How to Perform Cardiac Contrast-Enhanced Ultrasound (cCEUS): Part I
Abstract
1. Introduction
1.1. Brief History of Cardiac CEUS
1.2. Specific Terms for Cardiac Contrast-Enhanced Ultrasound
2. Ultrasound Enhancing Agents (UEAs), Doses, and Dynamics
2.1. UEA Contraindications and Pre-Procedure Assessment
2.2. Informed Consent
2.3. Safety of CEUS/How to Prepare for Adverse Events
2.4. Application of UEAs
2.4.1. Bolus Injection
2.4.2. Continuous Infusion via a Peripheral Line
2.4.3. Bolus Injection/Infusion via a Central Line
2.4.4. Multiple Injection/Infusions of UEA Boli or Restarting UEA Infusion
2.4.5. UEA Application in Patients with LVAD and ECMO Devices
2.5. Perspectives of New UEAs
2.6. What Should We Know About Contrast Phases?
3. Imaging Modalities for UEAs
Perspectives of Imaging Methods and Analysis Tools
4. Fundamentals of Practical Application
4.1. Left Ventricular Function
4.1.1. Background and Indications
4.1.2. Advantages of Contrast-Enhanced Echocardiography
4.2. Preparation and Performance
4.2.1. Technical Aspects and Settings
4.2.2. Pre-Assessment and Primary Scan Plane
4.2.3. CEUS Procedure
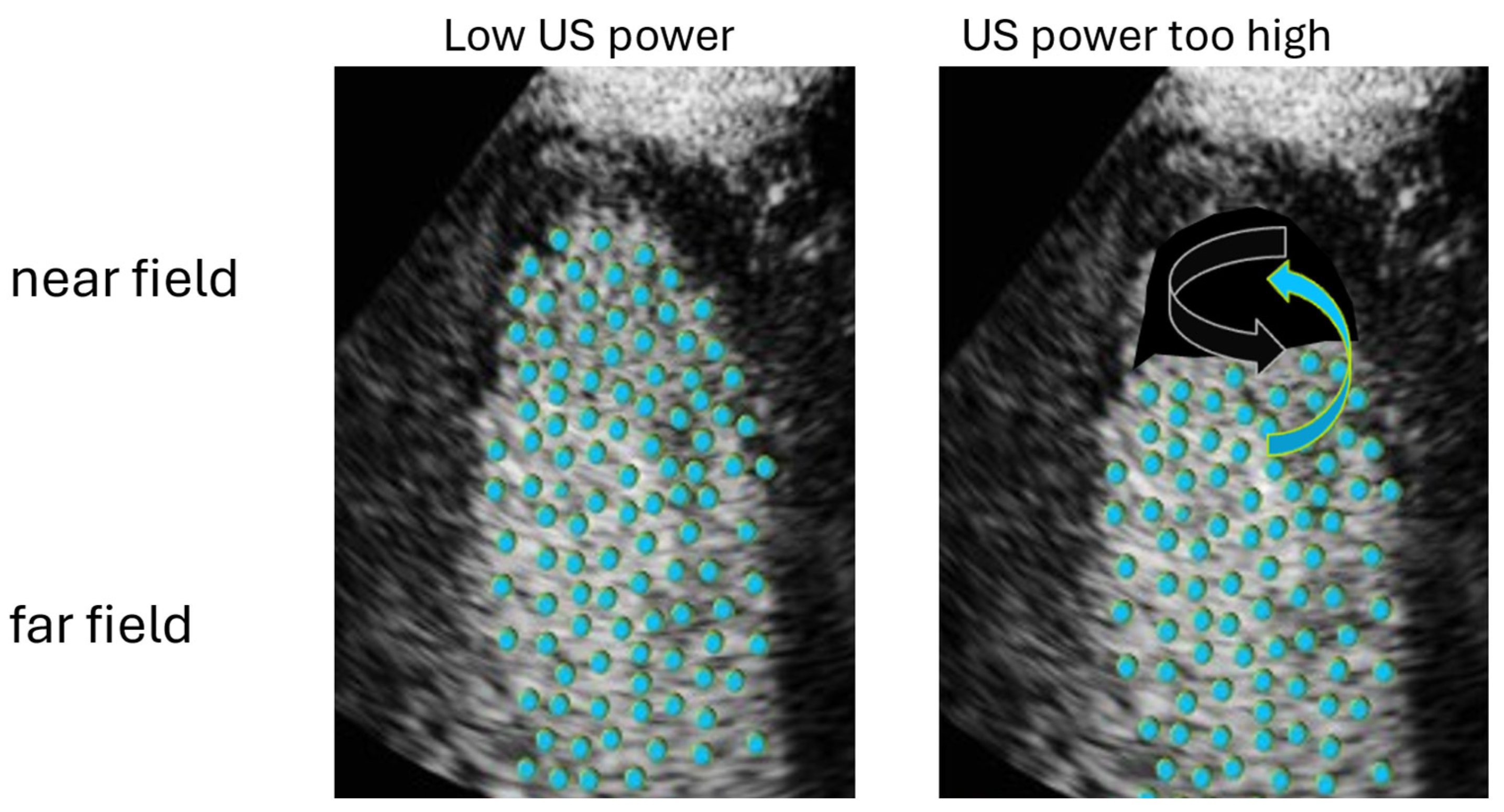
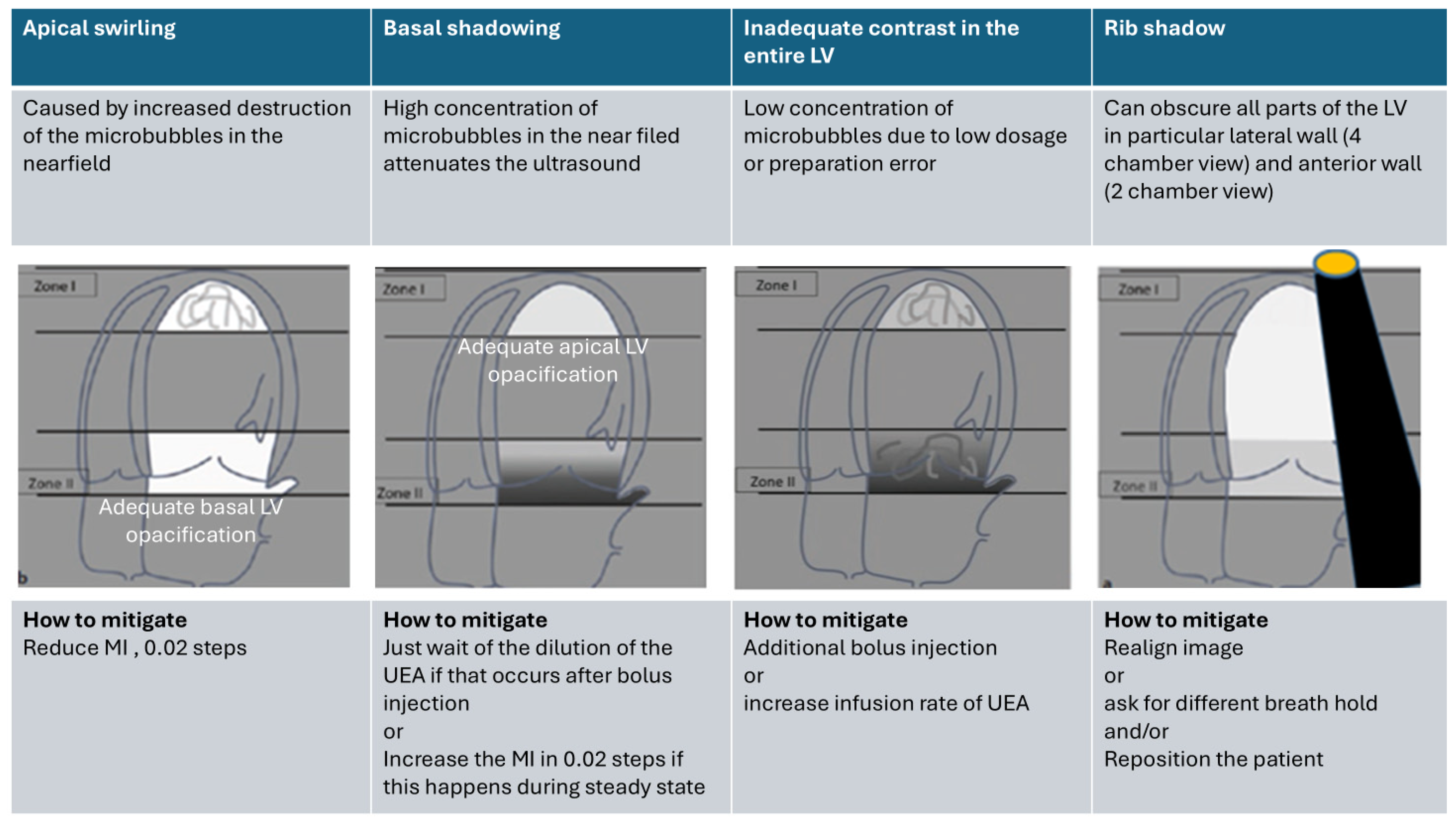
4.3. Optimization of LV Opacification
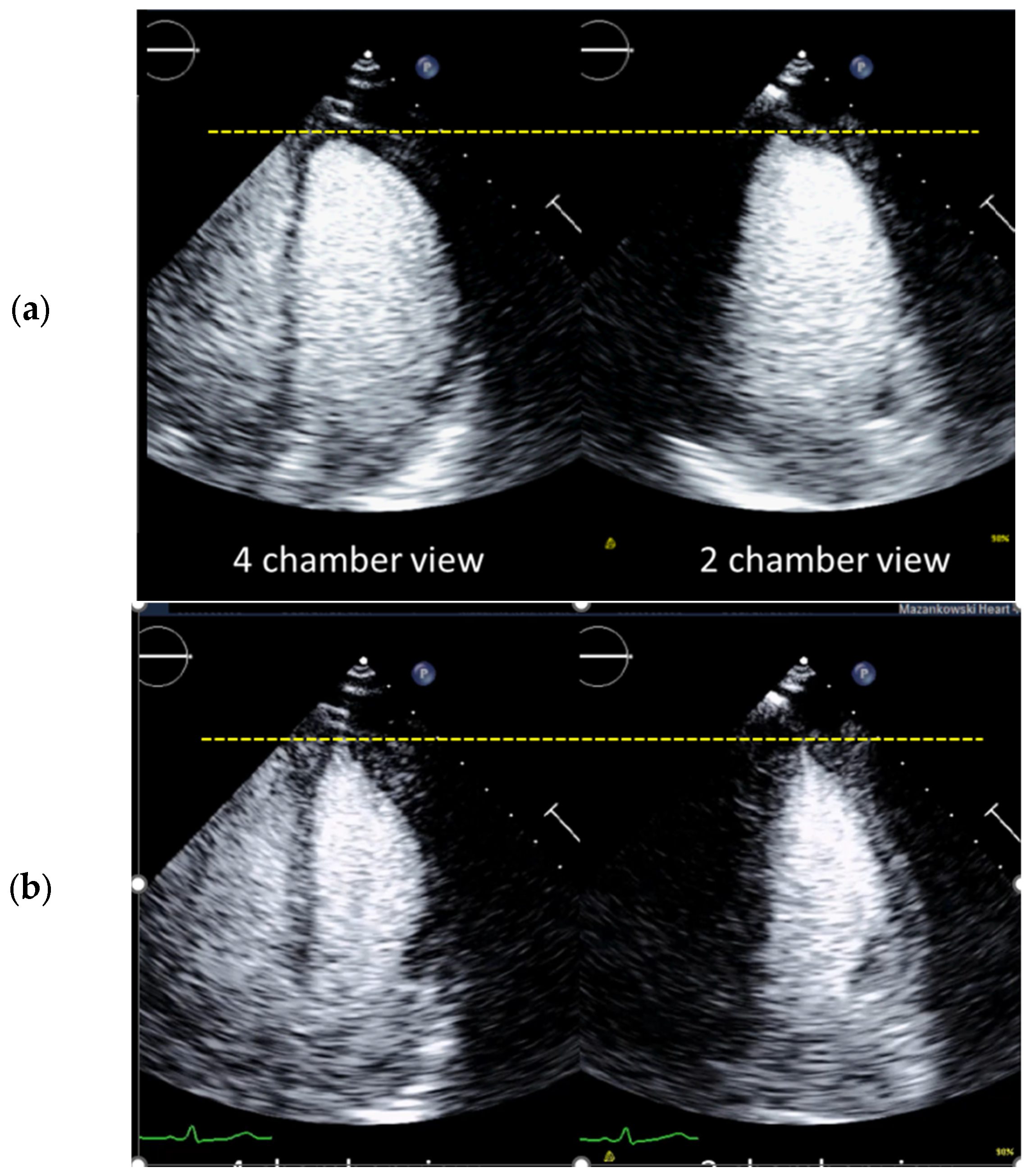
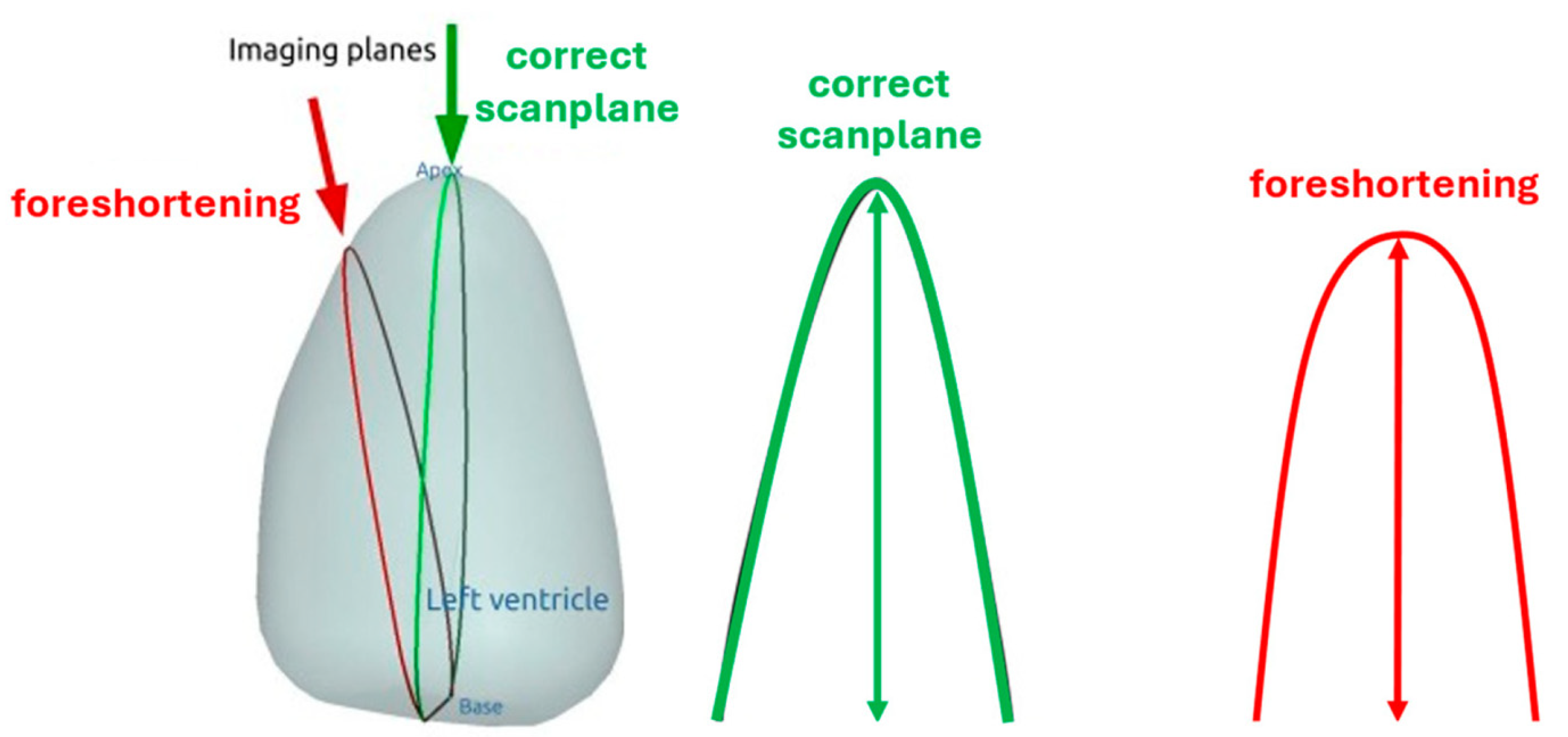
4.4. How to Avoid Foreshortened Imaging Planes
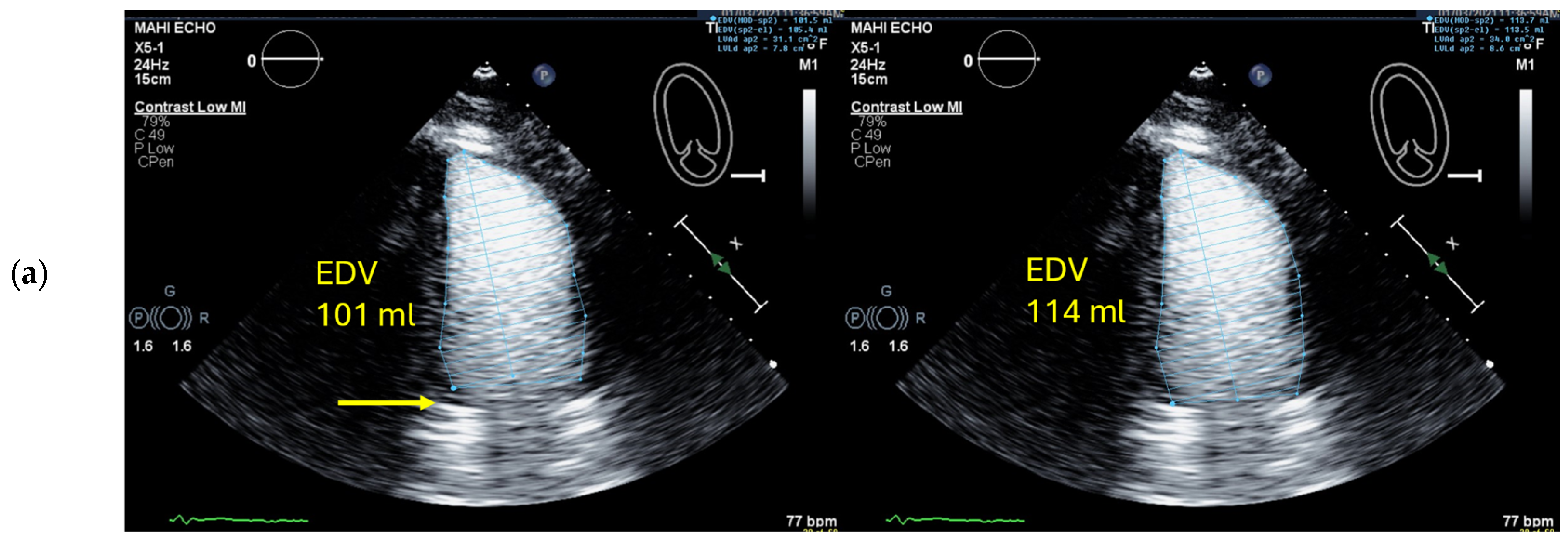
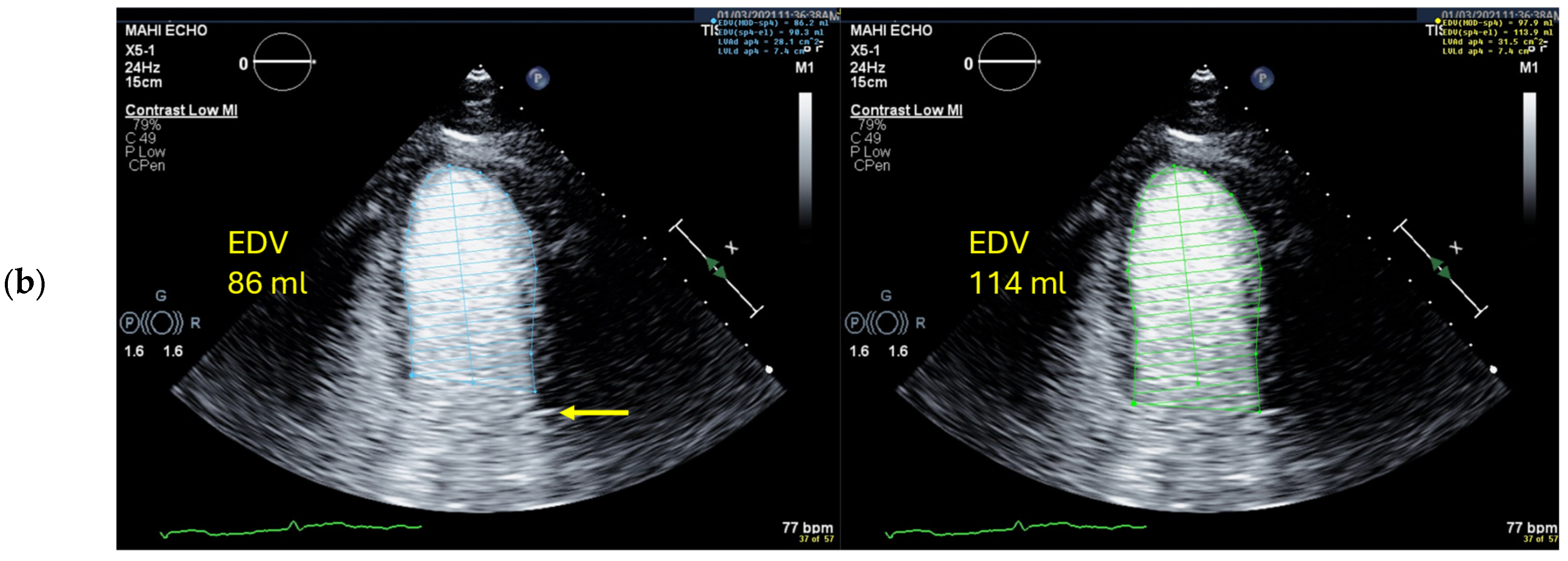
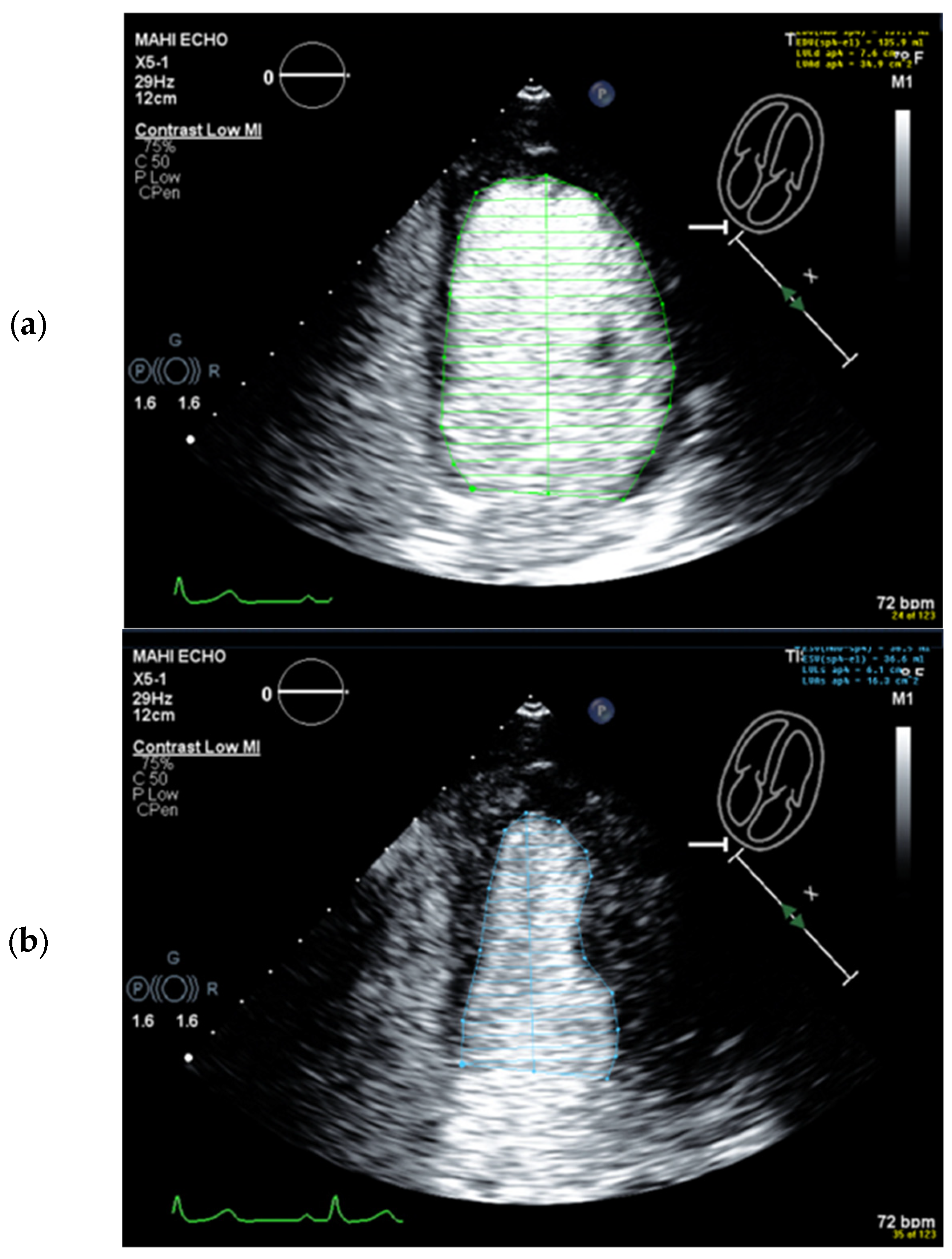
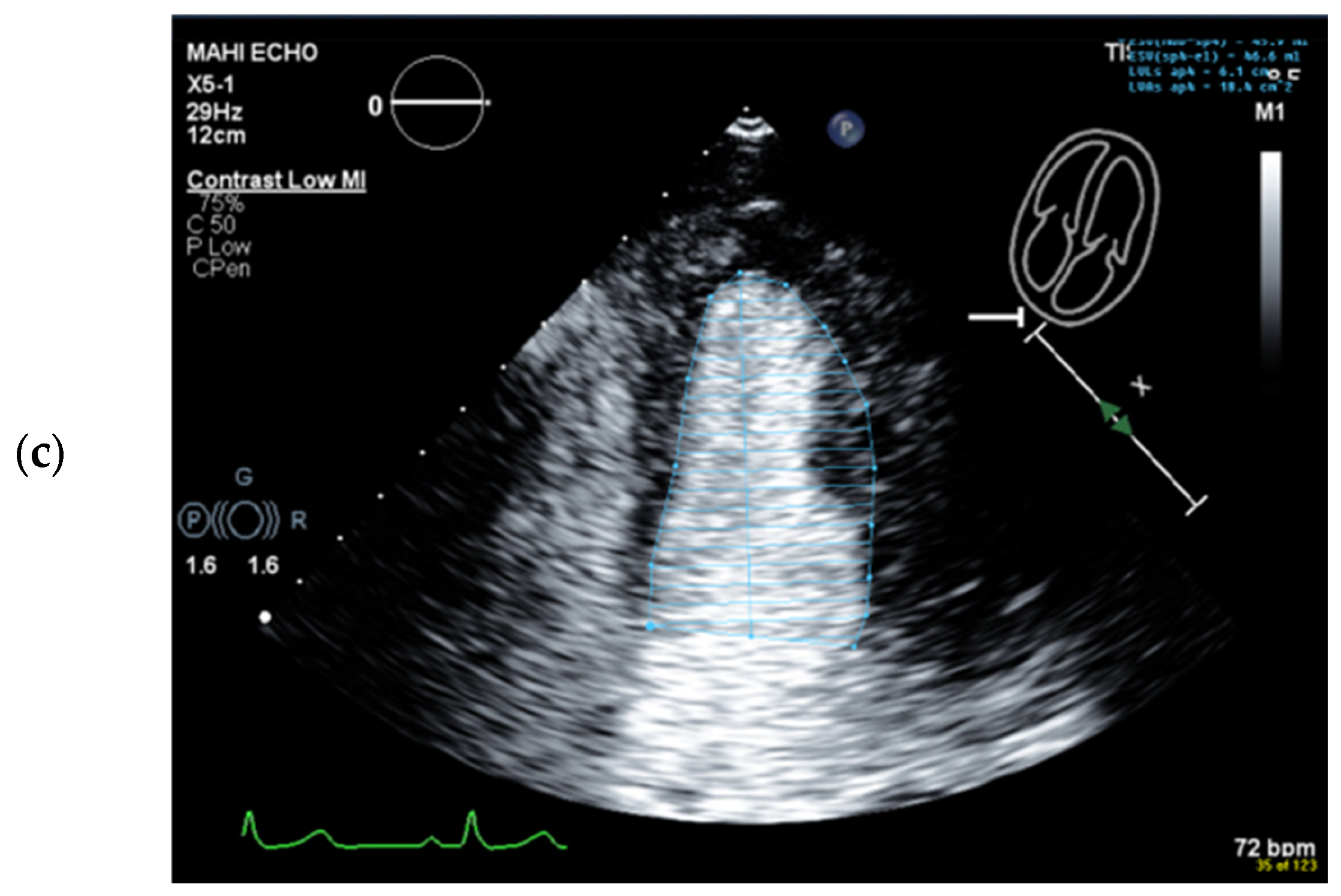
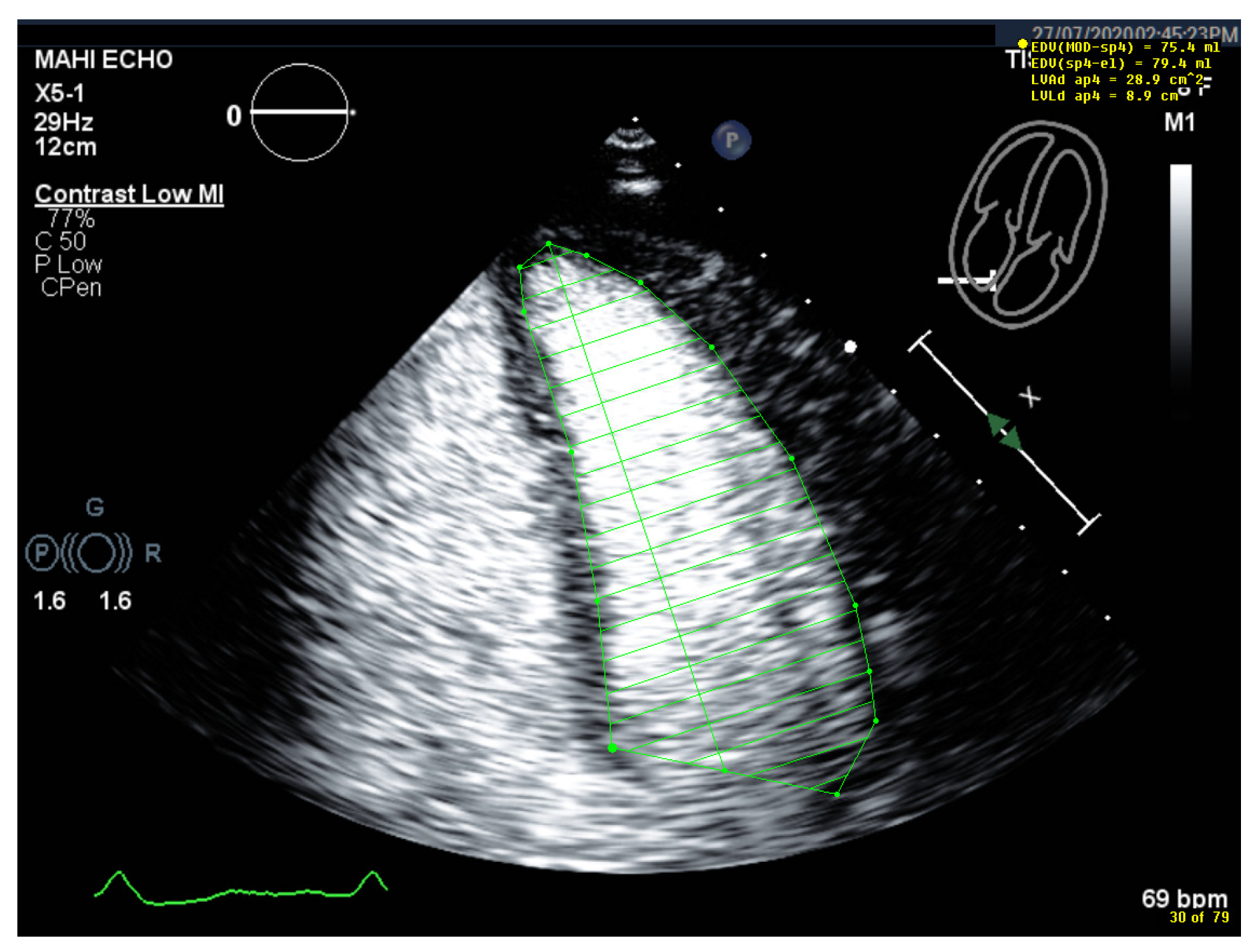
4.5. Left Ventricular Volume and Ejection Fraction Measurements
4.5.1. Pitfalls
Selection of Foreshortened Loops
Selection of False End-Diastolic and End-Systolic Frames
Starting Point for the LV Contour at the Mitral Valve Ring Is Incorrect
Wrong Contour/Papillary Muscle
4.6. Interpretation and Reporting
4.6.1. Global Systolic LV Function
4.6.2. Regional (Segmental) Wall Motion
4.6.3. Left Ventricular Strain Analysis
