiographic Changes Induced by Exercise in Healthy, Young Individuals with Early Repolarization Pattern. Diagnostics. 2025; 15(14):1755. https://doi.org/10.3390/diagnostics15141755
Chicago/Turabian StyleKocsis, Loránd, Zsuzsanna Pap, István Adorján Szabó, and Attila Frigy. 2025. "Dynamic Echocardiographic Changes Induced by Exercise in Healthy, Young Individuals with Early Repolarization Pattern" Diagnostics 15, no. 14: 1755. https://doi.org/10.3390/diagnostics15141755
APA StyleKocsis, L., Pap, Z., Szabó, I. A., & Frigy, A. (2025). Dynamic Echocardiographic Changes Induced by Exercise in Healthy, Young Individuals with Early Repolarization Pattern. Diagnostics, 15(14), 1755. https://doi.org/10.3390/diagnostics15141755







