Mobile Phone Auscultation Accurately Diagnoses Chronic Obstructive Pulmonary Disease Using Nonlinear Respiratory Biofluid Dynamics
Abstract
1. Introduction
2. Methods
2.1. Settings and Participants
2.2. Data Collection and Storage
2.3. Technology and Analytic Method
3. Results
3.1. COPD Diagnosis
3.2. Subgroup Accuracy
3.3. COPD Assessment Test Survey
3.4. Examination of Low-Dimensional Chaos
3.5. Case Description
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Zhang, M.; Li, Y.; Jiang, Y.; Zhao, W. Prevalence of COPD and its association with socioeconomic status in China: Findings from China Chronic Disease Risk Factor Surveillance 2007. BMC Public Health 2011, 11, 586. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.B.; Hansel, N.N.; Connett, J.E.; Scanlon, P.D.; Tashkin, D.P.; Wise, R.A. Spirometric predictors of lung function decline and mortality in early chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Decramer, M.; Miravitlles, M.; Price, D.; Román-Rodríguez, M.; Llor, C.; Welte, T.; Buhl, R.; Dusser, D.; Samara, K.; Siafakas, N. New horizons in early stage COPD—Improving knowledge, detection and treatment. Respir. Med. 2011, 105, 1576–1587. [Google Scholar] [CrossRef]
- Johnson, K.M.; Bryan, S.; Ghanbarian, S.; Sin, D.D.; Sadatsafavi, M. Characterizing undiagnosed chronic obstructive pulmonary disease: A systematic review and meta-analysis. Respir. Res. 2018, 19, 26. [Google Scholar] [CrossRef]
- Zhong, N.; Wang, C.; Yao, W.; Chen, P.; Kang, J.; Huang, S.; Chen, B.; Wang, C.; Ni, D.; Zhou, Y.; et al. Prevalence of chronic obstructive pulmonary disease in China: A large, population-based survey. Am. J. Respir. Crit. Care Med. 2007, 176, 753–760. [Google Scholar] [CrossRef]
- Cooper, B.G. Limitations to spirometry being performed in ‘the office’. Chronic Respir. Dis. 2005, 2, 113–115. [Google Scholar] [CrossRef]
- Richter, F. Charted: There Are More Mobile Phones Than People in the World. Web Article. World Economic Forum. Available online: https://www.weforum.org/stories/2023/04/charted-there-are-more-phones-than-people-in-the-world/ (accessed on 18 March 2024).
