Radiologic Predictors of Disease Recurrence in Nasopharyngeal Carcinoma: A Retrospective Evaluation of MRI and 18F-FDG-PET/CT Parameters
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NPC | Nasopharyngeal carcinoma |
| EBV | Epstein–Barr virus |
| IMRT | Intensity-modulated radiotherapy |
| CCRT | Concurrent chemoradiotherapy |
| PPS | Parapharyngeal space |
| SBI | Skull base invasion |
| ICS | Intracranial space |
| PNI | Perineural invasion |
| SUV | Standardized uptake value |
| TLG | Total lesion glycolysis |
| MTV | Metabolic tumor volume |
| PT | Primary Tumor |
| LN | Lymph node |
| DFS | Disease-free survival |
| LRRFS | Locoregional recurrence-free survival |
| DMFS | Distant metastasis-free survival |
| OS | Overall survival |
| GTV | Gross tumor volume |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Abdulamir, A.; Hafidh, R.; Abdulmuhaimen, N.; Abubakar, F.; Abbas, K. The distinctive profile of risk factors of nasopharyngeal carcinoma in comparison with other head and neck cancer types. BMC Public Health 2008, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Adham, M.; Kurniawan, A.N.; Muhtadi, A.I.; Roezin, A.; Hermani, B.; Gondhowiardjo, S.; Tan, I.B.; Middeldorp, J.M. Nasopharyngeal carcinoma in Indonesia: Epidemiology, incidence, signs, and symptoms at presentation. Chin. J. Cancer 2012, 31, 185. [Google Scholar] [CrossRef]
- Chang, E.T.; Ye, W.; Zeng, Y.-X.; Adami, H.-O. The evolving epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1035–1047. [Google Scholar] [CrossRef]
- Chua, M.L.K.; Wee, J.T.S.; Hui, E.P.; Chan, A.T.C. Nasopharyngeal carcinoma. Lancet 2016, 387, 1012–1024. [Google Scholar] [CrossRef]
- Jen, C.-W.; Tsai, Y.-C.; Wu, J.-S.; Chen, P.-L.; Yen, J.-H.; Chuang, W.-K.; Cheng, S.H.-C. Prognostic classification for patients with nasopharyngeal carcinoma based on American Joint Committee on Cancer staging system T and N categories. Ther. Radiol. Oncol. 2020, 4, 2. [Google Scholar] [CrossRef]
- Guo, L.-L.; Wang, H.-Y.; Zheng, L.-S.; Wang, M.-D.; Cao, Y.; Li, Y.; Liu, Z.-J.; Peng, L.-X.; Huang, B.-J.; Shao, J.-Y. Metastasis of nasopharyngeal carcinoma: What we know and do not know. Vis. Cancer Med. 2021, 2, 4. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, L.-H.; Chen, Y.-P.; Liu, X.; Zhou, G.-Q.; Lin, A.-H.; Sun, Y.; Ma, J. Chemoradiotherapy versus radiotherapy alone in stage II nasopharyngeal carcinoma: A systemic review and meta-analysis of 2138 patients. J. Cancer 2017, 8, 287. [Google Scholar] [CrossRef]