4.7. Alternative Imaging Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindner, J.R. Contrast echocardiography: Current status and future directions. Heart 2021, 107, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Averkiou, M.; Nielsen, M.B.; Barr, R.G.; Burns, P.N.; Calliada, F.; Cantisani, V.; Choi, B.; Chammas, M.C.; Clevert, D.A.; et al. How to perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int. Open 2018, 4, E2–E15. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Averkiou, M.A.; Correas, J.M.; Lassau, N.; Leen, E.; Piscaglia, F. An EFSUMB introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012, 33, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Correas, J.M.; Cui, X.W.; Dong, Y.; Havre, R.F.; Jenssen, C.; Jung, E.M.; Krix, M.; Lim, A.; Lassau, N.; et al. EFSUMB Technical Review—Update 2023: Dynamic Contrast-Enhanced Ultrasound (DCE-CEUS) for the Quantification of Tumor Perfusion. Ultraschall Med. 2024, 45, 36–46. [Google Scholar] [CrossRef]
- Fischer, C.; Krix, M.; Weber, M.A.; Loizides, A.; Gruber, H.; Jung, E.M.; Klauser, A.; Radzina, M.; Dietrich, C.F. Contrast-Enhanced Ultrasound for Musculoskeletal Applications: A World Federation for Ultrasound in Medicine and Biology Position Paper. Ultrasound Med. Biol. 2020, 46, 1279–1295. [Google Scholar] [CrossRef]
- Safai Zadeh, E.; Gorg, C.; Prosch, H.; Jenssen, C.; Blaivas, M.; Laursen, C.B.; Jacobsen, N.; Dietrich, C.F. WFUMB Technological Review: How to Perform Contrast-Enhanced Ultrasound of the Lung. Ultrasound Med. Biol. 2022, 48, 598–616. [Google Scholar] [CrossRef]
- Prada, F.; Vetrano, I.G.; Gennari, A.G.; Mauri, G.; Martegani, A.; Solbiati, L.; Sconfienza, L.M.; Quaia, E.; Kearns, K.N.; Kalani, M.Y.S.; et al. How to Perform Intra-Operative Contrast-Enhanced Ultrasound of the Brain-A WFUMB Position Paper. Ultrasound Med. Biol. 2021, 47, 2006–2016. [Google Scholar] [CrossRef]
- Helfen, A.; Becher, H. Geschichte der Kontrastechokardiographie. Lit. Kardiol. 2024, 18, 269–276. [Google Scholar]
