Long-Term Follow-Up of Professional Soccer Players: The Analyses of Left and Right Heart Morphology and Function by Conventional, Three-Dimensional, and Deformation Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Tracking the Seasonal Load Data
2.3. Transthoracic Echocardiography
2.4. Left Heart Morphology and Function
2.5. Right Heart Morphology and Function
2.6. Statistical Analysis
3. Results
4. Discussion
- (1)
- Most of the athletes showed normal LV and RV morphology and function at baseline, despite all athletes playing professional soccer for many years.
- (2)
- Neither conventional nor advanced echocardiographic parameters indicated the development of an athlete’s heart in this cohort.
4.1. Introduction to Cardiovascular Adaptations
4.2. Expected Adaptations in Soccer Athletes
4.3. Changes in Myocardial Deformation
4.4. Atrial Function
4.5. Differentiating Physiological Remodeling from Pathological Adaptation
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Bosscher, R.; Claeys, M.; Dausin, C.; Goetschalckx, K.; Claus, P.; Herbots, L.; Ghekiere, O.; Van De Heyning, C.; Paelinck, B.P.; Janssens, K.; et al. Three-Dimensional Echocardiography of the Athlete’s Heart: A Comparison with Cardiac Magnetic Resonance Imaging. Int. J. Cardiovasc. Imaging 2022, 39, 295–306. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Caselli, S.; Solari, M.; Pelliccia, A.; Cameli, M.; Focardi, M.; Padeletti, M.; Corrado, D.; Bonifazi, M.; Mondillo, S. Novel Echocardiographic Techniques for the Evaluation of Athletes’ Heart: A Focus on Speckle-Tracking Echocardiography. Eur. J. Prev. Cardiol. 2016, 23, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Larson, M.G.; McCabe, E.L.; Osypiuk, E.; Lehman, B.T.; Stanchev, P.; Aragam, J.; Benjamin, E.J.; Solomon, S.D.; Vasan, R.S. Reproducibility of Speckle-Tracking-Based Strain Measures of Left Ventricular Function in a Community-Based Study. J. Am. Soc. Echocardiogr. 2013, 26, 1258–1266.e2. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, T.; Biering-Sørensen, S.R.; Olsen, F.J.; Sengeløv, M.; Jørgensen, P.G.; Mogelvang, R.; Shah, A.M.; Jensen, J.S. Global Longitudinal Strain by Echocardiography Predicts Long-Term Risk of Cardiovascular Morbidity and Mortality in a Low-Risk General Population: The Copenhagen City Heart Study. Circ. Cardiovasc. Imaging 2017, 10, e005521. [Google Scholar] [CrossRef]