- Chen, L.; Hu, C.-S.; Chen, X.-Z.; Hu, G.-Q.; Cheng, Z.-B.; Sun, Y.; Li, W.-X.; Chen, Y.-Y.; Xie, F.-Y.; Liang, S.-B. Adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: Long-term results of a phase 3 multicentre randomised controlled trial. Eur. J. Cancer 2017, 75, 150–158. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Tang, L.-L.; Yang, Q.; Poh, S.-S.; Hui, E.P.; Chan, A.T.; Ong, W.-S.; Tan, T.; Wee, J.; Li, W.-F. Induction chemotherapy plus concurrent chemoradiotherapy in endemic nasopharyngeal carcinoma: Individual patient data pooled analysis of four randomized trials. Clin. Cancer Res. 2018, 24, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Colevas, A.D.; Yom, S.S.; Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J. NCCN guidelines insights: Head and neck cancers, version 1.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Vignon Whaley, J.J.; Afkhami, M.; Onyshchenko, M.; Massarelli, E.; Sampath, S.; Amini, A.; Bell, D.; Villaflor, V.M. Recurrent/metastatic nasopharyngeal carcinoma treatment from present to future: Where are we and where are we heading? Curr. Treat. Options Oncol. 2023, 24, 1138–1166. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, D.; Liao, X.; Lu, Y.; Yu, B.; Xu, M.; Bin, Y.; Zhou, P.; Yang, Z.; Liu, K. Failure patterns of recurrence and metastasis after intensity-modulated radiotherapy in patients with nasopharyngeal carcinoma: Results of a multicentric clinical study. Front. Oncol. 2022, 11, 693199. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Zhou, J.-Y.; Zhan, Z.-J.; Ke, L.-R.; Xia, W.-X.; Cao, X.; Cai, Z.-C.; Deng, Y.; Chen, X.; Zhang, L.-L. Tumor residue in patients with stage II–IVA nasopharyngeal carcinoma who received intensity-modulated radiation therapy: Development and validation of a prediction nomogram integrating postradiotherapy plasma Epstein–Barr virus deoxyribonucleic acid, clinical stage, and radiotherapy dose. BMC Cancer 2023, 23, 410. [Google Scholar]
- Zanoni, D.K.; Patel, S.G.; Shah, J.P. Changes in the 8th edition of the American Joint Committee on Cancer (AJCC) staging of head and neck cancer: Rationale and implications. Curr. Oncol. Rep. 2019, 21, 52. [Google Scholar] [CrossRef]
- Chen, W.-S.; Li, J.-J.; Hong, L.; Xing, Z.-B.; Wang, F.; Li, C.-Q. Comparison of MRI, CT and 18F-FDG PET/CT in the diagnosis of local and metastatic of nasopharyngeal carcinomas: An updated meta analysis of clinical studies. Am. J. Transl. Res. 2016, 8, 4532. [Google Scholar]