- Dietrich, C.F.; Bolondi, L.; Duck, F.; Evans, D.H.; Ewertsen, C.; Fraser, A.G.; Gilja, O.H.; Jenssen, C.; Merz, E.; Nolsoe, C.; et al. History of Ultrasound in Medicine from its birth to date (2022), on occasion of the 50 Years Anniversary of EFSUMB. A publication of the European Federation of Societies for Ultrasound In Medicine and Biology (EFSUMB), designed to record the historical development of medical ultrasound. Med. Ultrason. 2022, 24, 434–450. [Google Scholar] [CrossRef]
- Fraser, A.G.; Monaghan, M.J.; van der Steen, A.F.W.; Sutherland, G.R. A concise history of echocardiography: Timeline, pioneers, and landmark publications. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1130–1143. [Google Scholar] [CrossRef]
- Hampson, R.; Senior, R.; Ring, L.; Robinson, S.; Augustine, D.X.; Becher, H.; Anderson, N.; Willis, J.; Chandrasekaran, B.; Kardos, A.; et al. Contrast echocardiography: A practical guideline from the British Society of Echocardiography. Echo Res. Pract. 2023, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Mulvagh, S.L.; Rakowski, H.; Vannan, M.A.; Abdelmoneim, S.S.; Becher, H.; Bierig, S.M.; Burns, P.N.; Castello, R.; Coon, P.D.; Hagen, M.E.; et al. American Society of Echocardiography Consensus Statement on the Clinical Applications of Ultrasonic Contrast Agents in Echocardiography. J. Am. Soc. Echocardiogr. 2008, 21, 1179–1201, quiz 1281. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.R.; Abdelmoneim, S.; Belcik, J.T.; McCulloch, M.L.; Mulvagh, S.L.; Olson, J.J.; Porcelli, C.; Tsutsui, J.M.; Wei, K. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: A focused update from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.R.; Feinstein, S.B.; Senior, R.; Mulvagh, S.L.; Nihoyannopoulos, P.; Strom, J.B.; Mathias, W., Jr.; Gorman, B.; Rabischoffsky, A.; Main, M.L.; et al. CEUS cardiac exam protocols International Contrast Ultrasound Society (ICUS) recommendations. Echo Res. Pract. 2022, 9, 7. [Google Scholar] [CrossRef]
- Porter, T.R.; Mulvagh, S.L.; Abdelmoneim, S.S.; Becher, H.; Belcik, J.T.; Bierig, M.; Choy, J.; Gaibazzi, N.; Gillam, L.D.; Janardhanan, R.; et al. Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update. J. Am. Soc. Echocardiogr. 2018, 31, 241–274. [Google Scholar] [CrossRef]