- Tops, L.F.; Delgado, V.; Marsan, N.A.; Bax, J.J. Myocardial Strain to Detect Subtle Left Ventricular Systolic Dysfunction. Eur. J. Heart Fail. 2017, 19, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Utomi, V.; Oxborough, D.; Whyte, G.P.; Somauroo, J.; Sharma, S.; Shave, R.; Atkinson, G.; George, K. Systematic Review and Meta-Analysis of Training Mode, Imaging Modality and Body Size Influences on the Morphology and Function of the Male Athlete’s Heart. Heart 2013, 99, 1727–1733. [Google Scholar] [CrossRef]
- Palermi, S.; Cavarretta, E.; D’Ascenzi, F.; Castelletti, S.; Ricci, F.; Vecchiato, M.; Serio, A.; Cavigli, L.; Bossone, E.; Limongelli, G.; et al. Athlete’s Heart: A Cardiovascular Step-By-Step Multimodality Approach. Rev. Cardiovasc. Med. 2023, 24, 151. [Google Scholar] [CrossRef]
- Pelliccia, A.; Caselli, S.; Sharma, S.; Basso, C.; Bax, J.J.; Corrado, D.; D’Andrea, A.; D’Ascenzi, F.; Di Paolo, F.M.; Edvardsen, T.; et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) Joint Position Statement: Recommendations for the Indication and Interpretation of Cardiovascular Imaging in the Evaluation of the Athlete’s Heart. Eur. Heart J. 2018, 39, 1949–1969. [Google Scholar] [CrossRef]
- Osgnach, C.; Poser, S.; Bernardini, R.; Rinaldo, R.; Di Prampero, P.E. Energy Cost and Metabolic Power in Elite Soccer: A New Match Analysis Approach. Med. Sci. Sports Exerc. 2010, 42, 170–178. [Google Scholar] [CrossRef]
- García-Calvo, T.; Ponce-Bordón, J.C.; Pons, E.; López Del Campo, R.; Resta, R.; Raya-González, J. High Metabolic Load Distance in Professional Soccer According to Competitive Level and Playing Positions. PeerJ 2022, 10, e13318. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.-U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a Common Standard for 2D Speckle Tracking Echocardiography: Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 183–193. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of Left Atrial, Right Ventricular, and Right Atrial Deformation Imaging Using Two-Dimensional Speckle Tracking Echocardiography: A Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, B.J.; Spataro, A.; Proschan, M.A.; Spirito, P. The Upper Limit of Physiologic Cardiac Hypertrophy in Highly Trained Elite Athletes. N. Engl. J. Med. 1991, 324, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Caselli, S.; Di Paolo, F.M.; Pisicchio, C.; Di Pietro, R.; Quattrini, F.M.; Di Giacinto, B.; Culasso, F.; Pelliccia, A. Three-Dimensional Echocardiographic Characterization of Left Ventricular Remodeling in Olympic Athletes. Am. J. Cardiol. 2011, 108, 141–147. [Google Scholar] [CrossRef]
- Zaidi, A.; Ghani, S.; Sharma, R.; Oxborough, D.; Panoulas, V.F.; Sheikh, N.; Gati, S.; Papadakis, M.; Sharma, S. Physiological Right Ventricular Adaptation in Elite Athletes of African and Afro-Caribbean Origin. Circulation 2013, 127, 1783–1792. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, B.J.; Di Paolo, F.M.; Biffi, A.; Quattrini, F.M.; Pisicchio, C.; Roselli, A.; Caselli, S.; Culasso, F. Prevalence and Clinical Significance of Left Atrial Remodeling in Competitive Athletes. J. Am. Coll. Cardiol. 2005, 46, 690–696. [Google Scholar] [CrossRef]
- Rawlins, J.; Carre, F.; Kervio, G.; Papadakis, M.; Chandra, N.; Edwards, C.; Whyte, G.P.; Sharma, S. Ethnic Differences in Physiological Cardiac Adaptation to Intense Physical Exercise in Highly Trained Female Athletes. Circulation 2010, 121, 1078–1085. [Google Scholar] [CrossRef]
- Di Paolo, F.M.; Schmied, C.; Zerguini, Y.A.; Junge, A.; Quattrini, F.; Culasso, F.; Dvorak, J.; Pelliccia, A. The Athlete’s Heart in Adolescent Africans. J. Am. Coll. Cardiol. 2012, 59, 1029–1036. [Google Scholar] [CrossRef]
- Morganroth, J.; Maron, B.J.; Henry, W.L.; Epstein, S.E. Comparative Left Ventricular Dimensions in Trained Athletes. Ann. Intern. Med. 1975, 82, 521–524. [Google Scholar] [CrossRef]