- Heironimus, J.; Steiner, R.; Stromberg, A. Detecting Covid-19 Respiratory Markers with Ordinary Mobile Phones. Int. J. Digit. Healthc. 2022, 2, IJDH-109. [Google Scholar] [CrossRef]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Kline Leidy, N. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef]
- Huecker, M.; Schutzman, C.; French, J.; El-Kersh, K.; Ghafghazi, S.; Desai, R.; Frick, D.; Thomas, J.J. Accurate Modeling of Ejection Fraction and Stroke Volume With Mobile Phone Auscultation: Prospective Case-Control Study. JMIR Cardio 2024, 8, e57111. [Google Scholar] [CrossRef]
- SAEM24 Abstracts. Acad. Emerg. Med. 2024, 31, 8–401. [CrossRef]
- Morris, J.F. Spirometry in the evaluation of pulmonary function. West. J. Med. 1976, 125, 110–118. [Google Scholar] [PubMed]
- Kakavas, S.; Kotsiou, O.S.; Perlikos, F.; Mermiri, M.; Mavrovounis, G.; Gourgoulianis, K.; Pantazopoulos, I. Pulmonary function testing in COPD: Looking beyond the curtain of FEV1. NPJ Prim. Care Respir. Med. 2021, 31, 23. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, E.; Swart, F.; Maree, D.; Heydenreich; Van Heerden, J.; Esterhuizen, T.M.; Irusen, E.M.; Koegelenberg, C.F.N. The utility of hand-held mobile spirometer technology in a resource-constrained setting. S. Afr. Med. J. 2019, 109, 219–222. [Google Scholar] [CrossRef]
- Exarchos, K.P.; Gogali, A.; Sioutkou, A.; Chronis, C.; Peristeri, S.; Kostikas, K. Validation of the portable Bluetooth(R) Air Next spirometer in patients with different respiratory diseases. Respir. Res. 2020, 21, 79. [Google Scholar] [CrossRef]
- Jankowski, P.; Górska, K.; Mycroft, K.; Korczyński, P.; Soliński, M.; Kołtowski, Ł.; Krenke, R. The use of a mobile spirometry with a feedback quality assessment in primary care setting—A nationwide cross-sectional feasibility study. Respir. Med. 2021, 184, 106472. [Google Scholar] [CrossRef]