- Senior, R.; Becher, H.; Monaghan, M.; Agati, L.; Zamorano, J.; Vanoverschelde, J.L.; Nihoyannopoulos, P.; Edvardsen, T.; Lancellotti, P.; EACVI Scientific Documents Committee for 2014–16 and 2016–18. Clinical practice of contrast echocardiography: Recommendation by the European Association of Cardiovascular Imaging (EACVI) 2017. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1205–1205af. [Google Scholar] [CrossRef]
- Becher, H.; Helfen, A. Contrast Echocardiography. Echocardiography Compendium for Clinical Practice; Springer/Nature: London, UK, 2019. [Google Scholar]
- Hoffmann, R.; von Bardeleben, S.; ten Cate, F.; Borges, A.C.; Kasprzak, J.; Firschke, C.; Lafitte, S.; Al-Saadi, N.; Kuntz-Hehner, S.; Engelhardt, M.; et al. Assessment of systolic left ventricular function: A multi-centre comparison of cineventriculography, cardiac magnetic resonance imaging, unenhanced and contrast-enhanced echocardiography. Eur. Heart J. 2005, 26, 607–616. [Google Scholar] [CrossRef]
- Aggeli, C.; Giannopoulos, G.; Roussakis, G.; Christoforatou, E.; Marinos, G.; Toli, C.; Pitsavos, C.; Stefanadis, C. Safety of myocardial flash-contrast echocardiography in combination with dobutamine stress testing for the detection of ischaemia in 5250 studies. Heart 2008, 94, 1571–1577. [Google Scholar] [CrossRef]
- Dijkmans, P.A.; Senior, R.; Becher, H.; Porter, T.R.; Wei, K.; Visser, C.A.; Kamp, O. Myocardial contrast echocardiography evolving as a clinically feasible technique for accurate, rapid, and safe assessment of myocardial perfusion: The evidence so far. J. Am. Coll. Cardiol. 2006, 48, 2168–2177. [Google Scholar] [CrossRef]
- Siebelink, H.M.; Scholte, A.J.; Van de Veire, N.R.; Holman, E.R.; Nucifora, G.; van der Wall, E.E.; Bax, J.J. Value of contrast echocardiography for left ventricular thrombus detection postinfarction and impact on antithrombotic therapy. Coron. Artery Dis. 2009, 20, 462–466. [Google Scholar] [CrossRef]
- LUMASON. LUMASON (Sulfur Hexafluoride Lipid-Type A Microspheres) for Injectable Suspension, for Intravenous Use or Intravesical Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203684s009lbl.pdf (accessed on 1 July 2025).