- D’Andrea, A.; Riegler, L.; Cocchia, R.; Scarafile, R.; Salerno, G.; Gravino, R.; Golia, E.; Vriz, O.; Citro, R.; Limongelli, G.; et al. Left Atrial Volume Index in Highly Trained Athletes. Am. Heart J. 2010, 159, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.H.; Haskell, W.; Snell, P.; Van Camp, S.P. Task Force 8: Classification of Sports. J. Am. Coll. Cardiol. 2005, 45, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Riegler, L.; Morra, S.; Scarafile, R.; Salerno, G.; Cocchia, R.; Golia, E.; Martone, F.; Di Salvo, G.; Limongelli, G.; et al. Right Ventricular Morphology and Function in Top-Level Athletes: A Three-Dimensional Echocardiographic Study. J. Am. Soc. Echocardiogr. 2012, 25, 1268–1276. [Google Scholar] [CrossRef]
- Pelliccia, A.; Culasso, F.; Di Paolo, F.M.; Maron, B.J. Physiologic Left Ventricular Cavity Dilatation in Elite Athletes. Ann. Intern. Med. 1999, 130, 23–31. [Google Scholar] [CrossRef]
- Spirito, P.; Pelliccia, A.; Proschan, M.A.; Granata, M.; Spataro, A.; Bellone, P.; Caselli, G.; Biffi, A.; Vecchio, C.; Maron, B.J. Morphology of the “Athlete’s Heart” Assessed by Echocardiography in 947 Elite Athletes Representing 27 Sports. Am. J. Cardiol. 1994, 74, 802–806. [Google Scholar] [CrossRef]
- Oxborough, D.; Sharma, S.; Shave, R.; Whyte, G.; Birch, K.; Artis, N.; Batterham, A.M.; George, K. The Right Ventricle of the Endurance Athlete: The Relationship between Morphology and Deformation. J. Am. Soc. Echocardiogr. 2012, 25, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Russell, E.R.; Mackay, D.F.; Stewart, K.; MacLean, J.A.; Pell, J.P.; Stewart, W. Association of Field Position and Career Length with Risk of Neurodegenerative Disease in Male Former Professional Soccer Players. JAMA Neurol. 2021, 78, 1057. [Google Scholar] [CrossRef]
- Caselli, S.; Montesanti, D.; Autore, C.; Di Paolo, F.M.; Pisicchio, C.; Squeo, M.R.; Musumeci, B.; Spataro, A.; Pandian, N.G.; Pelliccia, A. Patterns of Left Ventricular Longitudinal Strain and Strain Rate in Olympic Athletes. J. Am. Soc. Echocardiogr. 2015, 28, 245–253. [Google Scholar] [CrossRef]