- Kupczyk, M.; Hofman, A.; Kołtowski, Ł.; Kuna, P.; Łukaszyk, M.; Buczyłko, K.; Bodzenta-Łukaszyk, A.; Nastałek, P.; Soliński, M.; Dąbrowiecki, P. Home self-monitoring in patients with asthma using a mobile spirometry system. J. Asthma. 2021, 58, 505–511. [Google Scholar] [CrossRef]
- Ramos Hernández, C.; Núñez Fernández, M.; Pallares Sanmartín, A.; Mouronte Roibas, C.; Cerdeira Domínguez, L.; Botana Rial, M.I.; Blanco Cid, N.; Fernández Villar, A. Validation of the portable Air-Smart Spirometer. PLoS ONE 2018, 13, e0192789. [Google Scholar] [CrossRef]
- Sekerel, B.E.; Unsal, H.; Sahiner, U.M.; Uysal Soyer, O.; Damadoglu, E.; Karakaya, G.; Kalyoncu, A.F. Clinical Validation of the Spirohome Clinic Ultrasonic Spirometer in Child and Adolescent Patients. J. Asthma Allergy. 2022, 15, 219–229. [Google Scholar] [CrossRef]
- Sharan, R.V.; Abeyratne, U.R.; Swarnkar, V.R.; Claxton, S.; Hukins, C.; Porter, P. Predicting spirometry readings using cough sound features and regression. Physiol. Meas. 2018, 39, 095001. [Google Scholar] [CrossRef]
- Irina, B.P.; Steluta, M.M.; Emanuela, T.; Diana, M.; Cristina, O.D.; Mirela, F.; Cristian, O. Respiratory muscle training program supplemented by a cell-phone application in COPD patients with severe airflow limitation. Respir. Med. 2021, 190, 106679. [Google Scholar] [CrossRef] [PubMed]
- Emokpae, L.E.; Emokpae, R.N., Jr.; Bowry, E.; Bin Saif, J.; Mahmud, M.; Lalouani, W.; Younis, M.; Joyner, R.L., Jr. A wearable multi-modal acoustic system for breathing analysis. J. Acoust. Soc. Am. 2022, 151, 1033. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, Y.; Yang, C.; Hu, H.; Xiong, Z. Mobile health applications in self-management of patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis of their efficacy. BMC Pulm. Med. 2018, 18, 147. [Google Scholar] [CrossRef] [PubMed]
- Glynn, L.; Mc Cann, M.; Mc Cabe, C. Smartphone applications supporting self-management programme for adults with Chronic Obstructive Pulmonary Disease: A Scoping Review. PLoS Digit. Health 2024, 3, e0000532. [Google Scholar] [CrossRef]