- European Medicines Agency. SonoVue. Available online: https://www.ema.europa.eu/en/documents/product-information/sonovue-epar-product-information_en.pdf (accessed on 1 July 2025).
- OPTISON. OPTISON (Perflutren Protein-Type A Microspheres Injectable Suspension, USP). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020899s015lbl.pdf (accessed on 1 July 2025).
- DEFINITY. DEFINITY®® (Perflutren Lipid Microsphere) Injectable Suspension. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/021064s025s029lbl.pdf (accessed on 1 July 2025).
- Gaibazzi, N.; Picano, E.; Suma, S.; Garibaldi, S.; Porter, T.R.; Botti, A.; Tuttolomondo, D.; Tedeschi, A.; Lorenzoni, V. Coronary Flow Velocity Reserve Reduction Is Associated with Cardiovascular, Cancer, and Noncancer, Noncardiovascular Mortality. J. Am. Soc. Echocardiogr. 2020, 33, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Cosyns, B.; Helfen, A.; Leong-Poi, H.; Senior, R. How to perform an ultrasound contrast myocardial perfusion examination? Eur. Heart J. Cardiovasc. Imaging 2022, 23, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Strom, J.; Main, M.; Wie, K.; Feinstein, S.; Fiano, R.; Gennarelli, R. Abstract 4121840: Contemporary Safety of Ultrasound Enhancing Agents in a Nationwide Analysis. Circulation 2024, 150, A4121840. [Google Scholar] [CrossRef]
- Muskula, P.R.; Main, M.L. Safety With Echocardiographic Contrast Agents. Circ. Cardiovasc. Imaging 2017, 10, e005459. [Google Scholar] [CrossRef]
- Wei, K.; Mulvagh, S.L.; Carson, L.; Davidoff, R.; Gabriel, R.; Grimm, R.A.; Wilson, S.; Fane, L.; Herzog, C.A.; Zoghbi, W.A.; et al. The safety of deFinity and Optison for ultrasound image enhancement: A retrospective analysis of 78,383 administered contrast doses. J. Am. Soc. Echocardiogr. 2008, 21, 1202–1206. [Google Scholar] [CrossRef]
- Abdelmoneim, S.S.; Bernier, M.; Scott, C.G.; Dhoble, A.; Ness, S.A.; Hagen, M.E.; Moir, S.; McCully, R.B.; Pellikka, P.A.; Mulvagh, S.L. Safety of contrast agent use during stress echocardiography: A 4-year experience from a single-center cohort study of 26,774 patients. JACC Cardiovasc. Imaging 2009, 2, 1048–1056. [Google Scholar] [CrossRef]
- Li, Q.; Yang, K.; Ji, Y.; Liu, H.; Fei, X.; Zhang, Y.; Li, J.; Luo, Y. Safety Analysis of Adverse Events of Ultrasound Contrast Agent Lumason/SonoVue in 49,100 Patients. Ultrasound Med. Biol. 2023, 49, 454–459. [Google Scholar] [CrossRef]
- Filippone, A.; Kirchin, M.A.; Monteith, J.; Storto, M.L.; Spinazzi, A. Safety of Lumason(R) (SonoVue(R)) in special populations and critically ill patients. Front. Cardiovasc. Med. 2023, 10, 1225654. [Google Scholar] [CrossRef]
- Elharram, M.; Choy, J.; Ian Paterson, D.; Pituskin, E. Low-Dose Application of Ultrasound-Enhancing Agents Without Flush in Echocardiography: Prospective Experience From the Edmonton Cancer Registry. J. Am. Soc. Echocardiogr. 2023, 37, 368–370. [Google Scholar] [CrossRef]
- Lindner, J.R.; Belcik, T.; Main, M.L.; Montanaro, A.; Mulvagh, S.L.; Olson, J.; Olyaei, A.; Porter, T.R.; Senior, R. Expert Consensus Statement from the American Society of Echocardiography on Hypersensitivity Reactions to Ultrasound Enhancing Agents in Patients with Allergy to Polyethylene Glycol. J. Am. Soc. Echocardiogr. 2021, 34, 707–708. [Google Scholar] [CrossRef]
- Wu, M.; Fields, J.J.; Sachdev, V.; Belcik, J.T.; Chen, J.; Reed, F.; Fu, X.; Hodovan, J.; Harmann, L.M.; Swistara, G.; et al. Increased Susceptibility for Adverse Reactions to Ultrasound Enhancing Agents in Sickle Cell Disease. J. Am. Soc. Echocardiogr. 2023, 36, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, M.B.; Tombak, A.; Orscelik, O.; Koseci, T.; Turker, A.; Basir, H.; Akdeniz, A.; Tiftik, E.N. Cardiac Chamber Quantification by Echocardiography in Adults With Sickle Cell Disease: Need Attention to Eccentric Hypertrophy. Cureus 2021, 13, e15592. [Google Scholar] [CrossRef] [PubMed]
- Oyarzabal, N.; Longo Areso, N.; Bernedo Belar, N.; Popolizio, I.; Arregui, A.; Balza de Vallejo, O. Anaphylactic Shock Due to Allergy to Macrogol 4000 Contained in SonoVue®. Case Rep. Clin. Med. 2017, 6, 143–147. [Google Scholar] [CrossRef][Green Version]
- Ali, M.T.; Johnson, M.; Irwin, T.; Henry, S.; Sugeng, L.; Kansal, S.; Allison, T.G.; Bremer, M.L.; Jones, V.R.; Martineau, M.D.; et al. Incidence of Severe Adverse Drug Reactions to Ultrasound Enhancement Agents in a Contemporary Echocardiography Practice. J. Am. Soc. Echocardiogr. 2024, 37, 276–284.e273. [Google Scholar] [CrossRef]
- Szebeni, J.; Bawa, R. Human Clinical Relevance of the Porcine Model of Pseudoallergic Infusion Reactions. Biomedicines 2020, 8, 82. [Google Scholar] [CrossRef]
- Main, M.L.; Goldman, J.H.; Grayburn, P.A. Thinking outside the “box”-the ultrasound contrast controversy. J. Am. Coll. Cardiol. 2007, 50, 2434–2437. [Google Scholar] [CrossRef]