- Chan, S.-C.; Yeh, C.-H.; Yen, T.-C.; Ng, S.-H.; Chang, J.T.-C.; Lin, C.-Y.; Yen-Ming, T.; Fan, K.-H.; Huang, B.-S.; Hsu, C.-L. Clinical utility of simultaneous whole-body 18 F-FDG PET/MRI as a single-step imaging modality in the staging of primary nasopharyngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1297–1308. [Google Scholar] [CrossRef]
- Xie, H.J.; Sun, X.S.; Zhang, X.; Xiao, B.B.; Lin, D.F.; Lin, X.P.; Lv, X.F.; Liu, L.Z.; Han, F.; Zou, R.H.; et al. Head and neck MRI-based T stage and [18F]FDG PET/CT-based N/M stage improved prognostic stratification in primary nasopharyngeal carcinoma. Eur. Radiol. 2023, 33, 7952–7966. [Google Scholar] [CrossRef]
- Peng, H.; Chen, L.; Tang, L.-L.; Li, W.-F.; Mao, Y.-P.; Guo, R.; Zhang, Y.; Liu, L.-Z.; Tian, L.; Zhang, X. Significant value of 18 F-FDG-PET/CT in diagnosing small cervical lymph node metastases in patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Chin. J. Cancer 2017, 36, 95. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Mao, Y.; Chen, L.; Tang, L.; Zhou, G.; Sun, Y.; Yue, D.; Lin, A.; Li, L. Prognostic value of magnetic resonance imaging-detected cranial nerve invasion in nasopharyngeal carcinoma. Br. J. Cancer 2014, 110, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Guan, W.; Huang, W.; Cui, C.; Li, H.; Ruan, G.; Liu, L.; Zhao, Q.; Ma, H. Carotid space involvement is a prognostic factor and marker for induction chemotherapy in patients with nasopharyngeal carcinoma. Oral Oncol. 2022, 135, 106230. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Quan, T.; Zhao, Q.; Li, S.; Cai, Y.; Zhou, J.; Luo, C.; Ruan, G.; Cui, C.; Liang, S. MRI of nasopharyngeal carcinoma: Parapharyngeal subspace involvement has prognostic value and influences T-staging in the IMRT era. Eur. Radiol. 2022, 32, 262–271. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Huang, Y.; Hu, X.F.; Chen, X.P.; Xu, T.; Liu, L.Z.; Wei, W.H.; Huang, G.S.; Zhou, M.M.; Huang, Z.L.; et al. Prognostic value of classifying parapharyngeal extension in nasopharyngeal carcinoma based on magnetic resonance imaging. Biomed. Res. Int. 2015, 2015, 749515. [Google Scholar] [CrossRef]
- Cheng, Y.-K.; Liu, L.-Z.; Jiang, N.; Yue, D.; Tang, L.-L.; Zhang, F.; Lin, L.; Liu, X.; Chen, L.; Ma, J. MRI-detected skull-base invasion. Strahlenther. Onkol. 2014, 190, 905. [Google Scholar] [CrossRef]
- Feng, Y.; Cao, C.; Hu, Q.; Chen, X. Grading of MRI-detected skull-base invasion in nasopharyngeal carcinoma with skull-base invasion after intensity-modulated radiotherapy. Radiat. Oncol. 2019, 14, 10. [Google Scholar] [CrossRef]