- Quach, S.; Benoit, A.; Oliveira, A.; Packham, T.L.; Goldstein, R.; Brooks, D. Features and characteristics of publicly available mHealth apps for self-management in chronic obstructive pulmonary disease. Digit Health 2023, 9. [Google Scholar] [CrossRef]
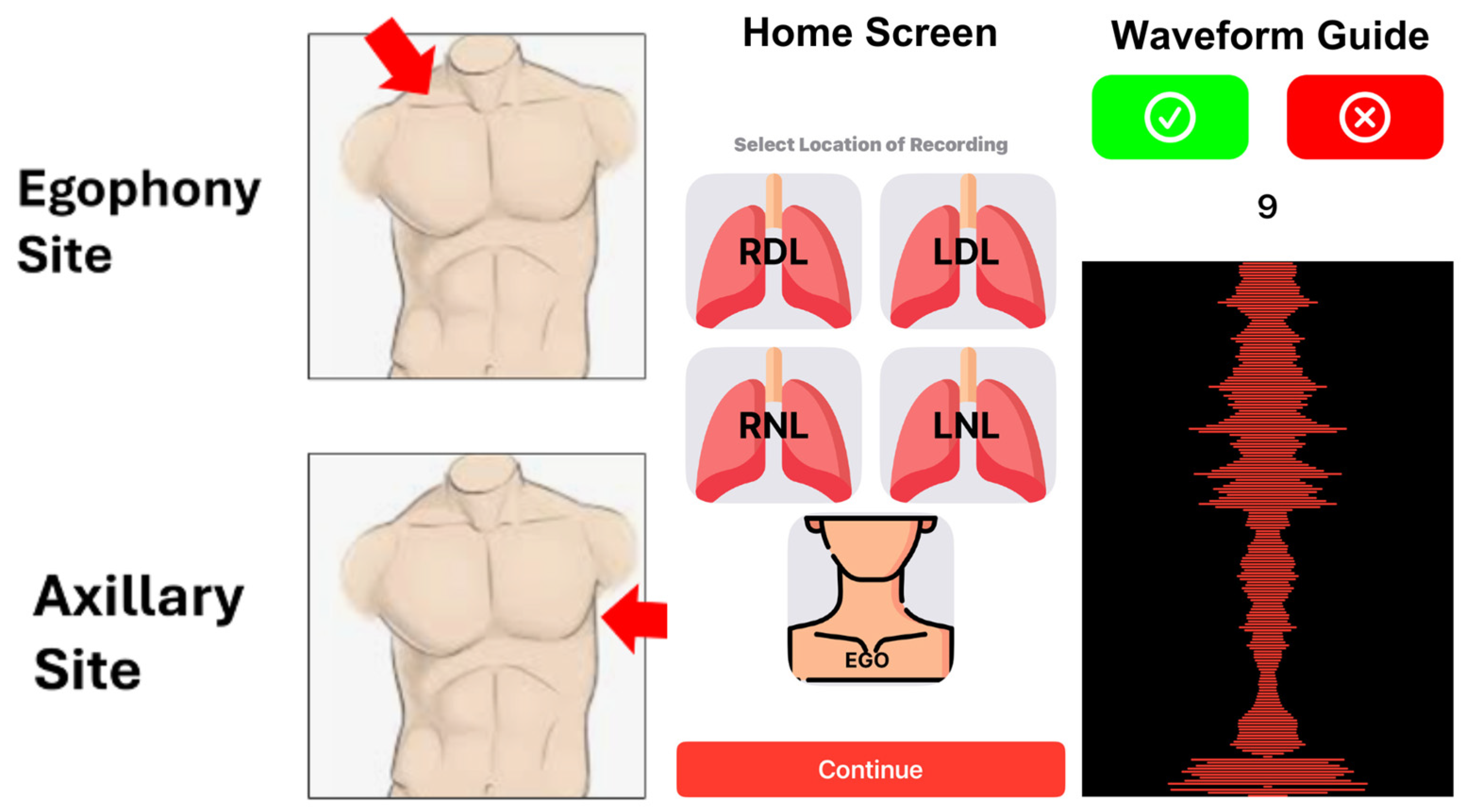

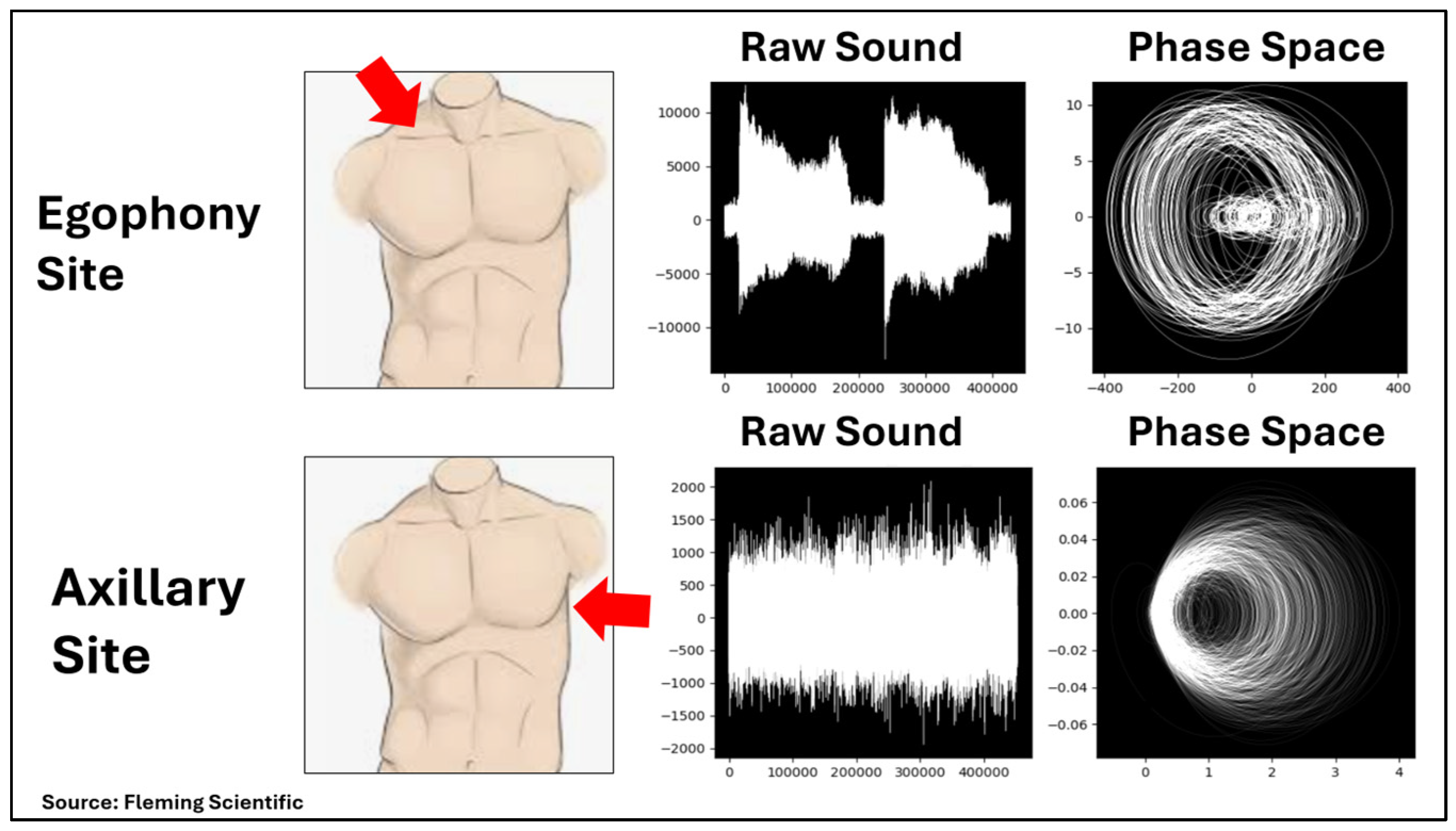
| Variable | Total (n = 108) | COPD Group (n = 52) | Comparison Group (n = 56) | p ** |
|---|---|---|---|---|
| Age—years, Median (IQR) | 61 (50–68.5) | 63 (56–70) | 57.5 (39–66.5) | 0.03 * |
| Female sex—n (%) | 71 (65.7) | 34 (65.4) | 37 (66.1) | 0.94 |
| Race—n (%) | ||||
| White | 64 (59.3) | 31 (59.6) | 33 (58.9) | 0.63 |
| Black/African American | 43 (39.8) | 21 (40.4) | 22 (39.3) | |
| Asian | 1 (0.9) | 0 (0.0) | 1 (1.8) | |
| Ethnicity, Non-Hispanic—n (%) | 108 (100) | 52 (100) | 56 (100) | NA |
| BMI kg/m2—N, median (IQR) | 29.7 (24.5–35.5) | 28.4 (23.5–31.2) | 31.2 (25.1–37.5) | 0.02 * |
| BMI Category—n (%) | n = 107 | n = 51 | n = 56 | |
| <18.5 | 3 (2.8) | 3 (5.9) | 0 (0.0) | 0.11 |
| 18.5–24.9 | 29 (27.1) | 15 (29.4) | 14 (25.0) | 0.65 |
| 25–25.9 | 23 (21.5) | 13 (25.5) | 10 (17.9) | 0.37 |
| 30–34.9 | 25 (23.4) | 14 (27.6) | 11 (19.6) | 0.37 |
| ≥35.0 | 27 (25.2) | 6 (11.8) | 21 (37.5) | 0.002 |
| Medical co-morbidities | ||||
| 0 | 4 (3.7) | 1 (1.9) | 3 (5.4) | 0.62 |
| 1 | 15 (13.9) | 7 (13.5) | 8 (14.3) | 0.90 |
| 2 | 23 (21.3) | 13 (25.0) | 10 (17.9) | 0.37 |
| 3 | 19 (17.6) | 11 (21.2) | 8 (14.3) | 0.35 |
| 4 | 11 (10.2) | 5 (9.6) | 6 (10.7) | 0.85 |
| 5+ | 35 (32.4) | 14 (26.9) | 21 (37.5) | 0.24 |
| Performance Test | Auscultation Source | |||||
|---|---|---|---|---|---|---|
| Egophony | Axillary | Composite | ||||
| Train | Test | Train | Test | Train | Test | |
| Area Under the Curve | 0.92 | 0.87 | 0.98 | 0.87 | 0.99 | 0.96 |
| Sensitivity | 0.93 | 0.83 | 0.98 | 0.83 | 1.00 | 0.92 |
| Specificity | 0.91 | 0.90 | 0.98 | 0.90 | 0.98 | 1.00 |
| BMI > 30 | BMI ≤ 30 | Age < 50 | Age ≥ 50 < 65 | Age ≥ 65 | Male | Female | White | Not White | |