- Whyte, A.F.; Soar, J.; Dodd, A.; Hughes, A.; Sargant, N.; Turner, P.J. Emergency treatment of anaphylaxis: Concise clinical guidance. Clin. Med. 2022, 22, 332–339. [Google Scholar] [CrossRef]
- Himbert, D.; Brochet, E.; Ducrocq, G.; Vahanian, A. Contrast echocardiography guidance for alcohol septal ablation of hypertrophic obstructive cardiomyopathy. Eur. Heart J. 2010, 31, 1148. [Google Scholar] [CrossRef]
- Prentice, R.; Ahmadian, H.; Thomas, D.; Berger, J.; Gore, R. Improved efficiency and diagnostic utility of inpatient transthoracic echocardiography following implementation of a sonographer-initiated perflutren-based contrast administration protocol. Cardiovasc. Ultrasound 2020, 18, 35. [Google Scholar] [CrossRef]
- Tang, A.; Chiew, S.K.; Rashkovetsky, R.; Becher, H.; Choy, J.B. Feasibility of sonographer-administered echocontrast in a large-volume tertiary-care echocardiography laboratory. Can. J. Cardiol. 2013, 29, 391–395. [Google Scholar] [CrossRef]
- Strom, J.B.; Appis, A.; Barr, R.G.; Chammas, M.C.; Clevert, D.A.; Darge, K.; Feinstein, L.; Feinstein, S.B.; Fowlkes, J.B.; Gorman, B.; et al. Multi-societal expert consensus statement on the safe administration of ultrasound contrast agents. Echo Res. Pract. 2025, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Fine, N.M.; Abdelmoneim, S.S.; Dichak, A.; Kushwaha, S.S.; Park, S.J.; Mulvagh, S.L. Safety and feasibility of contrast echocardiography for LVAD evaluation. JACC Cardiovasc. Imaging 2014, 7, 429–430. [Google Scholar] [CrossRef]
- Schinkel, A.F.L.; Akin, S.; Strachinaru, M.; Muslem, R.; Soliman, O.I.I.; Brugts, J.J.; Constantinescu, A.A.; Manintveld, O.C.; Caliskan, K. Safety and feasibility of contrast echocardiography for the evaluation of patients with HeartMate 3 left ventricular assist devices. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 690–693. [Google Scholar] [CrossRef]
- Baeza-Herrera, L.A.; Hernandez-Reyes, J.P.; Lazcano-Diaz, E.A.; Orihuela-Sandoval, C.; Gonzalez-Ruiz, F.J.; Manzur-Sandoval, D.; Ramos-Enriquez, A.; Terrazas-Cervantes, E.; Rojas-Velasco, G. A contrast echocardiography-based protocol to rule out thrombus in Venous-Arterial ECMO: A proof of concept. Echocardiography 2023, 40, 299–302. [Google Scholar] [CrossRef]
- Bleakley, C.; Smith, R.; Garfield, B.; Jackson, T.; Remmington, C.; Patel, B.V.; Price, S. Contrast Echocardiography in VV-ECMO-Dependent Patients with COVID-19. J. Am. Soc. Echocardiogr. 2020, 33, 1419–1420. [Google Scholar] [CrossRef]
- Janus, S.E.; Hajjari, J.; Al-Kindi, S.; Elgudin, Y.; Hoit, B.D. Contrast-enhanced echocardiographic detection of severe aortic insufficiency in venoarterial extracorporeal membrane oxygenation. Echocardiography 2020, 37, 905–907. [Google Scholar] [CrossRef]
- Lashin, H.; Shepherd, S.; Smith, A. Contrast-Enhanced Echocardiography Application in Patients Supported by Extracorporeal Membrane Oxygenation (ECMO): A Narrative Review. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2080–2089. [Google Scholar] [CrossRef]
- Frinking, P.; Segers, T.; Luan, Y.; Tranquart, F. Three Decades of Ultrasound Contrast Agents: A Review of the Past, Present and Future Improvements. Ultrasound Med. Biol. 2020, 46, 892–908. [Google Scholar] [CrossRef]
- Soliman, O.I.; Geleijnse, M.L.; Meijboom, F.J.; Nemes, A.; Kamp, O.; Nihoyannopoulos, P.; Masani, N.; Feinstein, S.B.; Ten Cate, F.J. The use of contrast echocardiography for the detection of cardiac shunts. Eur. J. Echocardiogr. 2007, 8, S2–S12. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, J.; Zhang, Z.; Zhang, Y.; Zhu, S.; Liu, M.; Zhang, Z.; Wu, C.; Yang, X.; Cheng, J.; et al. Automatic view classification of contrast and non-contrast echocardiography. Front. Cardiovasc. Med. 2022, 9, 989091. [Google Scholar] [CrossRef]
- Medvedofsky, D.; Lang, R.M.; Kruse, E.; Guile, B.; Weinert, L.; Ciszek, B.; Jacobson, Z.; Negron, J.; Volpato, V.; Prado, A.; et al. Feasibility of Left Ventricular Global Longitudinal Strain Measurements from Contrast-Enhanced Echocardiographic Images. J. Am. Soc. Echocardiogr. 2018, 31, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Kitano, T.; Nabeshima, Y.; Otsuji, Y.; Negishi, K.; Takeuchi, M. Accuracy of Left Ventricular Volumes and Ejection Fraction Measurements by Contemporary Three-Dimensional Echocardiography with Semi- and Fully Automated Software: Systematic Review and Meta-Analysis of 1,881 Subjects. J. Am. Soc. Echocardiogr. 2019, 32, 1105–1115.e1105. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Smistad, E.; Ostvik, A.; Salte, I.M.; Melichova, D.; Nguyen, T.M.; Haugaa, K.; Brunvand, H.; Edvardsen, T.; Leclerc, S.; Bernard, O.; et al. Real-Time Automatic Ejection Fraction and Foreshortening Detection Using Deep Learning. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 2595–2604. [Google Scholar] [CrossRef]