- Ni, W.; Qi, W.; Xu, F.; Cao, W.; Xu, C.; Chen, J.; Gao, Y. Prognostic value of nasopharynx tumour volume in local-regional advanced nasopharyngeal carcinoma. Ann. Transl. Med. 2020, 8, 223. [Google Scholar] [CrossRef]
- Tian, Y.M.; Xiao, W.W.; Bai, L.; Liu, X.W.; Zhao, C.; Lu, T.X.; Han, F. Impact of primary tumor volume and location on the prognosis of patients with locally recurrent nasopharyngeal carcinoma. Chin. J. Cancer 2015, 34, 247–253. [Google Scholar] [CrossRef]
- Qin, L.; Wu, F.; Lu, H.; Wei, B.; Li, G.; Wang, R. Tumor volume predicts survival rate of advanced nasopharyngeal carcinoma treated with concurrent chemoradiotherapy. Otolaryngol. Head Neck Surg. 2016, 155, 598–605. [Google Scholar] [CrossRef]
- Sze, W.-M.; Lee, A.W.; Yau, T.-K.; Yeung, R.M.; Lau, K.-Y.; Leung, S.K.; Hung, A.W.; Lee, M.C.; Chappell, R.; Chan, K. Primary tumor volume of nasopharyngeal carcinoma: Prognostic significance for local control. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 21–27. [Google Scholar] [CrossRef]
- Shen, C.; Lu, J.J.; Gu, Y.; Zhu, G.; Hu, C.; He, S. Prognostic impact of primary tumor volume in patients with nasopharyngeal carcinoma treated by definitive radiation therapy. Laryngoscope 2008, 118, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Fei, Z.; Pan, J.; Bai, P.; Chen, L. Significance of primary tumor volume and T-stage on prognosis in nasopharyngeal carcinoma treated with intensity-modulated radiation therapy. Jpn. J. Clin. Oncol. 2011, 41, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Sun, Y.; Yu, X.-L.; Yin, W.-J.; Li, W.-F.; Chen, Y.-Y.; Mao, Y.-P.; Liu, L.-Z.; Li, L.; Lin, A.-H. Is primary tumor volume still a prognostic factor in intensity modulated radiation therapy for nasopharyngeal carcinoma? Radiother. Oncol. 2012, 104, 294–299. [Google Scholar] [CrossRef]
- Guo, C.; Luo, J.; Liang, M.; Xiao, J. Correlation of 18F-FDG PET/CT metabolic parameters with Ki-67 expression and tumor staging in nasopharyngeal carcinoma. Nucl. Med. Commun. 2025, 46, 437–443. [Google Scholar] [CrossRef]
- Aktan, M.; Kanyilmaz, G.; Yavuz, B.B.; Koc, M.; Eryilmaz, M.A.; Adli, M. Prognostic value of pre-treatment (18)F-FDG PET uptake for nasopharyngeal carcinoma. Radiol. Med. 2017, 130, 4–12. [Google Scholar] [CrossRef]
- Jin, Y.-N.; Yao, J.-J.; Wang, S.-Y.; Zhang, W.-J.; Zhou, G.-Q.; Zhang, F.; Cheng, Z.-B.; Ma, J.; Mo, H.-Y.; Sun, Y. Prognostic value of primary gross tumor volume and standardized uptake value of 18F-FDG in PET/CT for distant metastasis in locoregionally advanced nasopharyngeal carcinoma. Tumor Biol. 2017, 39, 1010428317717843. [Google Scholar] [CrossRef]