|---|---|---|---|---|---|---|---|---|---|
| N | 53 | 55 | 24 | 47 | 37 | 37 | 71 | 64 | 44 |
| N—Train | 42 | 44 | 16 | 39 | 31 | 26 | 60 | 49 | 37 |
| N—Test | 11 | 11 | 8 | 8 | 6 | 11 | 11 | 15 | 7 |
| Train Accuracy | 100% | 98% | 100% | 100% | 97% | 96% | 100% | 100% | 97% |
| Test Accuracy | 91% | 100% | 100% | 100% | 83% | 91% | 100% | 93% | 100% |
| Hx Asthma | Hx No Asthma | Hx ILD | Hx No ILD | Hx HTN | Hx No HTN | Hx CAD | Hx No CAD | ||
| N | 33 | 75 | 19 | 89 | 58 | 50 | 16 | 92 | |
| N—Train | 25 | 61 | 16 | 70 | 44 | 42 | 13 | 73 | |
| N—Test | 8 | 14 | 3 | 19 | 14 | 8 | 3 | 19 | |
| Train Accuracy | 100% | 98% | 94% | 100% | 98% | 100% | 99% | 99% | |
| Test Accuracy | 100% | 93% | 100% | 95% | 93% | 100% | 100% | 100% | |
| Hx HLD | Hx No HLD | Hx DM2 | Hx No DM2 | Hx CKD/ESRD | Hx No CKD/ESRD | Hx HF | Tob Ever | Tob Never | |
| N | 49 | 59 | 25 | 83 | 17 | 91 | 11 | 80 | 28 |
| N—Train | 41 | 45 | 18 | 68 | 14 | 72 | 9 | 66 | 20 |
| N—Test | 8 | 14 | 7 | 15 | 3 | 19 | 2 | 14 | 8 |
| Train Accuracy | 98% | 100% | 100% | 99% | 93% | 100% | 100% | 98% | 100% |
| Test Accuracy | 88% | 100% | 86% | 100% | 100% | 95% | 100% | 93% | 100% |
| Variable | Total | COPD Group | Comparison Group | p |
|---|---|---|---|---|
| Spirometry | ||||
| Forced Vital Capacity (FVC) | ||||
| Pre Z-score—n, mean (SD) | 108, −1.04 (1.37) | 52, −1.24 (1.45) | 56, −0.86 (1.27) | 0.15 |
| Pre—n, median (IQR) | 108, 2.80 (2.19–3.42) | 52, 2.62 (2.14–3.22) | 56, 2.86 (2.31–3.78) | 0.07 |
| Pre-percent predicted—n, mean (SD) | 108, 84.6 (20.1) | 52, 81.7 (21.9) | 56, 87.4 (17.9) | 0.14 |
| Post Z-score—n, mean (SD) | 50, −0.63 (1.36) | 21, −0.62 (1.58) | 29, −0.64 (1.21) | 0.96 |
| Post—n, mean (SD) | 57, 3.30 (1.13) | 26, 3.11 (1.10) | 31, 3.45 (1.14) | 0.27 |
| Post-percent predicted—n, mean (SD) | 57, 90.5 (20.6) | 26, 90.3 (24.6) | 31, 90.6 (17.0) | 0.97 |
| Forced Expiratory Volume in one second (FEV1) | ||||
| Z-score—n, mean (SD) | 108, −1.64 (1.60) | 52, −2.43 (1.66) | 56, −0.90 (1.14) | <0.0001 |