- Suwatanaviroj, T.; He, W.; Mirhadi, E.; Paakanen, R.; Pituskin, E.; Paterson, I.; Choy, J.; Becher, H. Variability of left ventricular volume and ejection fraction measurements using contrast echocardiography: The influence of the left ventricular length measurements in a large cohort of patients during monitoring cardiotoxic effects of chemotherapy. Echocardiography 2018, 35, 322–328. [Google Scholar] [CrossRef]
- Gaibazzi, N.; Tuttolomondo, D.; Rabia, G.; Lorenzoni, V.; Benatti, G.; De Rosa, F. Standard echocardiography versus very-low mechanical index contrast-imaging: Left ventricle volumes and ejection fraction multi-reader variability and reference values in a subgroup with no risk factors or cardiac disease. Heart Vessel. 2019, 35, 544–554. [Google Scholar] [CrossRef]
- Dobson, R.; Ghosh, A.K.; Ky, B.; Marwick, T.; Stout, M.; Harkness, A.; Steeds, R.; Robinson, S.; Oxborough, D.; Adlam, D.; et al. BSE and BCOS Guideline for Transthoracic Echocardiographic Assessment of Adult Cancer Patients Receiving Anthracyclines and/or Trastuzumab. J. Am. Coll. Cardiol. CardioOnc. 2021, 3, 1–16. [Google Scholar] [CrossRef]
- Kolla, B.C.; Roy, S.S.; Duval, S.; Weisdorf, D.; Valeti, U.; Blaes, A. Cardiac Imaging Methods for Chemotherapy-related Cardiotoxicity Screening and Related Radiation Exposure: Current Practice and Trends. Anticancer Res. 2017, 37, 2445–2449. [Google Scholar] [CrossRef]
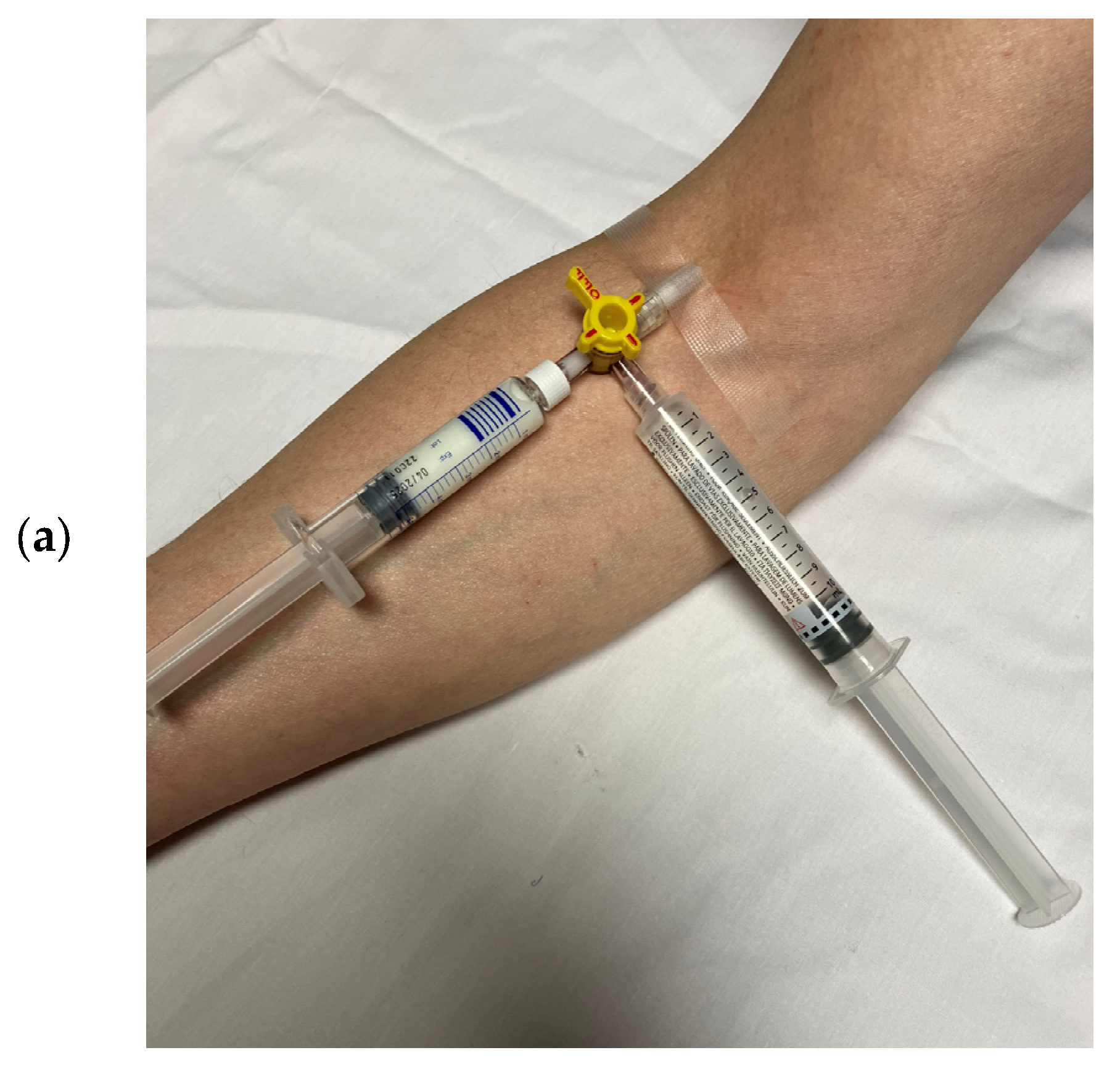
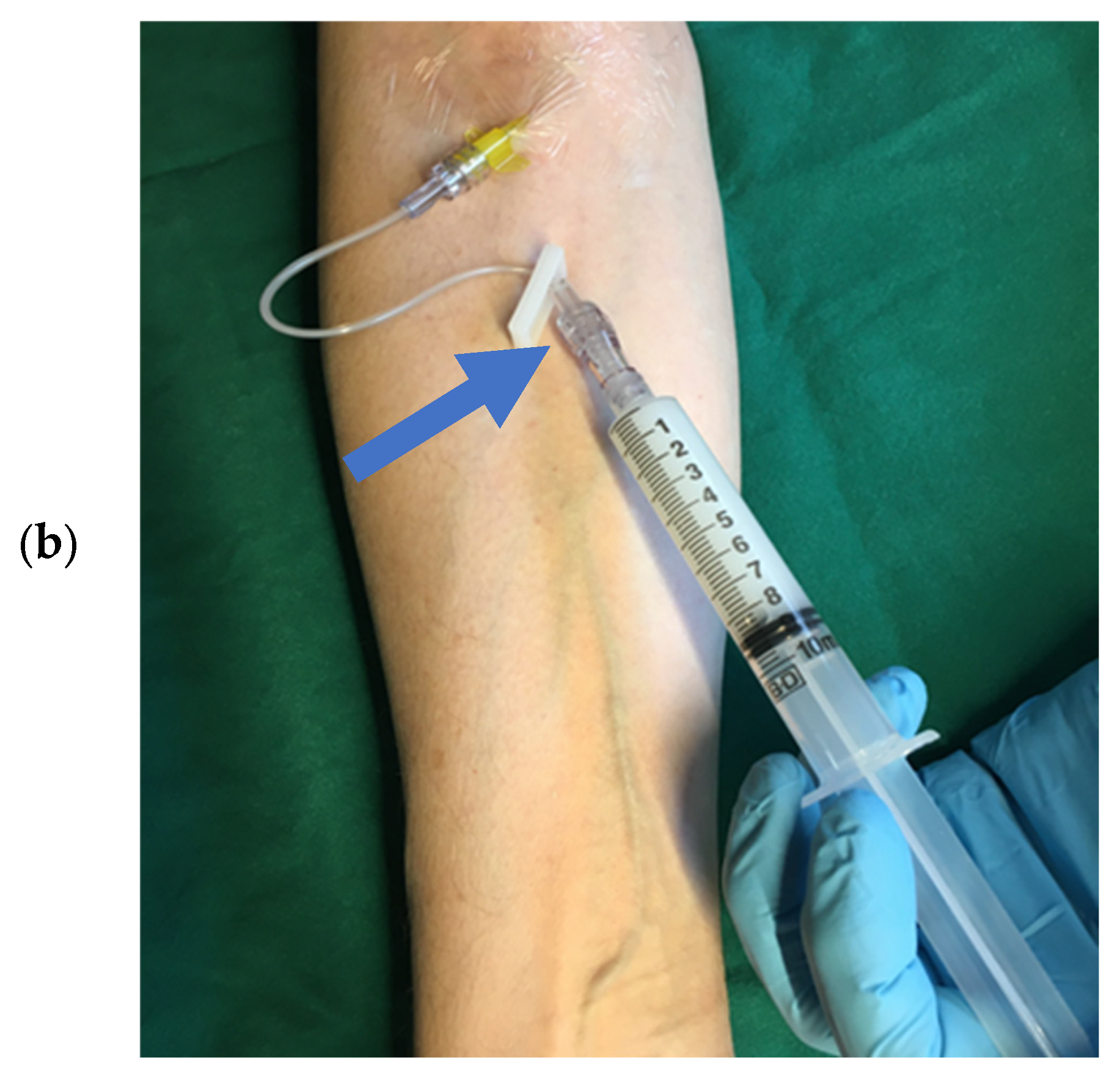


| SonoVue®/Lumason® Bracco Imaging, Milan, Italy [22,23] | Optison® GE Healthcare, Marlborough, MA, USA [24] | Luminity®/Definity® Lantheus Medical Imaging, North Billerica, MA, USA [25] | |
|---|---|---|---|
| Gas | Sulfur hexafluoride, SF6 | Perflutren, C3F8/octafluoropropan | Perflutren, C3F8, octafluoropropan |
| Shell | Phospholipid | Human albumin | Natural lipids (DPPC, DPPA), synthetic lipid MPEG 500 DPPE |
| Gas/mL | 45 μg/mL | 220 +/− 110 μg/mL | 150 μg/mL |
| Mean size of microbubbles | 1.5–2.5 μm | 3–4.5 μm | 1.1–3.3 μm |
| Bubble concentration | 1.5–5.6 × 108/mL | 5–8 × 108/mL | Up to 1.2 × 1010/mL |
| Storage | Room temperature | Refrigerator, 2°–8 °C | Refrigerator, 2°–8 °C Room temperature for Definity®RT * |
| Withdrawal from the vial, preparation # | No venting needle required; a special spike is provided in the package When a UEA is left in the vial for later injection, close the spike | Minimize positive and negative pressure by venting the vial and slowly withdrawing the UEA from the vial Remove the venting needle after withdrawal of the UEA | Minimize positive and negative pressure by venting the vial and slowly withdrawing the UEA from the vial Remove the venting needle after withdrawal of the UEA |
| SonoVue®/Lumason® [22,23] | Optison® [24] | Luminity®/Definity® [25] | |
|---|---|---|---|
| Bolus injection package inserts * | 2 mL undiluted suspension followed by 5 mL 0.9% sodium chloride flush | 0.5 mL followed by slow flush of 0.9% sodium chloride injection or 5% dextrose injection | 10 microL/kg intravenously over 30 s to 60 s followed by a 10 mL flush of 0.9% sodium chloride injection; |
| Bolus injection for current state-of-the-art scanner ** | 0.5 mL undiluted suspension or 1 mL diluted suspension (1 vial + 5 mL 0.9% saline flush) # | 0.3–0.5 mL with slow 5- to 10-mL saline flush (15) | 1–2 mL of diluted Definity® (1.3 mL activated Definity with 8.7 mL of preservative-free saline in a 10 mL syringe) 1 mL of the diluted suspension (0.5 mL in 9.5 mL 0.9% NaCl) # |
| Initial infusion rate ** | 0.7 mL/min of undiluted suspension or 1.4 mL/min of diluted suspension (1:1 with 0.9% NaCl) # | 1–4 mL/min of diluted suspension (3 mL in 30 mL 0.9% NaCl) | 4 mL/min of diluted suspension (1 vial, 1.3 mL in 50 mL 0.9% NaCl -saline bag) #; not to exceed 10 mL/min 1 mL/min of diluted suspension (1 vial in 30 mL 0.9% NaCl syringe) # |
| Maximum dosage | Max. 2 mL/undiluted dose; a second dosage may be administered. | The maximum total dose should not exceed 5.0 mL in any 10 min period. The maximum total dose should not exceed 8.7 mL in any one patient study. | Two bolus doses of undiluted Definity (5 to 30 min apart, depending on EU, CA, or US) or one single infusion. |
| All UEAs | Pregnant and lactating women have not been included in studies for clinical license. |
| SonoVue® * | Previous hypersensitivity reactions to SonoVue®/Lumason® components of UEAs such as polyethylene glycol (PEG, macrogol), or PEG-containing products such as certain bowel preparations for colonoscopy or laxatives. Severe pulmonary hypertension (>90 mmHg). Acute respiratory distress syndrome (ARDS). Known right-to-left shunt. In combination with dobutamine in patients with conditions suggesting cardiovascular instability where dobutamine is contraindicated. |
| Luminity®/Definity® | Previous hypersensitivity reactions to these UEAs, components of UEAs such as polyethylene glycol (PEG, macrogol), or PEG-containing products such as certain bowel preparations for colonoscopy or laxatives. CAVEAT: Patients with sickle cell disease are particularly susceptible to adverse nociceptive events at high doses. |