- Huang, C.L.; Chen, Y.; Guo, R.; Mao, Y.P.; Xu, C.; Tian, L.; Liu, L.Z.; Lin, A.H.; Sun, Y.; Ma, J.; et al. Prognostic value of MRI-determined cervical lymph node size in nasopharyngeal carcinoma. Cancer Med. 2020, 9, 7100–7106. [Google Scholar] [CrossRef]
- Chen, B.; Zhan, Z.; Pan, J.; Xiao, Y.; Tang, L.; Guo, Q.; Xu, Y.; Zong, J.; Zhang, R.; Xu, H.; et al. Re-evaluation of the prognostic significance of retropharyngeal node metastasis in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Asia Pac. J. Clin. Oncol. 2022, 18, e173–e181. [Google Scholar] [CrossRef]
- Jiang, C.; Gao, H.; Zhang, L.; Li, H.; Zhang, T.; Ma, J.; Liu, B. Distribution pattern and prognosis of metastatic lymph nodes in cervical posterior to level V in nasopharyngeal carcinoma patients. BMC Cancer 2020, 20, 667. [Google Scholar] [CrossRef]
- Wang, R.-Z.; Zhu, L.-R.; Xu, Y.-C.; Chen, M.-W.; Liang, Z.-G.; Chen, K.-H.; Li, L.; Zhu, X.-D. Development and Validation of a Nomogram Based on the Different Grades of Cervical Lymph Node Necrosis to Predict Overall Survival in Patients with Lymph Node-Positive Locally Advanced Nasopharyngeal Carcinoma. Acad. Radiol. 2025, 32, 3647–3658. [Google Scholar] [CrossRef]
- Fu, Y.C.; Liang, S.B.; Huang, W.J.; Chen, L.S.; Chen, D.M.; Liu, L.Z.; Luo, M.; Zhong, X.F.; Xu, X.Y. Prognostic Value of Lymph Node Necrosis at Different N Stages in Patients with Nasopharyngeal Carcinoma. J. Cancer 2023, 14, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Bin, Y.; Meng, Z.; Huang, L.L.; Hu, X.Y.; Song, J.M.; Xie, Y.T.; Kang, M.; Wang, R.S. Prognostic value of the cervical lymph node necrosis ratio in nasopharyngeal carcinoma. Radiother. Oncol. 2022, 177, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liang, S.; Cui, C.; Zhang, Y.; Xie, F.; Zhou, J.; Dong, A.; Chen, M.; Xie, C.; Li, H.; et al. Prognostic significance of quantitative metastatic lymph node burden on magnetic resonance imaging in nasopharyngeal carcinoma: A retrospective study of 1224 patients from two centers. Radiother. Oncol. 2020, 151, 40–46. [Google Scholar] [CrossRef]
- Zhu, Y.-L.; Deng, X.-L.; Zhang, X.-C.; Tian, L.; Cui, C.-Y.; Lei, F.; Xu, G.-Q.; Li, H.-J.; Liu, L.-Z.; Ma, H.-L. Predicting distant metastasis in nasopharyngeal carcinoma using gradient boosting tree model based on detailed magnetic resonance imaging reports. World J. Radiol. 2024, 16, 203. [Google Scholar] [CrossRef]
- Lin, G.W.; Wang, L.X.; Ji, M.; Qian, H.Z. The use of MR imaging to detect residual versus recurrent nasopharyngeal carcinoma following treatment with radiation therapy. Eur. J. Radiol. 2013, 82, 2240–2246. [Google Scholar] [CrossRef]