| Pre—n, median (IQR) | 108, 1.9 (1.40–2.47) | 52, 1.40 (1.00–1.90) | 56, 2.30 (1.81–2.93) | <0.0001 |
| FEV1 percent predicted—n, median (IQR) | 108, 0.73 (0.54–0.92) | 52, 0.54 (0.40–0.71) | 56, 0.85 (0.73–1.00) | <0.0001 |
| FEV1/FVC—n, mean (SD) | 58, 68.7 (15.4) | 27, 56.30 (10.47) | 31, 79.52 (9.75) | <0.0001 |
| Lung Volumes—median (IQR) | ||||
| Total Lung Volumes (TLC = Total Lung Capacity) | n = 98 | n = 47 | n = 51 | |
| TLC ULN | 6.60 (5.88–8.24) | 6.51 (5.83–8.24) | 6.93 (5.97–8.37) | 0.53 |
| TLC LLN | 4.46 (3.91–5.40) | 4.38 (3.85–5.40) | 4.69 (3.95–5.54) | 0.54 |
| TLC Z-Score | −0.54 (−1.95–0.39) | 0.17 (−0.51–0.63) | −1.54 (−2.5–−0.48) | <0.0001 |
| TLC Pre | 5.19 (4.39–6.31) | 5.67 (5.01–6.51) | 4.70 (3.76–5.78) | 0.0004 |
| TLC Pre-percent predicted | 93 (78–105) | 103 (94–108) | 83 (70–94) | <0.0001 |
| Residual Volumes (RV) | n = 97 | n = 47 | n = 50 | |
| RV ULN | 2.73 (2.50–3.06) | 2.78 (2.57–3.05) | 2.71 (2.4–3.1) | 0.5159 |
| RV LLN | 1.08 (0.97–1.28) | 1.09 (1.03–1.32) | 1.07 (0.79–1.28) | 0.2451 |
| RV Pre | 2.13 (1.54–2.70) | 2.70 (2.17–3.63) | 1.65 (1.15–2.10) | <0.0001 |
| RV Pre-percent predicted | 110 (85–140) | 141 (117–191) | 88 (75–106) | <0.0001 |
| Residual volume percent Total Lung volume | n = 97 | n = 47 | n = 50 | |
| RV% TLC ULN | 45 (38–49) | 46 (40–48) | 43 (35–50) | 0.2373 |
| RV% TLC LLN | 23 (18–25) | 24 (19–25) | 21 (16–26) | 0.2826 |
| RV% TLC Pre | 39 (31–48) | 47 (41–58) | 34 (28–38) | <0.0001 |
| RV% TLC Pre-percent predicted | 121 (105–151) | 146 (126–172) | 110 (92–121) | <0.0001 |
| Diffusing Capacity—n, median (IQR) | ||||
| DLCO Z-score | 98, −2.36 (−4.22–−1.09) | 48, −3.15 (−4.52–−1.93) | 50, −1.39 (−3.92–−0.11) | 0.0058 |
| DLCO Pre | 97, 13.96 (9.00–20.16) | 47, 13.05 (8.84–15.51) | 50, 16.65 (9.83–23.83) | 0.0115 |
| DLCO Pre-percent predicted | 98, 67 (44–84) | 48, 59 (44–73) | 50, 79 (44–97) | 0.0103 |
| Variable | Total n = 108 | COPD n = 52 | Control n = 56 | p |
|---|---|---|---|---|
| Symptoms | ||||
| Cough | 3 (1.5–4) | 3 (1.5–4) | 2 (1.5–4) | 0.6889 |