- Wu, V.C.-C.; Takeuchi, M. Echocardiographic Assessment of Right Ventricular Systolic Function. Cardiovasc. Diagn. Ther. 2018, 8, 70–79. [Google Scholar] [CrossRef]
- Pagourelias, E.D.; Kouidi, E.; Efthimiadis, G.K.; Deligiannis, A.; Geleris, P.; Vassilikos, V. Right Atrial and Ventricular Adaptations to Training in Male Caucasian Athletes: An Echocardiographic Study. J. Am. Soc. Echocardiogr. 2013, 26, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Richand, V.; Lafitte, S.; Reant, P.; Serri, K.; Lafitte, M.; Brette, S.; Kerouani, A.; Chalabi, H.; Dos Santos, P.; Douard, H.; et al. An Ultrasound Speckle Tracking (Two-Dimensional Strain) Analysis of Myocardial Deformation in Professional Soccer Players Compared with Healthy Subjects and Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2007, 100, 128–132. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, K.H.; Rink, L.; Hornsby, K.; Cho, J.Y.; Cho, G.-Y.; Lee, J.-H.; Seong, I.-W.; Jeong, M.H.; Cho, J.G.; et al. Left Atrial Enlargement and Its Association with Left Atrial Strain in University Athletes Participated in 2015 Gwangju Summer Universiade. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Donal, E.; Cameli, M.; Sade, L.E. The Atrium: Central Part of a Building—A Definition, Cardiologists Should Not Forget. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 873–875. [Google Scholar] [CrossRef]
- Trivedi, S.J.; Claessen, G.; Stefani, L.; Flannery, M.D.; Brown, P.; Janssens, K.; Elliott, A.; Sanders, P.; Kalman, J.; Heidbuchel, H.; et al. Differing Mechanisms of Atrial Fibrillation in Athletes and Non-Athletes: Alterations in Atrial Structure and Function. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Maitra, N.S.; Hassan Virk, H.U.; Farrell, A.; Hamzeh, I.; Arya, B.; Pressman, G.S.; Wang, Z.; Marwick, T.H. Normal Ranges of Right Atrial Strain. JACC Cardiovasc. Imaging 2023, 16, 282–294. [Google Scholar] [CrossRef]
- Oxborough, D.; George, K.; Cooper, R.; Bhatia, R.; Ramcharan, T.; Zaidi, A.; Gati, S.; Prakash, K.; Rakhit, D.; Robinson, S.; et al. Echocardiography in the Cardiac Assessment of Young Athletes: A 2025 Guideline from the British Society of Echocardiography (Endorsed by Cardiac Risk in the Young). Echo Res. Pract. 2025, 12, 7. [Google Scholar]