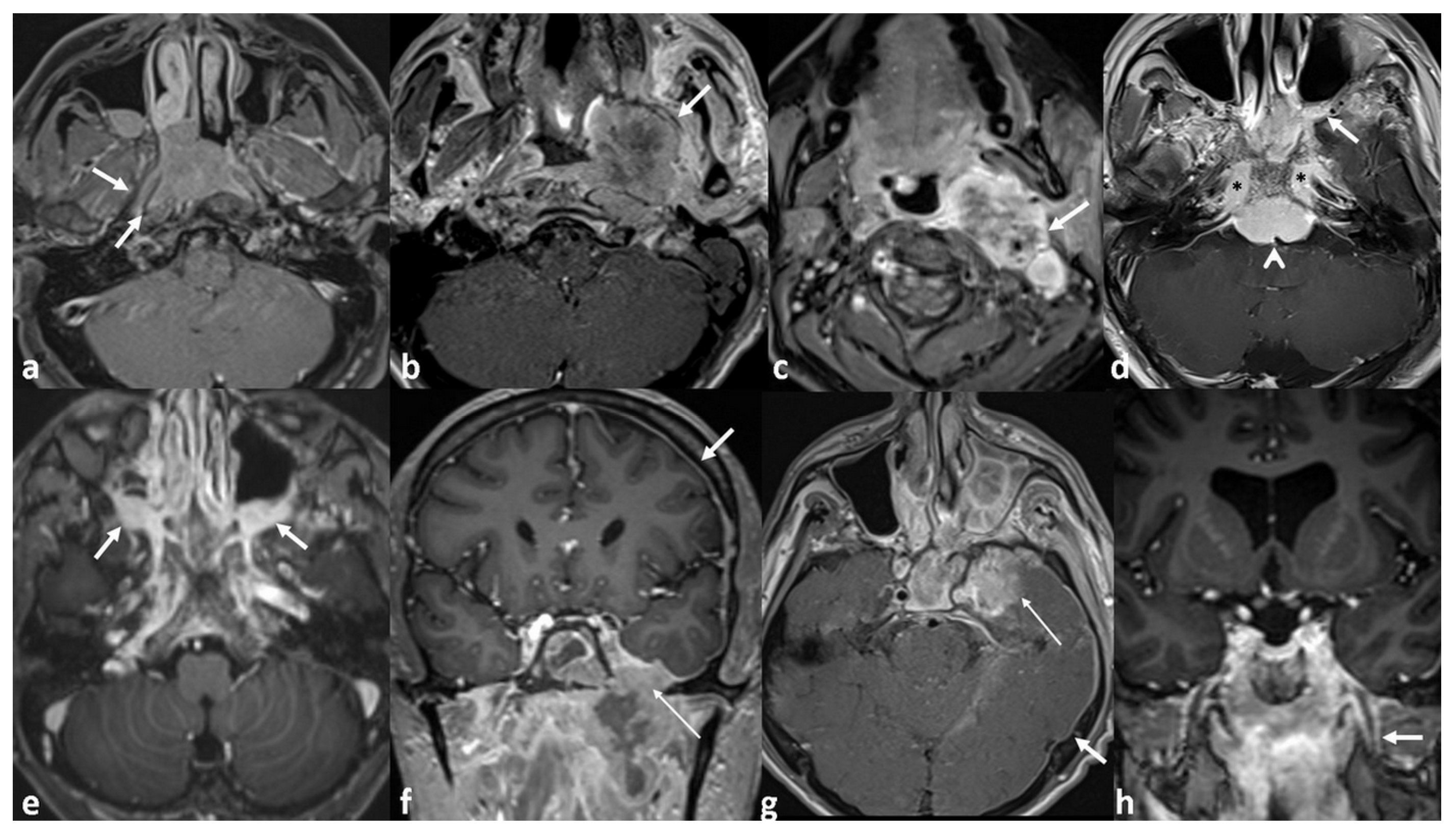
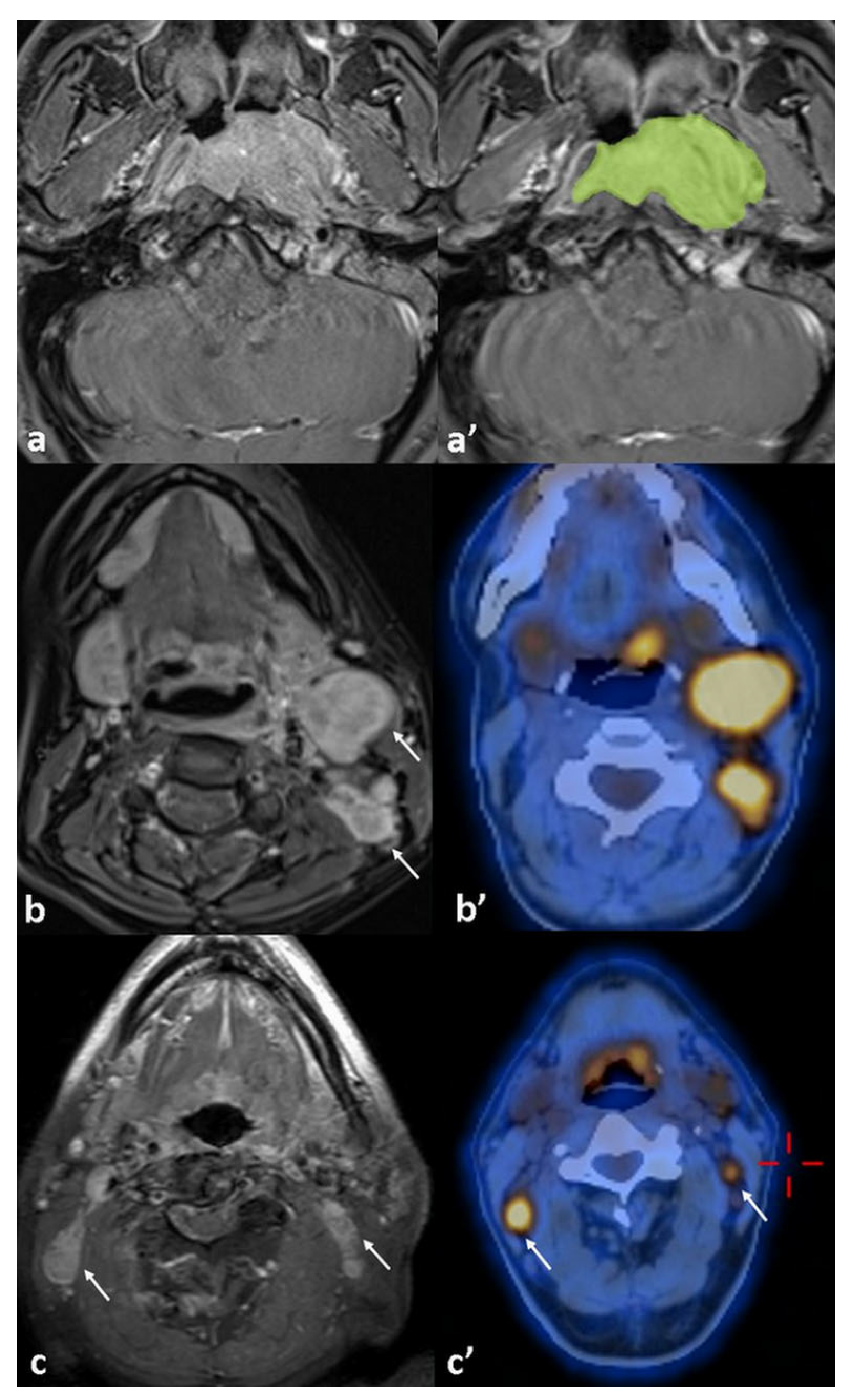
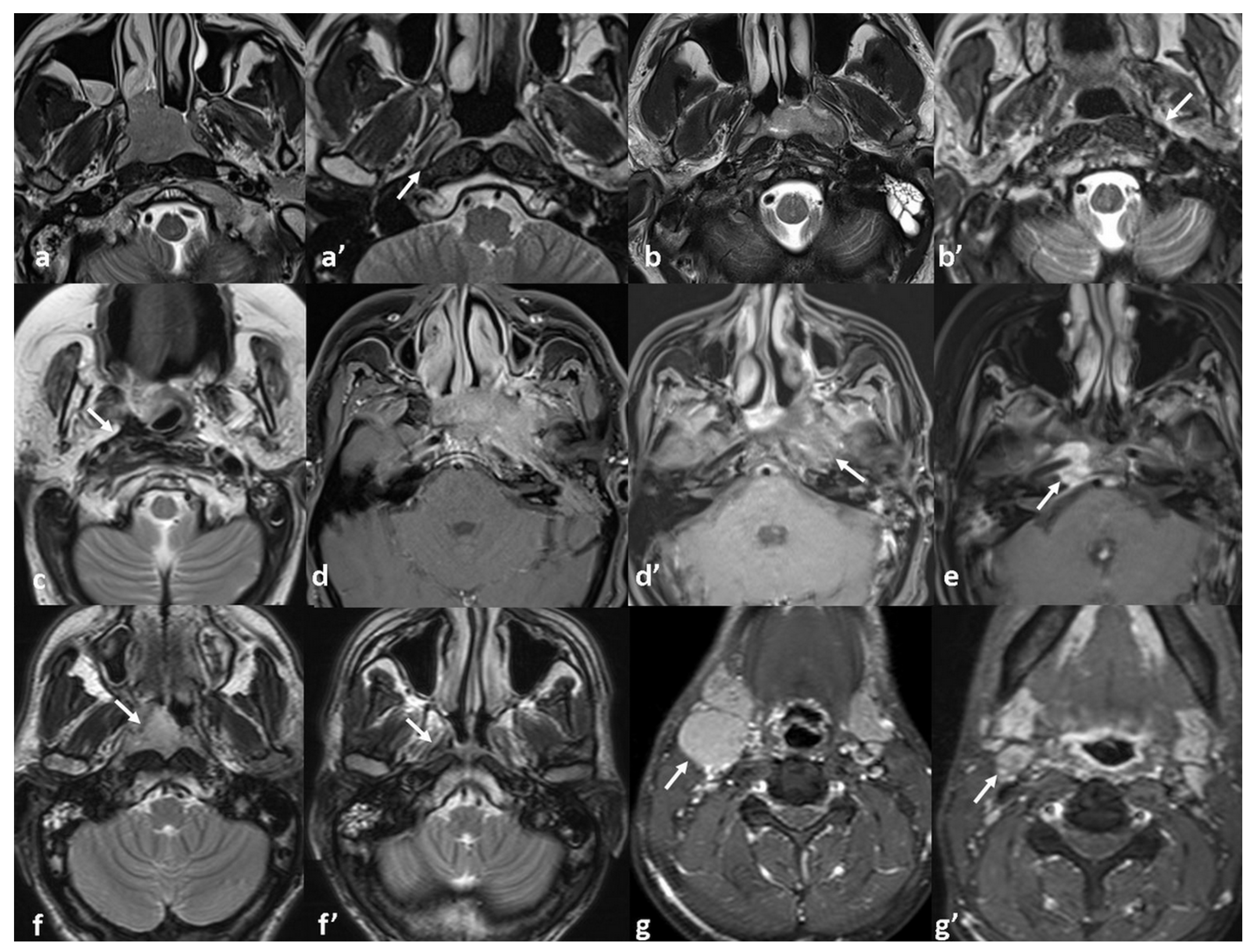
| Classification | Number of Patients (%) | Classification | Number of Patients (%) |
|---|---|---|---|
| Limited PPS invasion Parapharyngeal fat Pre-styloid space Medial pterygoid muscle Retropharygeal space Prevertebral space Nasal cavity Medial pterygoid plate | 25 (48%) 2 (3%) 2 (3%) 4 (7%) 8 (15%) 3 (5%) 5 (5%) | Extensive PPS invasion Carotid canal Lateral pterygoid muscle Infratemporal fossa Temporal muscle Lateral pterygoid plate Pterygomaxillary fissure Middle ear Mastoid space Paranasal sinus Orbital cavity Para-oropharyngeal space | 13 (25%) 3 (5%) 4 (7%) 2 (5%) 5 (5%) 2 (3%) 1 (2%) 1 (2%) 2 (3%) 1 (2%) 1 (2%) |
| 27/52 | 16/52 | ||
| Limited SB invasion Base of sphenoid bone Clivus Petrous bone apex Sphenoid bone greater wing medial border; bony structure around -Vidian canal -Foramen lacerum | 13 (15%) 10 (19%) 2 (3%) 4 (7%) | Extensive SB invasion Pterygopalatine fossa Tegmen tympani Sphenoid bone greater wing and bony structure around -Foramen rotundum -Foramen ovale -Jugular foramen -Hypoglossal canal -Foramen magnum | 4 (7%) 1 (2%) 6 (12%) |
| 13/52 | 7/52 | ||
| Limited ICS invasion Cavernous sinus Retro-clival space Dura mater (around middle cranial fossa) | 7 (13%) 2 (3%) 4 (7%) | Extensive ICS invasion Orbital cavity through orbital fissure and canal Distant dura mater (exp. lateral to temporal lobe) Brain parenchyma | 1 (2%) 3 (5%) |
| 8/52 | 3/52 |
| Patients’ Characteristics | No. of Patients (%) |
|---|---|
| Gender (M/F) | 43/9 |
| Age (yrs), mean ± sd | 46 ± 12 |
| Pathology (Nonkeratinizing SCC) | |
| Differentiated | 31 |
| Undifferentiated | 21 |
| T-Stage (%) | |
| T1 | 7/52 (14%) |
| T2 | 24/52 (46%) |
| T3 | 11/52 (21%) |
| T4 | 10/52 (19%) |
| N-stage | |
| N0 | 5/52 (9%) |
| N1 | 18/52 (35%) |
| N2 | 17/52 (33%) |
| N3 | 12/52 (23%) |
| Overall-Stage (TNM-AJCC 8th addition) | |
| 2 | 13/52 (25%) |
| 3 | 15/52 (29%) |
| 4a | 16/52 (31%) |
| 4b | 8/52 (15%) |
| Metastasis (n) | 8/52 (15%) |
| Bone | 2 (1 = Cervical 5, 1 = Thoracic 6) |
| Lung | 1 |
| Bone + distant LN | 2 (n = 1, axilla; n = 1, retroperitoneum) |
| Bone + Lung | 1 |
| Bone + Liver | 2 |
| MRI and 18F-FDGPET/CT | |
| Time interval (d), mean ± sd | 3 ± 5 |
| Treatment (n) | |
| CCRT | 28 (54%) |
| Induction CT + CCRT | 10 (19%) |
| Induction CT + CCRT + maintenance CT | 14 (27%) |