| Optison® | Previous hypersensitivity reaction to Optison®. Allergy to blood products or albumin. |
| Low-MI Method * | Intermediate-MI Method * | |
|---|---|---|
| Mechanical Index (MI) | <0.2 * | 0.2–0.5 |
| Applications | First-line method for all LV indications | May be used in addition for LV thrombi; excessive LV trabeculation |
| Strengths | High sensitivity for detecting UEA Myocardial tissue signals minimized Display of contrast in myocardial vessels for assessment of perfusion | Higher spatial resolution |
| Limitations | Spatial resolution is worse than in the intermediate-MI method | More microbubble destruction; apical swirling is more likely Myocardial tissue signals, which limit delineation of cavity/myocardium |
|
|
|
| 1 | Select unforeshortened loops avoid 4-chamber views, which include anterolateral papillary muscles | When several loops are available, select the one with the longest long axis which impairs tracing of the lateral LV wall |
| 2 | Select the end-diastolic frame | The first frame after mitral valve closure or the largest LV cavity |
| 3 | Trace the LV border: end diastole | Starting on the septal mitral ring (4-chamber view) and inferior mitral ring (2-chamber view), following the dark/bright interface until the lateral/anterior mitral ring The start and end points of the contour at the mitral ring should be connected by a straight line TIP The mitral leaflets may be obscured by the UEA. Then look for the bright signals of the mitral ring, which are more clearly delineated and follow the entire loop rather than only in the end-diastolic frame. |
| 4 | Select the end-systolic frame | Smallest cavity—the mitral valve closure may be obscured by the UEA! TIP: When scrolling through the systolic frames of the loop, look for the frame when the cavity becomes larger. The frame before it is the end-systolic frame. |
| 5 | Trace the LV border: end systole | Starting on the septal mitral ring (4-chamber view) and inferior mitral ring (2-chamber view), following the dark/bright interface until the lateral/anterior mitral ring The start and end points of the contour at the mitral ring should be connected with a straight line |
| 6 | Check the LV length (distance between the middle of the line connecting the mitral ring and the apex) | When the difference in diastolic LV length between 4- and 2-chamber views is <0.5 cm, no major foreshortening can be assumed When the difference is >5 mm, the recording of the view with the shorter LV length is probably not optimal. The other recordings of this view should be reviewed to find the one with the longest LV [59]. |
| Imaging method | Low-MI contrast-specific imaging. Additional intermediate-MI imaging in case of apical aneurysm for the assessment of thrombi. |
| Sector depth/width | Adjust to display the entire LV and 1/3 of LA. |
| Focus | Midventricular or basal. |
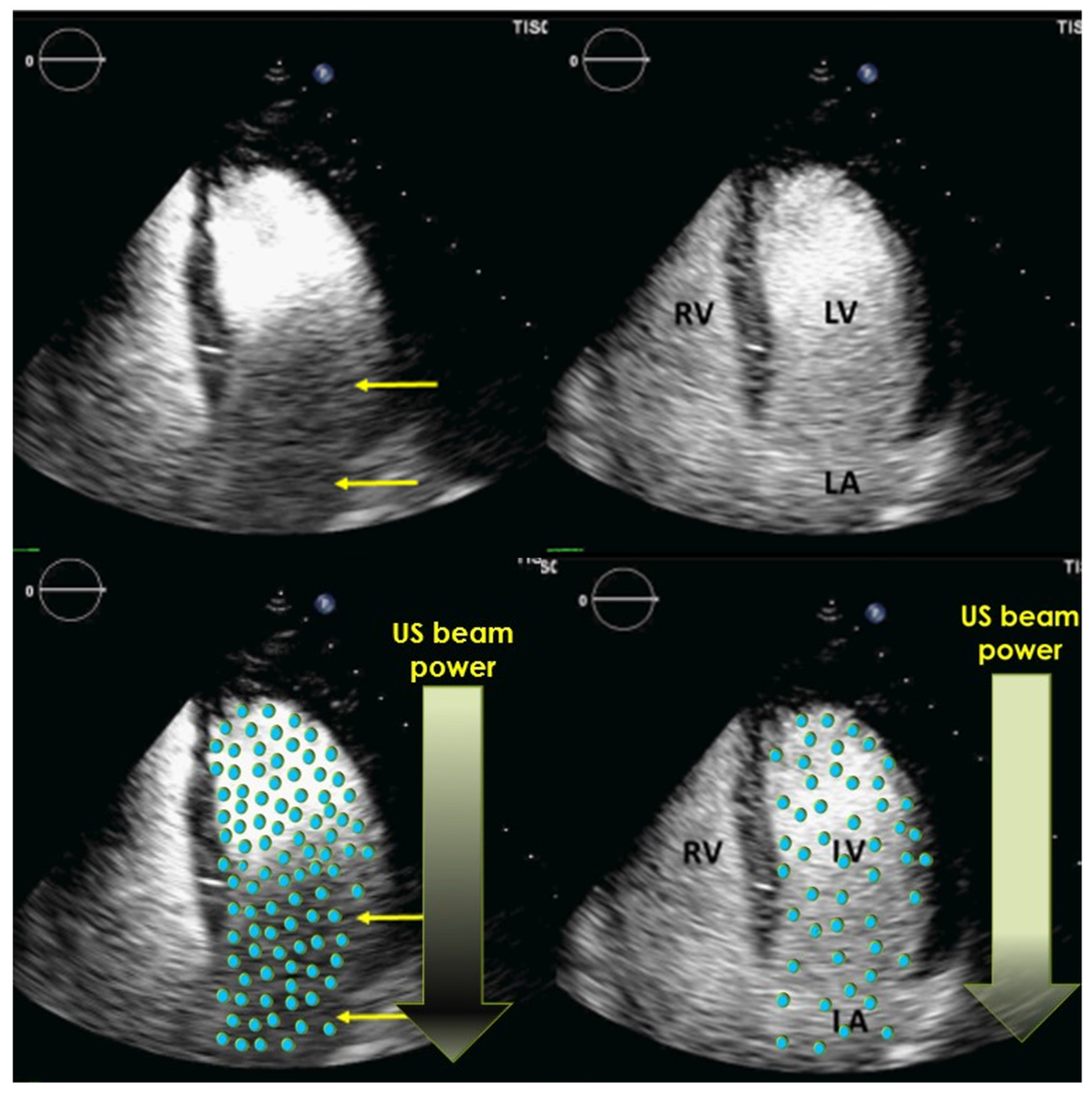
| Compression | Adjust to enhance any signals from contrast agents; a high dynamic range is not necessary. The preset of the manufacturers is usually adequate. |
| Gain | Adjust to enhance the delineation of the LV. Often, a reduction in the gain is required to reduce the myocardial contrast signals. |
| Automated segmentation and volume calculation | Currently introduced by one manufacturer. |
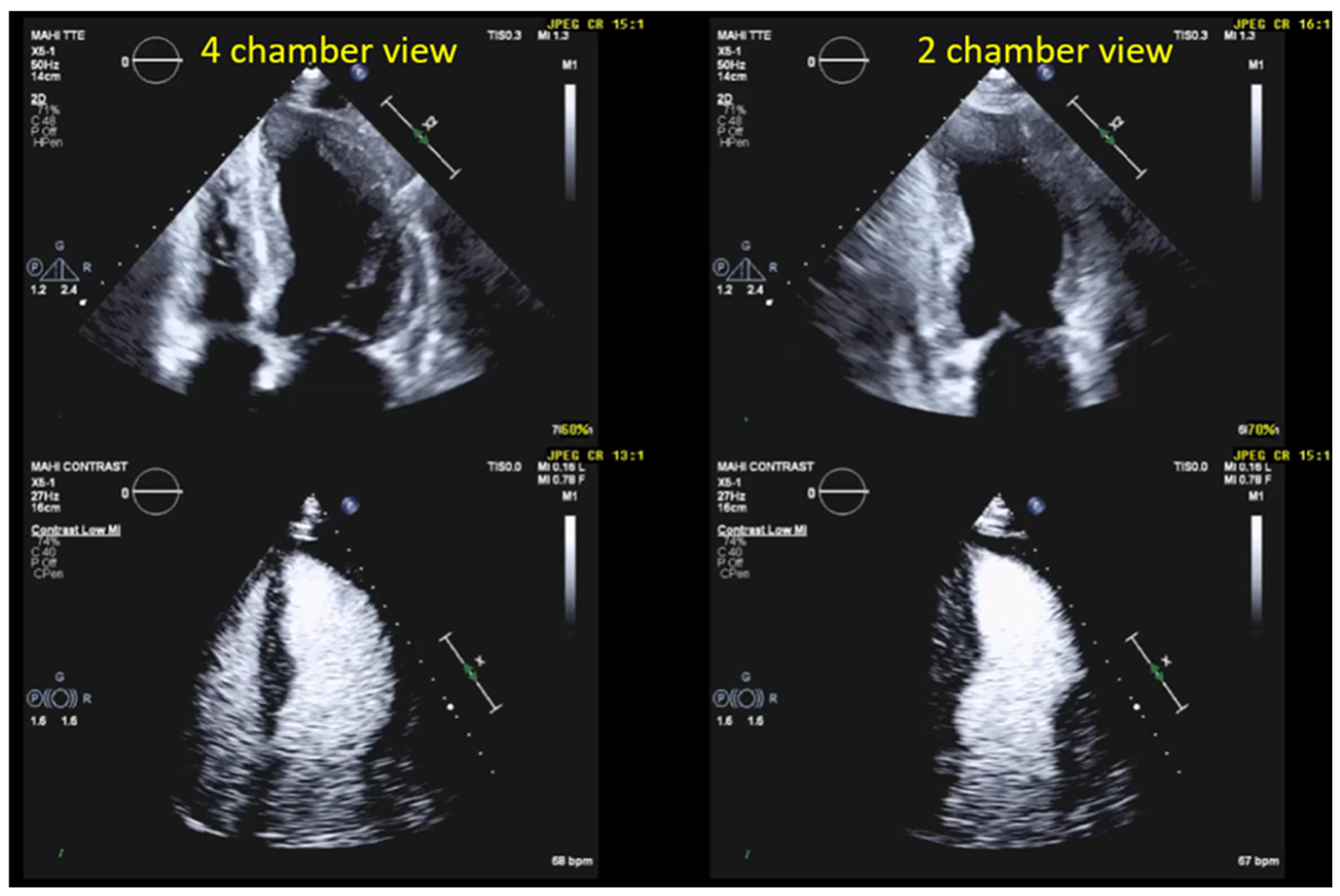
| Imaging planes | Standard apical views and apical sweeps to assess the LV cavity and search for thrombi. The aneurysm may only be displayed in non-standard views [Figure 12]. |
| Contrast application | Bolus injections. |
| Typical findings | LV cavity is well delineated during the entire cardiac cycle. |
| Alternative imaging | MRI when there are contraindications to UEAs or inadequate recordings despite the use of UEAs. Gated blood pool scintigraphy and CT in patients with contraindications for MRIs and UEAs. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becher, H.; Helfen, A.; Michels, G.; Gaibazzi, N.; Senior, R.; Dietrich, C.F. How to Perform Cardiac Contrast-Enhanced Ultrasound (cCEUS): Part I. Diagnostics 2025, 15, 1743. https://doi.org/10.3390/diagnostics15141743
Becher H, Helfen A, Michels G, Gaibazzi N, Senior R, Dietrich CF. How to Perform Cardiac Contrast-Enhanced Ultrasound (cCEUS): Part I. Diagnostics. 2025; 15(14):1743. https://doi.org/10.3390/diagnostics15141743
Chicago/Turabian StyleBecher, Harald, Andreas Helfen, Guido Michels, Nicola Gaibazzi, Roxy Senior, and Christoph Frank Dietrich. 2025. "How to Perform Cardiac Contrast-Enhanced Ultrasound (cCEUS): Part I" Diagnostics 15, no. 14: 1743. https://doi.org/10.3390/diagnostics15141743
APA StyleBecher, H., Helfen, A., Michels, G., Gaibazzi, N., Senior, R., & Dietrich, C. F. (2025). How to Perform Cardiac Contrast-Enhanced Ultrasound (cCEUS): Part I. Diagnostics, 15(14), 1743. https://doi.org/10.3390/diagnostics15141743