- Zorzi, A.; Calore, C.; Vio, R.; Pelliccia, A.; Corrado, D. Accuracy of the ECG for Differential Diagnosis between Hypertrophic Cardiomyopathy and Athlete’s Heart: Comparison between the European Society of Cardiology (2010) and International (2017) Criteria. Br. J. Sports Med. 2018, 52, 667–673. [Google Scholar] [CrossRef]
- Gomez, S.E.; Perez, M.V.; Wheeler, M.T.; Hadley, D.; Hwang, C.E.; Kussman, A.; Kim, D.S.; Froelicher, V. Classification of Premature Ventricular Contractions in Athletes During Routine Preparticipation Exams. Circ. Arrhythmia Electrophysiol. 2024, 17, e012835. [Google Scholar] [CrossRef]
- Brosnan, M.J.; Te Riele, A.S.J.M.; Bosman, L.P.; Hoorntje, E.T.; Van Den Berg, M.P.; Hauer, R.N.W.; Flannery, M.D.; Kalman, J.M.; Prior, D.L.; Tichnell, C.; et al. Electrocardiographic Features Differentiating Arrhythmogenic Right Ventricular Cardiomyopathy from an Athlete’s Heart. JACC Clin. Electrophysiol. 2018, 4, 1613–1625. [Google Scholar] [CrossRef]
- Efremidis, M.; Vlachos, K.; Kyriakopoulou, M.; Mililis, P.; Martin, C.A.; Bazoukis, G.; Dragasis, S.; Megarisiotou, A.; Unger, P.; Frontera, A.; et al. The RV1-V3 Transition Ratio: A Novel Electrocardiographic Criterion for the Differentiation of Right versus Left Outflow Tract Premature Ventricular Complexes. Heart Rhythm O2 2021, 2, 521–528. [Google Scholar] [CrossRef] [PubMed]
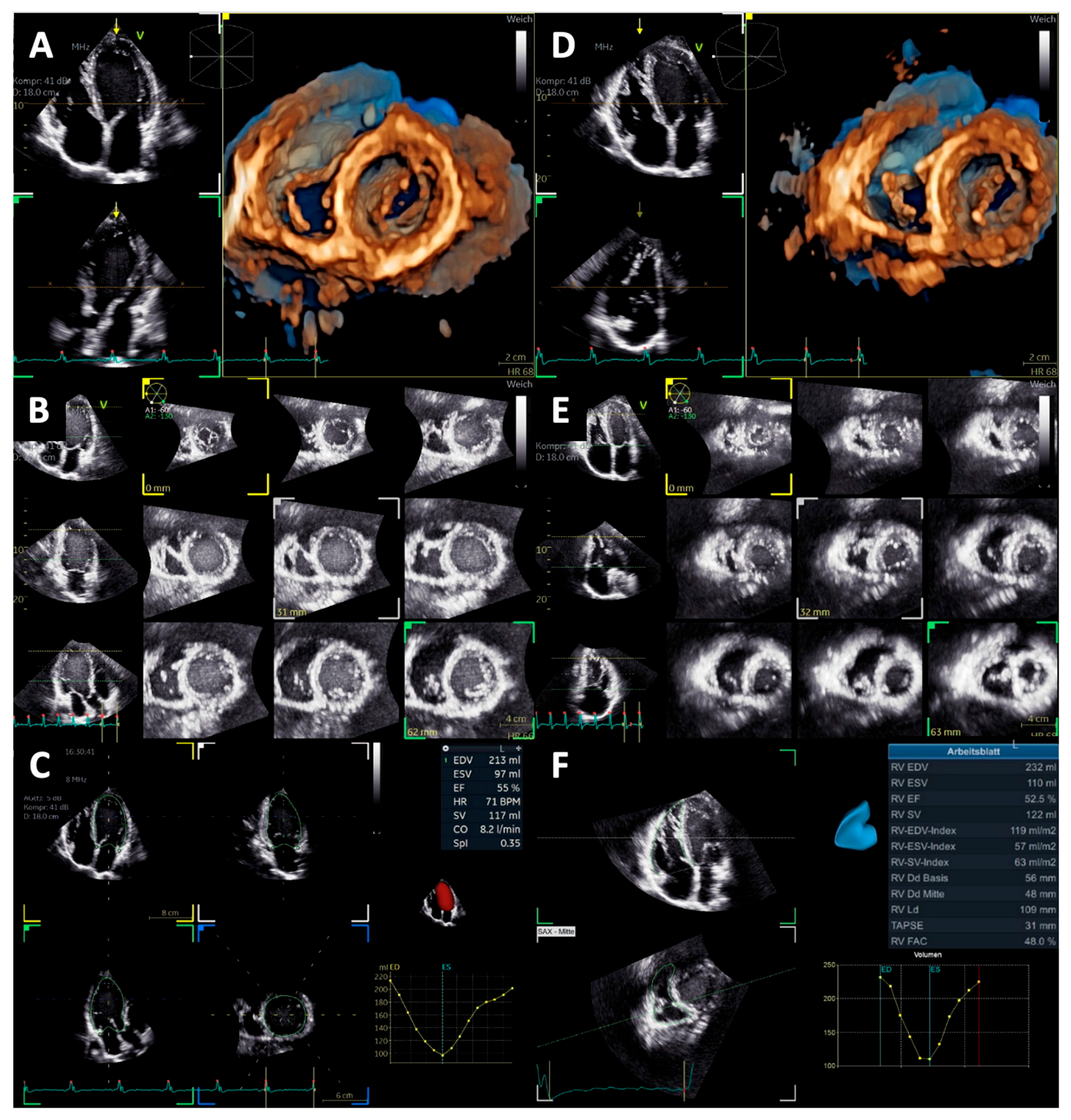
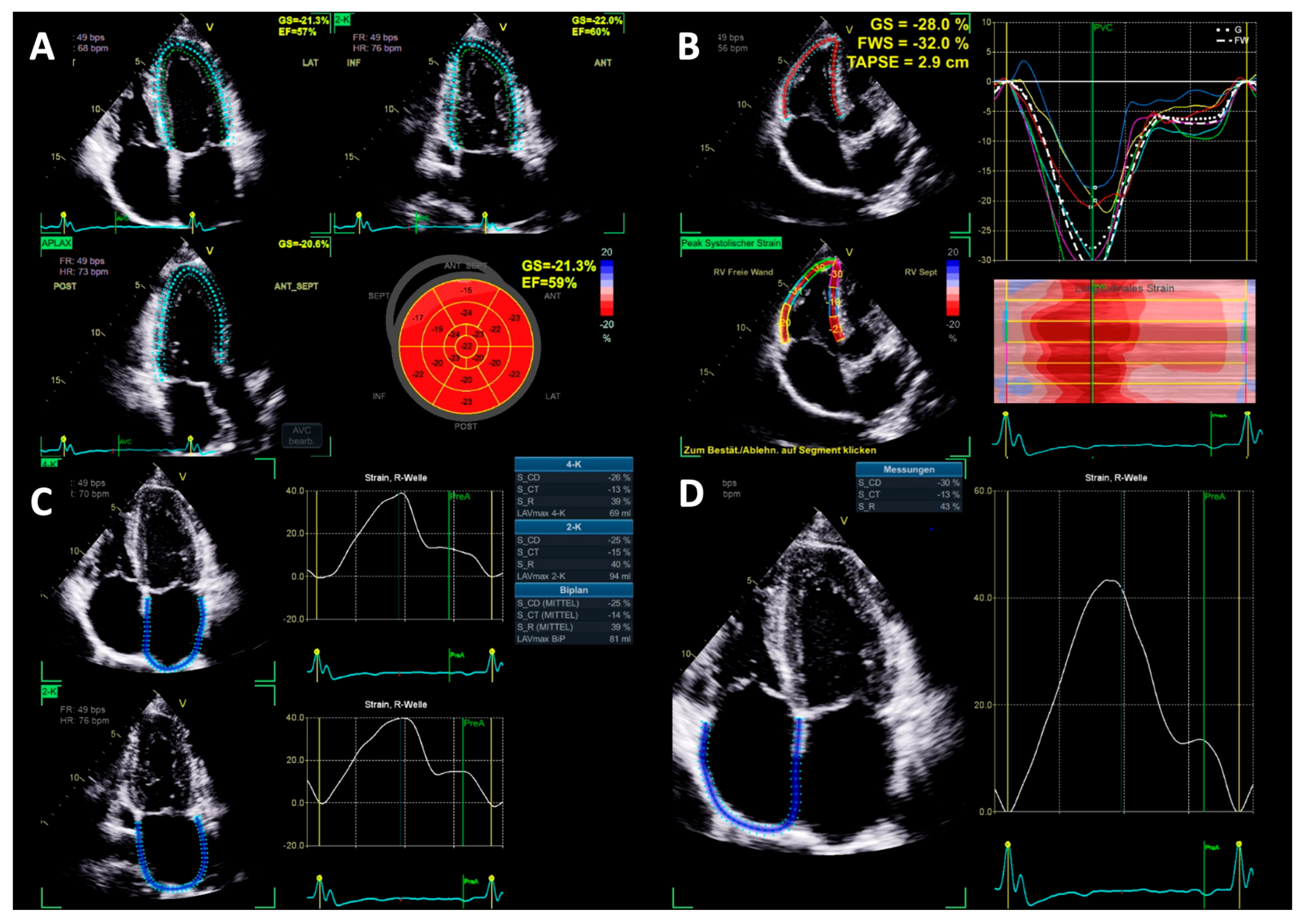
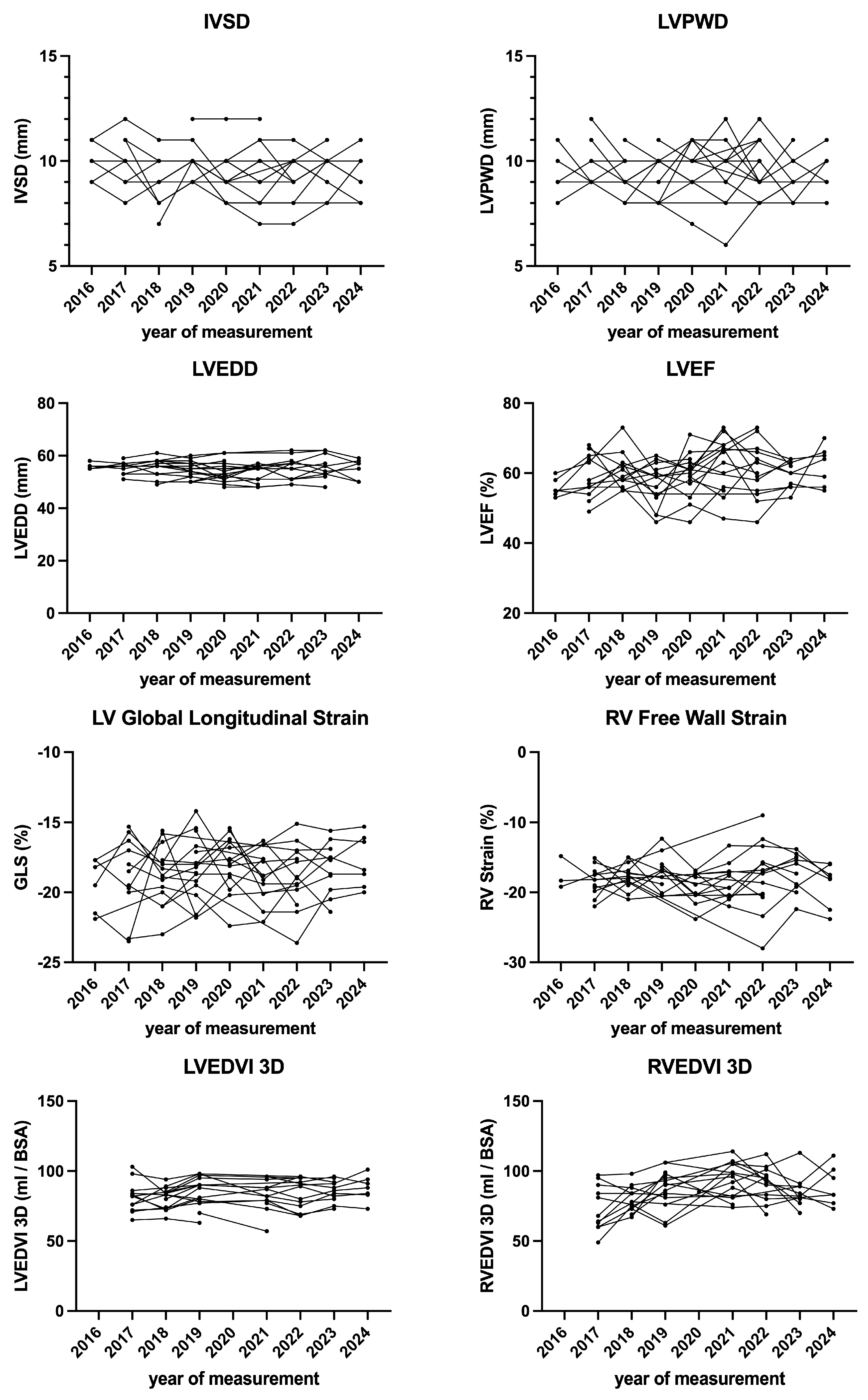
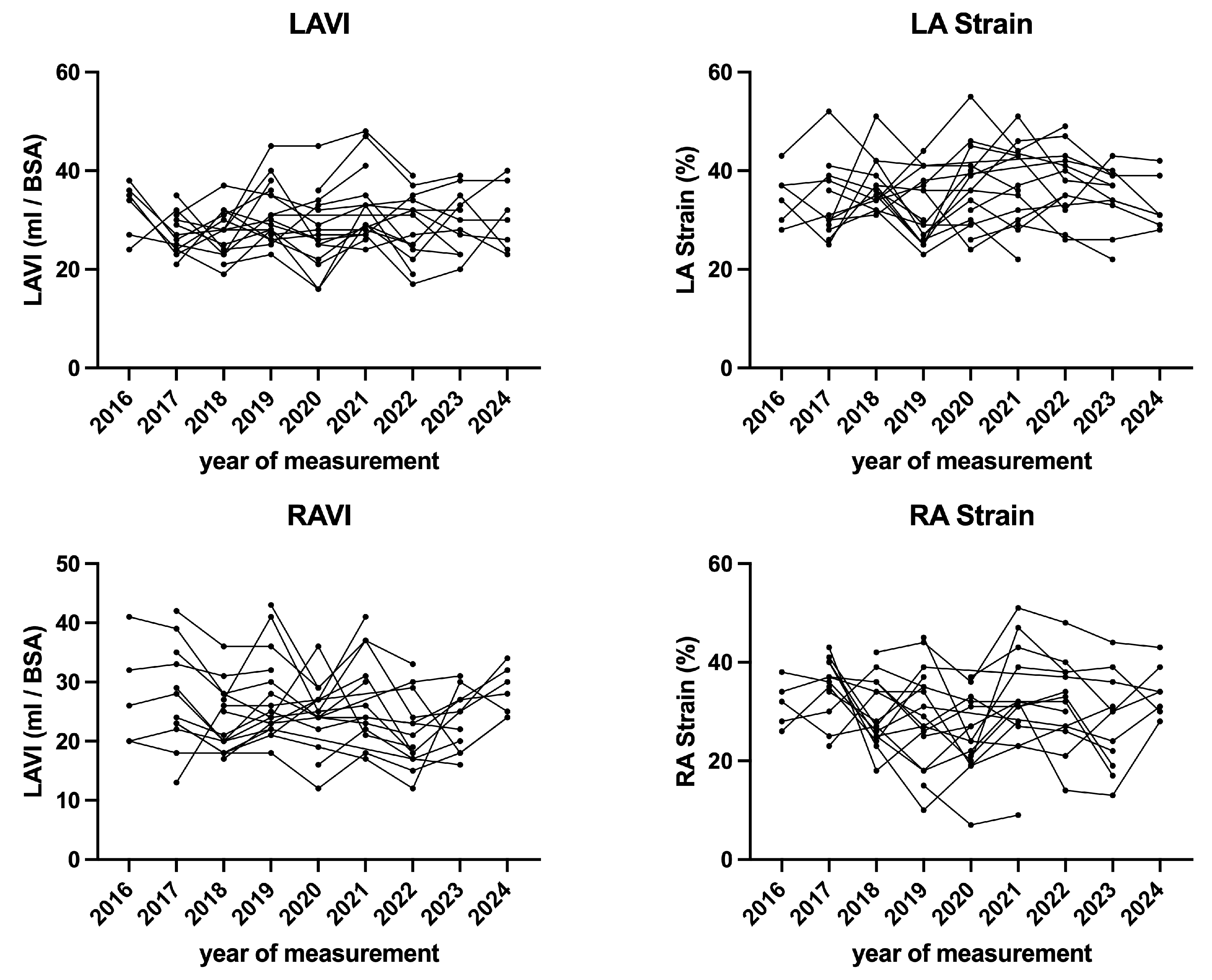
| Parameter | Baseline | End of Follow-Up | p Value |
|---|---|---|---|
| Age (years) | 21.2 ± 3.4 | 26.8 ± 4.1 | <0.001 |
| Male (%) | 20 (100) | - | - |
| Height (cm) | 182.7 ± 6.1 | 183.0 ± 6.6 | 0.163 |
| Weight (kg) | 78.9 ± 6.7 | 80.2 ± 8.6 | 0.088 |
| BSA (m2) | 2.01 ± 0.12 | 2.02 ± 0.14 | 0.094 |
| Heart rate (/min) | 56.4 ± 9.8 | 60.1 ± 9.7 | 0.169 |
| European origin (%) | 13 (65%) | - | - |
| African origin (%) | 7 (35%) | - | - |
| Parameters | Baseline | End of Follow-Up | p Value |
|---|---|---|---|