| Disease recurrence | 14/52 (27%) |
| Local | 8/14 (57%) |
| Distant | 5/14 (36%) |
| Both | 1/14 (7%) |
| Second recurrence | 8/14 (57%) |
| Outcome | |
| Death | 7 (13%) |
| Survive | 43 (83%) |
| Not known | 2 (%4) |
| MRI Response Patterns | Local Tumor Stage | Total | No Recurrence | Recurrence | Total | |||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |||||
| Complete resolution without any sequel signal abnormality | 72% (n = 5) | 29% (n = 7) | 0% (n = 0) | 0% (n = 0) | 23% (n = 12) | 92% (n = 11) | 8% (n = 1) | n = 12 |
| Thin fibrosis at the primary tumor site | 14% (n = 1) | 21% (n = 5) | 36% (n = 4) | 0% (n = 0) | 19% (n = 10) | 90% (n = 9) | 10% (n = 1) | n = 10 |
| Bulky fibrosis without discernible soft tissue or enhancement | 0% (n = 0) | 21% (n = 5) | 46% (n = 5) | 10% (n = 1) | 21% (n = 11) | 73% (n = 8) | 27% (n = 3) | n = 11 |
| Heterogeneous signal with subtle contrast enhancement | 0% (n = 0) | 12% (n = 3) | 18% (n = 2) | 30% (n = 3) | 15% (n = 8) | 50% (n = 4) | 50% (n = 4) | n = 8 |
| Residual enhancing soft tissue | 0% (n = 0) | 0% (n = 0) | 0% (n = 0) | 60% (n = 6) | 12% (n = 6) | 17% (n = 1) | 83% (n = 5) | n = 6 |
| Complete resolution at the primary site with persistent nodal disease | 14% (n = 1) | 17% (n = 4) | 0% (n = 0) | 0% (n = 0) | 10% (n = 5) | 100% (n = 5) | 0% (n = 0) | n = 5 |
| Total | 100% (n = 7) | 100% (n = 24) | 100% (n = 11) | 100% (n = 10) | 100% (n = 52) | 73% (n = 38) | 27% (n = 14) | 100% (n = 52) |
| p < 0.001 * (tau-b = 0.43) | p = 0.004 * (χ2) | |||||||
| Variables, n (%) | Univariate Analysis p | Multivariate Analysis p | |
|---|---|---|---|
| T-stage | 0.060 | ||
| T1 | 1/7 (14%) | ||
| T2 | 4/24 (16.6%) | ||
| T3 | 3/11 (27.2%) | ||
| T4 | 6/10 (60%) | ||
| PT laterality | 0.034 * | 0.692 | |
| Unilateral | 8/35 (22.8%) | ||
| Bilateral | 6/11 (54.5%) | ||
| Central | 0/6 (0%) | ||
| PT Growth Pattern | 0.014 * | 0.897 | |
| Mass-forming | 9/44 (20.4%) | ||
| Infiltrative | 5/8 (62.5%) | ||
| PPS invasion | 0.001 * | 0.027 * OR: 1.23 95%CI [1.03–1.49] | |
| No | 1/9 (11.1%) | ||
| Local | 3/27 (11.1%) | ||
| Extensive | 10/16 (62.5%) | ||
| SB invasion | 0.521 | ||
| No | 7/32 (21.8%) | ||
| Local | 5/13 (38.4%) | ||
| Extensive | 2/7 (28.5%) | ||
| ICS invasion | 0.008 * | 0.076 | |
| No | 7/41 (17%) | ||
| Local | 5/8 (62.5%) | ||
| Extensive | 2/3 (66.6%) | ||
| Perineural invasion | <0.001 * | 0.029 * OR: 1.60 95%CI [1.05–2.43] | |
| Positive | 7/43 (16.2%) | ||
| Negative | 7/9 (77.7%) | ||
| GTV cm3 (mean) | 0.019 * | 0.042 * OR: 1.08 95%CI [1.02–1.16] | |