| Phlegm | 2 (0.5–3.0) | 2.5 (1–4) | 2 (0–3) | 0.1706 |
| Breathlessness | 3 (2–5) | 4 (2–5) | 3 (2–5) | 0.6273 |
| Chest tightness | 1 (0–3) | 1 (0–3) | 1 (0–3) | 0.7245 |
| Activities | 3 (0–4) | 3 (1–4) | 2 (0–3.5) | 0.1743 |
| Confidence | 0 (0–1.5) | 0 (0–2) | 0 (0–1) | 0.5134 |
| Sleep | 2 (0–3) | 2 (0–3.5) | 2 (0–3) | 0.6216 |
| Energy | 3 (2–4) | 3 (2–4) | 3 (2–3.5) | 0.3302 |
| CAT Score Total | 16 (11.5–25) | 17 (12–26) | 16 (10–23.5) | 0.2883 |
| Health Impact Category—n (%) | ||||
| Low | 25 (23.1) | 9 (17.3) | 16 (28.6) | 0.17 |
| Medium | 45 (41.7) | 22 (42.3) | 23 (41.1) | 0.90 |
| High | 30 (27.8) | 17 (32.7) | 13 (23.2) | 0.27 |
| Very High | 8 (7.4) | 4 (7.7) | 4 (7.1) | 0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gosser, C.E.; Daniel, L.; Huecker, M.; Cavallazzi, R.; Rivas, H.; Thomas, J.J.; Close, R. Mobile Phone Auscultation Accurately Diagnoses Chronic Obstructive Pulmonary Disease Using Nonlinear Respiratory Biofluid Dynamics. Diagnostics 2025, 15, 1550. https://doi.org/10.3390/diagnostics15121550
Gosser CE, Daniel L, Huecker M, Cavallazzi R, Rivas H, Thomas JJ, Close R. Mobile Phone Auscultation Accurately Diagnoses Chronic Obstructive Pulmonary Disease Using Nonlinear Respiratory Biofluid Dynamics. Diagnostics. 2025; 15(12):1550. https://doi.org/10.3390/diagnostics15121550
Chicago/Turabian StyleGosser, Caroline Emily, Luther Daniel, Martin Huecker, Rodrigo Cavallazzi, Hiram Rivas, Jarred Jeremy Thomas, and Ryan Close. 2025. "Mobile Phone Auscultation Accurately Diagnoses Chronic Obstructive Pulmonary Disease Using Nonlinear Respiratory Biofluid Dynamics" Diagnostics 15, no. 12: 1550. https://doi.org/10.3390/diagnostics15121550
APA StyleGosser, C. E., Daniel, L., Huecker, M., Cavallazzi, R., Rivas, H., Thomas, J. J., & Close, R. (2025). Mobile Phone Auscultation Accurately Diagnoses Chronic Obstructive Pulmonary Disease Using Nonlinear Respiratory Biofluid Dynamics. Diagnostics, 15(12), 1550. https://doi.org/10.3390/diagnostics15121550