| IVSD (mm) | 9.9 ± 1.0 | 9.9 ± 1.2 | 0.878 |
| LVPWD (mm) | 9.7 ± 1.3 | 9.8 ± 1.7 | 0.707 |
| LVEDD (mm) | 54.5 ± 3.1 | 54.6 ± 3.9 | 0.868 |
| RWT | 0.35 ± 0.05 | 0.36 ± 0.09 | 0.539 |
| LVMi (g/m2) | 104.8 ± 16.6 | 101.5 ± 17.5 | 0.507 |
| GLS (%) | −18.7 ± 2.2 | −18.4 ± 1.9 | 0.670 |
| DLVOT (mm) | 23.0 ± 1.2 | 23.9 ± 1.5 | 0.001 |
| VTILVOT (cm) | 21.9 ± 2.8 | 22.9 ± 2.8 | 0.067 |
| LVSViDoppler (mL/m2) | 44.9 ± 5.6 | 50.1 ± 5.0 | <0.001 |
| Cardiac Index (L/min/m2) | 2.5 ± 0.6 | 3.0 ± 0.5 | 0.006 |
| LVEDVibiplane (mL/m2) | 80.3 ± 8.6 | 78.3 ± 9.5 | 0.398 |
| LVESVibiplane (mL/m2) | 34.1 ± 6.5 | 30.1 ± 7.1 | 0.064 |
| LVSVibiplane (mL/m2) | 48.2 ± 11.6 | 48.2 ± 5.1 | 0.979 |
| LVEFbiplane (%) | 57.8 ± 5.4 | 61.9 ± 5.6 | 0.032 |
| MAPSE (mm) | 13.0 ± 3.0 | 12.8 ± 2.4 | 0.746 |
| LVEDVi3D (mL/m2) | 82.2 ± 11.1 | 83.5 ± 12.2 | 0.982 |
| LVESVi3D (mL/m2) | 36.3 ± 9.7 | 33.1 ± 10.6 | 0.157 |
| LVSVi3D (mL/m2) | 45.9 ± 5.8 | 46.2 ± 12.3 | 0.715 |
| LVEF3D (mL/m2) | 56.3 ± 7.5 | 58.6 ± 4.2 | 0.218 |
| Parameters | Baseline | End of Follow-Up | p Value |
|---|---|---|---|
| E/A-Ratio | 2.01 ± 0.50 | 1.75 ± 0.41 | 0.035 |
| E/e’ septal | 5.8 ± 0.9 | 5.5 ± 1.0 | 0.266 |
| E/e’ lateral | 4.5 ± 1.0 | 4.2 ± 0.8 | 0.278 |
| e’ septal | 0.14 ± 0.02 | 0.14 ± 0.02 | 0.948 |
| e’ lateral | 0.18 ± 0.03 | 0.18 ± 0.03 | 0.733 |
| E Wave (m/s) | 0.79 ± 0.16 | 0.73 ± 0.09 | 0.189 |
| A Wave (m/s) | 0.40 ± 0.09 | 0.43 ± 0.08 | 0.210 |
| TR-Vmax (m/s) | 2.3 ± 0.2 | 2.3 ± 0.2 | 0.828 |
| sPAP (mmHg) | 20.4 ± 3.7 | 20.5 ± 3.1 | 0.866 |
| LA Strain CD (%) | −23.2 ± 3.8 | −23.6 ± 6.0 | 0.909 |
| LA Strain CT (%) | −8.9 ± 2.7 | −10.3 ± 3.2 | 0.064 |
| LA Strain R (%) | 32.1 ± 5.3 | 33.9 ± 6.9 | 0.301 |
| LA Volmax (mL) | 58.5 ± 9.9 | 62.8 ± 15.9 | 0.305 |
| LAVi (mL/m2) | 29.8 ± 5.2 | 31.1 ± 7.4 | 0.401 |
| Parameters | Baseline | End of Follow-Up | p Value |
|---|---|---|---|
| DRVOT (mm) | 23.2 ± 1.7 | 23.7 ± 1.7 | 0.096 |
| VTIRVOT (cm) | 21.0 ± 2.7 | 21.7 ± 2.3 | 0.185 |
| RVSViDoppler (mL/m2) | 43.3 ± 6.7 | 47.1 ± 4.4 | 0.037 |
| RA Strain CD (%) | −23.9 ± 5.6 | −20.6 ± 7.3 | 0.085 |