| No recurrence/recurrence | 11.6 (±1.6)/30.9 (±8.2) | ||
| N-stage | 0.212 | ||
| N0 | 2/5 (40%) | ||
| N1 | 2/18 (11.1%) | ||
| N2 | 7/17 (41.2%) | ||
| N3 | 3/12 (25%) | ||
| Nodal laterality | 0.178 | ||
| Unilateral inv. | 3/22 (14%) | ||
| Bilateral inv. | 9/25 (36%) | ||
| LN diameters (mean ± sd) | |||
| (No recurrence/recurrence) | |||
| Short axis | 22.5 (±2)/23.1 (±2.6) | 0.432 | |
| Long axis | 34.7 (±3.6)/34.4 (±3.9) | 0.481 | |
| LN necrosis | 0.023 * | 0.443 | |
| Absent | 6/35 (17.1%) | ||
| Present | 8/17 (47%) | ||
| No. of Metastatic LN | 0.020 * | 0.031 * OR: 1.20 95%CI [0.02–0.43] | |
| No recurrence/recurrence | 5 (±0.8)/9 (±1.6) | ||
| 8th AJCC TNM Stage | 0.004 * | ||
| Stage 2 | 1/13 (7.7%) | ||
| Stage 3 | 3/15 (20%) | ||
| Stage 4a | 5/16 (31.2%) | ||
| Stage 4b | 5/8 (62.5%) | ||
| 18FDG-PET/CT (PT) | 0.915 0.757 | ||
| MTV | 21.7 (±3.1)/42.2 (±11) | 0.049 * | |
| TLG | 164.4 (±27)/394.2 (±121) | 0.045 * | |
| SUV-max | 14.9 (±1.1)/15.9 (±2) | 0.328 | |
| SUV-mean | 6.8 (±0.4)/7.6 (0.7) | 0.195 | |
| SUV-peak | 12.2 (±0.9)/13.4 (±2) | 0.281 | |
| 18FDG-PET/CT (LN) | |||
| MTV | 19.7 (±4.8)/16.3 (±3.9) | 0.295 | |
| TLG | 149.4 (±38.8)/139.6 (±42.5) | 0.445 | |
| SUV-max | 12 (±1.1)/13.5 (±1.9) | 0.261 | |
| SUV-mean | 5.9 (±0.5)/6.4 (±0.9) | 0.314 | |
| SUV-peak | 9.4 (±1)/10.9 (±1.8) | 0.240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaalioğlu, B.; Çakır, T.; Yazıcı, Ö.; Tekin, M.S.; Karcı, E. Radiologic Predictors of Disease Recurrence in Nasopharyngeal Carcinoma: A Retrospective Evaluation of MRI and 18F-FDG-PET/CT Parameters. Diagnostics 2025, 15, 1646. https://doi.org/10.3390/diagnostics15131646
Karaalioğlu B, Çakır T, Yazıcı Ö, Tekin MS, Karcı E. Radiologic Predictors of Disease Recurrence in Nasopharyngeal Carcinoma: A Retrospective Evaluation of MRI and 18F-FDG-PET/CT Parameters. Diagnostics. 2025; 15(13):1646. https://doi.org/10.3390/diagnostics15131646
Chicago/Turabian StyleKaraalioğlu, Banu, Tansel Çakır, Ömer Yazıcı, Mustafa S. Tekin, and Ebru Karcı. 2025. "Radiologic Predictors of Disease Recurrence in Nasopharyngeal Carcinoma: A Retrospective Evaluation of MRI and 18F-FDG-PET/CT Parameters" Diagnostics 15, no. 13: 1646. https://doi.org/10.3390/diagnostics15131646
APA StyleKaraalioğlu, B., Çakır, T., Yazıcı, Ö., Tekin, M. S., & Karcı, E. (2025). Radiologic Predictors of Disease Recurrence in Nasopharyngeal Carcinoma: A Retrospective Evaluation of MRI and 18F-FDG-PET/CT Parameters. Diagnostics, 15(13), 1646. https://doi.org/10.3390/diagnostics15131646