| RA Strain CT (%) | −8.1 ± 4.9 | −9.4 ± 4.4 | 0.394 |
| RA Strain R (%) | 32.2 ± 8.5 | 30.0 ± 7.8 | 0.352 |
| RA Volmax (mL) | 53.9 ± 18.0 | 54.6 ± 13.0 | 0.732 |
| RAVi (mL/m2) | 26.7 ± 8.5 | 26.9 ± 6.2 | 0.803 |
| RV Free Wall Strain (%) | −17.9 ± 2.2 | −18.5 ± 3.2 | 0.801 |
| RVEDVi3D (mL/m2) | 84.8 ± 14.3 | 80.6 ± 29.8 | 0.401 |
| RVESVi3D (mL/m2) | 40.4 ± 11.1 | 37.9 ± 15.5 | 0.574 |
| RVSVi3D (mL/m2) | 44.3 ± 5.7 | 47.4 ± 5.7 | 0.671 |
| RVEF3D (%) | 53.0 ± 6.7 | 53.3 ± 5.3 | 0.988 |
| TAPSE (mm) | 22.6 ± 3.3 | 23.3 ± 4.1 | 0.497 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandels, J.; Metze, M.; Stöbe, S.; Do, L.; Möbius-Winkler, M.N.; Antoniadis, M.; Hagendorff, A.; Marshall, R.P. Long-Term Follow-Up of Professional Soccer Players: The Analyses of Left and Right Heart Morphology and Function by Conventional, Three-Dimensional, and Deformation Analyses. Diagnostics 2025, 15, 1745. https://doi.org/10.3390/diagnostics15141745
Kandels J, Metze M, Stöbe S, Do L, Möbius-Winkler MN, Antoniadis M, Hagendorff A, Marshall RP. Long-Term Follow-Up of Professional Soccer Players: The Analyses of Left and Right Heart Morphology and Function by Conventional, Three-Dimensional, and Deformation Analyses. Diagnostics. 2025; 15(14):1745. https://doi.org/10.3390/diagnostics15141745
Chicago/Turabian StyleKandels, Joscha, Michael Metze, Stephan Stöbe, Lisa Do, Maximilian Nicolas Möbius-Winkler, Marios Antoniadis, Andreas Hagendorff, and Robert Percy Marshall. 2025. "Long-Term Follow-Up of Professional Soccer Players: The Analyses of Left and Right Heart Morphology and Function by Conventional, Three-Dimensional, and Deformation Analyses" Diagnostics 15, no. 14: 1745. https://doi.org/10.3390/diagnostics15141745
APA StyleKandels, J., Metze, M., Stöbe, S., Do, L., Möbius-Winkler, M. N., Antoniadis, M., Hagendorff, A., & Marshall, R. P. (2025). Long-Term Follow-Up of Professional Soccer Players: The Analyses of Left and Right Heart Morphology and Function by Conventional, Three-Dimensional, and Deformation Analyses. Diagnostics, 15(14), 1745. https://doi.org/10.3390/diagnostics15141745






